Abstract
Introduction: Substance use is an important risk factor for HIV, with both concentrated in certain vulnerable and marginalized populations. Although their management differs, there may be opportunities to integrate services for substance use and HIV. In this paper we systematically review evidence from studies that sought to integrate care for people living with HIV and substance use problems.
Methods: Studies were included if they evaluated service integration for substance use and HIV. We searched multiple databases from inception until October 2015. Articles were screened independently by two reviewers and assessed for risk of bias.
Results and discussion: 11,057 records were identified, with 7616 after removal of duplicates. After screening titles and abstracts, 51 met the inclusion criteria. Integration models were categorized by location (HIV, substance use and other facilities), level of integration from mirco (integrated care delivered to individuals) to macro (system level integrations) and degree of integration from least (screening and counselling only) to most (care for HIV, substance use and/or other illnesses at the same facility). Most reported descriptive or cohort studies; in four randomized control trials integrated activities improved patient outcomes. There is potential for integrating services at all facility types, including mobile health services. While services offering screening only can achieve synergies, there are benefits from delivering integrated treatment for HIV and substance use, including ease of referral to other mental health and social services.
Conclusions: Our review used a wide range of databases and conference archives to increase representation of papers from low- and middle-income countries. Limitations include the overrepresentation of studies from the United States, and the descriptive nature of the majority of papers. The evidence reviewed shows that greater integration offers important benefits in both patient and service outcomes but further research and outcome reporting is needed to better understand innovative and holistic care models at the complex intersection of substance use and HIV services.
Keywords: substance use, HIV, integration, public health, systematic review
Introduction
People living with HIV often have other health-related problems, either as a consequence of immune suppression, treatment effects, shared risk factors, or a combination thereof [1–4]. This recognition has stimulated interest in the scope for integrating services traditionally provided separately where there is demonstrable patient population overlap. One such area is the intersection of substance use, especially involving exchange of blood, and the risk of HIV. Persons Who Inject Drugs (PWID) are 22 times more likely to acquire HIV than adults in the general population [5]. Indeed, despite decreasing global HIV transmission, PWID in many regions are experiencing marginally increasing HIV infections and worldwide an estimated 1.7 million PWIDs live with HIV [6–9]. PWID often face service exclusion, poor or fragmented care access and discrimination, which when coupled with punitive laws and reluctance to fund harm reduction programs, contribute to their growing HIV burden [9]. The overlap with substance use extends beyond injecting drug use, heavy episodic drinking of alcohol has been linked to risk behaviours such as unsafe sex and other drug use [10,11]. Although the clinical management of HIV and substance use differ, there is, theoretically, a case for exploring synergies in their management; however, caution and contextual sensitivity are warranted as there is no universal formula for integration, the overlap between conditions is only partial and in many societies both are stigmatizing in different ways [7].
The argument for service integration partially draws on general interest in health services integration, both at the policy level, where programs addressing single health problems can be brought together, and at the operational level, ensuring efficient use of scarce resources; thereby offering a way to improve access, responsiveness to patients’ needs, increase coverage, reduce inequalities, and improve health outcomes [12]. Integration is seen as particularly promising in high HIV burden and low resource settings, helping ensure complex health needs are addressed, building on care delivery and drug distribution system commonalities, facility sharing, and aligning funding mechanisms [13,14].
Calls to integrate HIV services with services for other health needs, such as the growing burden of non-communicable diseases (NCDs) in ageing populations living with HIV, have been relatively uncontentious. However, substance use presents a complicated situation as illicit drug policies are highly contested. Some governments advocate a criminal justice response and others a public health response. In 2016, governments attending the UN General Assembly Special Session (UNGASS on the World Drug Problem) reaffirmed their commitment to policies prohibiting the production, trafficking, possession and use of illicit drugs, nevertheless they have committed to provision of medical assisted therapy and clean injecting equipment [15,16]. The 2016 UN Political Declaration on HIV and AIDS, while acknowledging progress in health-related risk and harm reduction programs in some countries, noted the worldwide need for progress in reducing PWID HIV transmission and called for commitment to tailored prevention interventions [17]. This is echoed by the Johns Hopkins-Lancet Commission on Drug Policy and Health which calls for a harm reduction response, drawing on a growing body of evidence of effectiveness and embedded in a commitment to human rights [18].
While recognizing the need to consider how political factors may influence service integration, this review is part of a larger review that questioned when and in what circumstances are there benefits to bringing non-communicable disease and HIV services together. Consequently, in this paper we systematically review evidence from studies that sought to integrate care for people living with HIV and substance use problems.
There have been several global and regional level systematic reviews of HIV amongst PWID. However, these differed in scope and focus, examining the two conditions’ epidemiology [19–21] or treatment adherence determinants [22]. One assessed the impact of alcohol use disorders on HIV medication adherence, health care utilization and treatment outcomes while two, focusing on Africa, explored the association between alcohol use and HIV infection risk [23–25]. None, to our knowledge, has looked at integrating HIV and substance use services and programs.
Methods
Definitions
We drew on the integration definition proposed by Briggs, Atun and Legido-Quigley [26–28] whereby managerial or operational changes to health systems bring together inputs, delivery, management and organization of particular service functions with the aim of improving coverage access, quality, acceptability and/or (cost)-effectiveness. We included studies describing: service integration interventions; service delivery point integration; different levels of service delivery integration; process modifications; introduction of technologies aimed at aiding integration; and integration of management decisions [29,30]. We drew on an integration typology defining “service integration” as that integrating different clinical services through teams of multidisciplinary professionals and “clinical integration” as that integrating care into a single or coherent process with shared guidelines and protocols within and/or across professionals [31]. This typology differentiated integration at the macro level, as involving integration of the health system or major elements within it; the meso level, as involving organizations or professionals working together delivering integrated care to particular groups or populations, and the micro level, as involving providers delivering integrated person-centered care to individuals (Box 1) [31].
Box 1.
Illustrative examples of integration typologies
| Micro | Meso | Macro | |
|---|---|---|---|
|
Clinical integration Single or coherent process delivered by a provider who is equipped with shared guidelines and protocols |
A physician in a methadone maintenance program clinic also provides HIV counselling and testing services | A nurse working in a mobile health van for underserved populations is trained to provide both HIV counselling and DART with a clear referral pathway to a partnering HIV clinic, as well as substance use counselling with a clear referral pathway to a methadone maintenance clinic | N/A |
|
Service integration Teams providing different clinical services within the same organisation |
An HIV/AIDS clinic employs a substance use counsellor and physician and provides a methadone maintenance program for patients | A substance use program offering needle exchange sites hires HIV counsellors to provide outreach, education and referral to communities that utilize needle exchange services | N/A |
|
Systems integration Coordination between multi-location, multi-professional organisations to develop systems for the delivery of services for multiple conditions |
N/A | N/A | The HIV/AIDS Bureau, the Bureau of Drug Rehabilitation and the Bureau of Communicable Disease Control collaborate together and coordinate with their service providers, community members and stakeholders to enact policies and shared plans to decrease fragmentation of care for HIV/AIDS, substance use and hepatitis care |
Inclusion criteria
The review was conducted in accordance with PRISMA guidelines. We included quantitative and qualitative studies, as well as conference abstracts that reported the effects of health system level arrangements (service characteristics, interventions, policies or programmes) in different integrated care models for adults living with HIV and substance use. All studies describing or evaluating a managerial or organizational change to an existing health system that sought to increase integration of HIV and substance use services were included. For the present purposes we include within substance use harmful or hazardous psychoactive substances, alcohol and illicit drugs (morphine, heroin, tramadol, oxycodone and methadone) including non-medical use of prescription drugs. Companion papers (under review) discuss studies integrating mental health conditions or chronic medical conditions care with HIV.
We did not exclude reports based on study design; nor did we require outcome measures. There were no date or language restrictions. Studies that met inclusion criteria for this review were in English, French and Spanish language. No studies were excluded based on degree of assessed bias, although this was noted in interpreting the findings.
Search strategy
The search strategy was developed with an information specialist for consistency with methods used in other health services integration systematic reviews [28,30]. The databases Global Health, Medline and Embase were searched from inception until October 2015. Key words (MeSH terms) and free text terms were developed for three themes: HIV, integration and chronic diseases and combined in the search strategy (Supplemental File 1). To ensure coverage of low- and middle-income countries, databases were searched using a simplified search strategy: Cochrane library, LILACs, Africa Wide, WHOLIS and abstracts from the International AIDS Society (IAS) Online Resource Library (2006–2015), the HIV Implementers meetings (2007–2012) and international conferences on NCDs including the 2014 Annual Meeting of the College on Problems of Drug Dependence and the 2015 Annual Scientific Meeting of the Research Society on Alcoholism among others.
Search and retrieval of studies
Two reviewers independently reviewed the list generated by the electronic database search to identify relevant articles by title or title and abstract. Two reviewers independently assessed retrieved articles to determine whether they met inclusion criteria. Disagreements were resolved by discussion with a third reviewer.
Data synthesis
Four reviewers independently extracted data from included studies using standardized forms developed to capture qualitative and quantitative study characteristics and results data. Data extraction or study interpretation differences were resolved by discussion and consensus. Data were extracted including information on: (1) study characteristics (study design, setting and sample size); (2) participants characteristics (age, gender, ethnicity and country of origin); (3) program or intervention integration activities; (4) results and outcome measure type (clinical, procedural and behavioural outcomes); and (5) integration activities’ advantages and disadvantages. Data were compared and disparities resolved. We conducted a narrative synthesis of study findings whereby after data extraction and using the extraction table we reviewed and sorted the studies into emergent groups. These groupings, which were based on location of the integration, then informed creation of the models.
Risk of bias assessment
Four reviewers independently assessed risk of bias in studies that reported evaluative findings using the Cochrane risk of bias tool for randomized studies, a simple observational study proforma or an adapted checklist for qualitative studies [32]. We classified studies that had low risk of bias in all domains as low overall risk of bias. Studies that had high or unclear risk of bias in one or more domains were classified as overall high or unclear risk of bias.
Results and discussion
Database searching identified 11,057 records, with 7616 remaining after duplicate removal, screened for inclusion by title and abstract, yielding 340 articles which were retrieved as full texts (Figure 1). Fifty-one articles met eligibility criteria, of which 47 were articles and four conference abstracts. We did not conduct a meta-analysis due to heterogeneity of study design, interventions, participants and outcomes, but instead present a descriptive summary of interventions, results and where available, outcomes.
Figure 1.
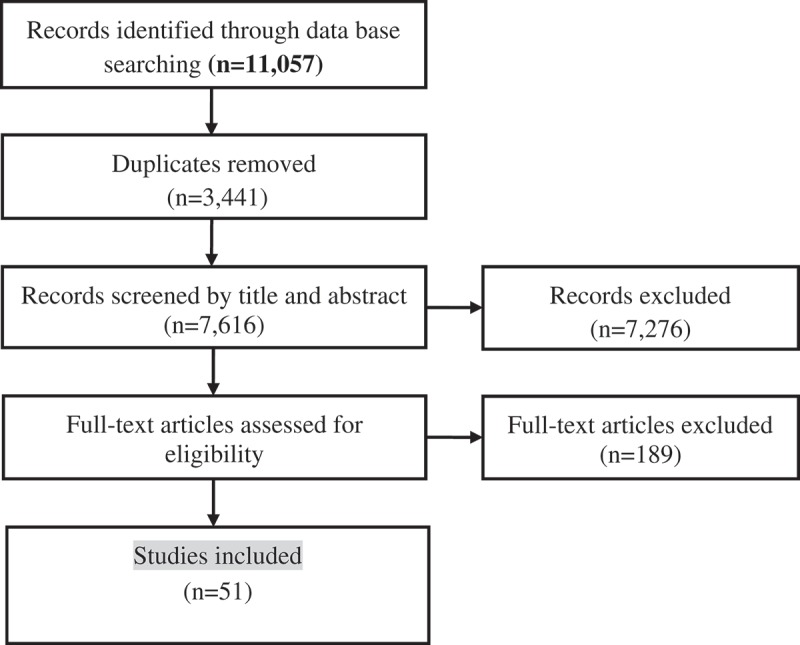
Study flow diagram.
Models of integration
Integration models were defined by entry point at which a patient receives care; HIV facilities, substance use facilities and other facilities. We identified three integration types within these models (Figure 2). Type 1 integration includes facilities combining screening and counselling without further shared service provision. We consider screening-only to be least integrated, as it is less resource intensive and less complex than other forms of integration. Type 2 integration incorporates some treatment aspect, such as antiretroviral therapy (ART) provision in substance use facilities or substance use treatment in HIV facilities. The most integrated type combines substance use and HIV treatment, with other health care provision or social services; for example, drug and HIV treatment integration with harm reduction and palliative care [33,34].
Figure 2.
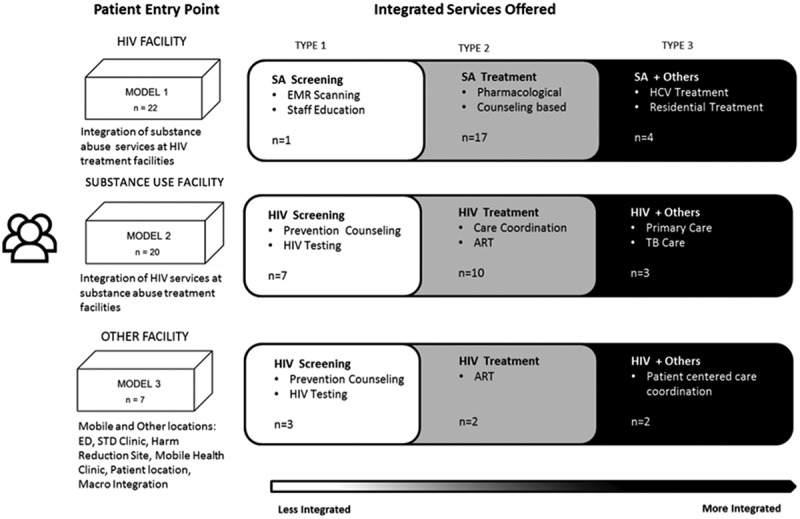
Models of integration of HIV and substance use services.
Characteristics of included studies
Fifty-one papers met the inclusion criteria; 22 papers described integration at HIV facilities, 20 at substance use facilities and seven at other facilities (sexually transmitted disease (STD) clinic, syringe access sites, emergency department and mobile health van). Two papers discussed patients’ perspectives on integration as a broader concept. Across sites, 11 involved screening activities, 29 treatment integration, and nine included services for other comorbidities. There were 18 descriptive studies; 14 cohort studies, seven qualitative studies, four RCT’s, one case-control, two mixed-methods and one cost analysis.
The majority of studies (n = 39) were conducted in the United States of America (USA) with two in Canada [33–74]. There were four European studies, two from Spain, one from Ireland and one from the Ukraine [75–78]. Two studies were from Africa, one each from Kenya and Tanzania [61,79]. There were four studies from Asia, one each from India, Indonesia, Taiwan and Vietnam [80–83]. The USA studies integrated care in all three facility types. Of the 41 studies from North America, 21 were located in HIV facilities. Elsewhere, only Kenya reported integration in an HIV facility [61]. Beyond North America, the majority of studies were in substance use facilities. Of ten non-North American studies, six were located in substance use facilities [75–77,79,80,82].
Most integration examples were from areas with a low to moderate burden of HIV infection amongst people who inject drugs (PWID); no studies were from countries with over 50% of PWID living with HIV (Figure 3). Studies from Indonesia, Spain and Tanzania, which fall in the moderate range of PWID living with HIV (25.01–50%) involved HIV care integration into drug treatment programs [76,79,80]. Examples from countries with a lower range (10–25% PWID living with HIV) such as Canada, Kenya, and the Ukraine involved integration into HIV facilities, except for Ireland which integrated HIV services into addiction services [33,34,61,75].
Figure 3.
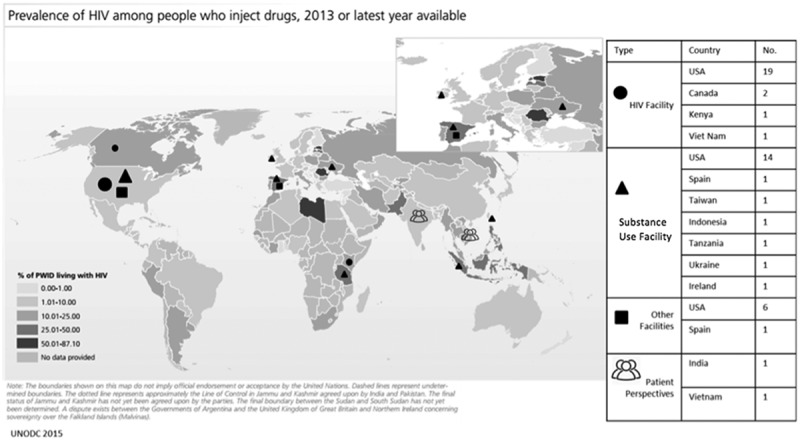
Map of integration by type and prevalence of HIV amongst persons who inject drugs.
Published examples were mostly from countries that allow access to both methadone (MMT) and buprenorphine maintenance treatment (BMT); only two studies were from areas that only offered MMT – Kenya and Vietnam [61,83]. India, which only offers BMT, was represented in one study [81].
Six papers offered an integration definition [45,55,57,63,69,75]. Two papers drew on Blount’s integrated service criteria where medical and behavioural elements are integrated into a treatment plan [57,63] and the remaining papers provided alternate definitions and criteria (Table 1).
Table 1.
Definitions of integration from studies included in the review
| Author | Definition of integration |
|---|---|
| Lombard et al. [57] and Proescholdbell et al. [63] | Blount (2003) criteria: integration of behavioural and medical elements into one treatment plan |
| Cheever et al. [45] | Integration as an ongoing process requiring assessment, planning, intervention and evaluation |
| Bachireddy [75] | Integration occurring on a spectrum with service co-location as “simple” integration and cross-disciplinary case management as more integrated |
| Sullivan [69] | Provider integration, where integration at the clinic level involves those services provided by different clinicians occurring at a single site, whereas individual integration is one where the treatment service is provided by the same clinician at a single site |
| Hoffman [55] | A formalized, collaborative process among services and systems with the goal of decreasing fragmentation of care and improving coordination |
Integrations occurred at all system levels, but most (n = 30) described micro level service integration [33–41,44,47–49,51–53,57–59,61–63,66–71,76,77,79,80,82] (Table 2). Within HIV facilities, 15 studies explored micro level integration [33–37,48,49,52,61–63,67,69–71], one described meso level clinical integration [60] and five described macro level integration [45,50,57,73,74]. Similarly, within substance use facilities, the majority of studies were micro level integrations (n = 12) [40,41,44,47,51,58,59,66,68,76,77,79,80,82], with three meso level integrations [46,56,65]. In other facilities, three studies described micro level integration [38,39,53], two described meso level integration [42,43] and two described macro level integration [55,78].
Table 2.
Overview of integration type by model from studies included in the review
| Integration location | Integration type | n = |
|---|---|---|
| HIV facility | Micro-service integration [33–37,48,49,52,61–63,67,69–71] | 15 |
| Macro-systems integration [33–37,48,49,52,61–63,67,69–71] | 5 | |
| Meso-clinical integration [60] | 1 | |
| MLa [72] | 1 | |
| Substance use facility |
Micro-service Integration [41,44,47,51,58,59,66,68,76,77,79,80] | 12 |
| Meso-clinical integration [46,56,65] | 3 | |
| ML [54,64,75] | 3 | |
| Micro-clinical integration [40,82] | 2 | |
| Other facility | Micro-service integration [38,39,53] | 3 |
| Meso-clinical integration [42,43] | 2 | |
| Macro-systems integration [55,78] | 2 | |
| Patient perspectives | ML [81,83] | 2 |
aML describes those studies that explored multiple levels.
Risk of bias assessment
We conducted risk of bias assessments for studies that evaluated service integration and reported outcome measures or qualitative results (Supplemental File 2). This included 23 studies; 13 cohort studies [36–40,42,62,63,66,68,76,79,80], four randomized control studies [35,52,61,69], two cross sectional studies [75,77], two qualitative studies [48,57], one case control study [59], and one cost analysis [67]. Fifteen had high risk of bias [36,37,39,40,48,62,63,68,69,75,77,79,80], six had moderate risk of bias [35,42,52,61,66,76] and two had low risk of bias [57,59].
Model 1: integration of substance use services at an HIV facility
Twenty-two studies described integrated HIV and substance use care at HIV facilities. We define an HIV facility as an HIV clinic, HIV primary care clinic or other health care setting established to deliver HIV care. Within this model, the HIV facility offers different activities for substance use screening or treatment (Table 3).
Table 3.
Summary of integrations provided at HIV facilities
| Type | Activity | Author | n = |
|---|---|---|---|
| 1. Substance use screening | Screening | O’Neill 2007 (USA) | 1 |
| 2. Substance use treatment | BUP/NX | Cheever 2011 (USA), Turner 2005 (USA), Weiss 2011A (USA), Weiss 2011B (USA), Altice 2011 (USA), Finkelstein 2011 (USA), Schackman 2011 (USA), Egan 2014 (USA), Sullivan 2006 (USA), Draioni 2014 (USA) | 10 |
| Counselling/MI | Hasin 2013 (USA), Aharanovich 2006 (USA), Aharanovich 2012 (USA) Lombard 2009 (USA), Proschold-Bell 2010 (USA), Parsons 2005 (USA), Papas 2011 (Kenya) | 7 | |
| 3. Substance use treatment + other treatments | HCV treatment | Taylor 2005 (USA), Taylor 2012 (USA) | 2 |
| Residential Care | Krusi 2009 (Canada), McNeil 2014 (Canada) | 2 |
Type 1: substance use screening at an HIV facility
One study reported only substance use screening within an HIV clinic in the USA, where electronic medical record scanning identified patients misusing substances and clinic staff referred them for substance or mental health evaluation [60].
Type 2: substance use treatment at an HIV facility
Eighteen papers incorporated substance use screening with treatment, including 10 studies from the USA that used buprenorphine naloxone (BUP/NX) for treatment. One sought to determine feasibility and efficacy of BUP/NX and psychosocial support in an HIV clinic, with no clear results [69].
Six papers reported on the American Buprenorphine HIV Evaluation and Support Initiative (BHIVES), which integrates BUP/NX into HIV care and involves a multisite evaluation with cross-site education and support [74]. Nine sites reported positive perceptions and described successful integration despite challenges with patient comorbidities and organizational barriers to program implementation, such as incorporating new procedures into established practice and limited adoption [73]. Patients had positive perceptions of BHIVES and attributed greater engagement with both HIV and substance use care to the integration [49]. Other studies reported positive perceptions of BUP/NX integration and the importance of counselling and social support in conjunction with pharmacological treatment [48].
Seven papers described counselling-focused interventions at HIV facilities. Telehealth motivational interviewing (MI) was used in three studies, two aiming to reduce drinking and one to reduce drug use; all reported positive patient outcomes [35,36,52]. One study explored an MI intervention, one a cognitive behavioral therapy (CBT) intervention and two described a combination MI and CBT approach provided to HIV patients at HIV facilities with positive results [61–63]. One study, conducted at HIV facilities in the USA, explored provider perceptions of clinic adaptations to integrate counselling; barriers including clinic size differences, space constraints, services offered, process design, professional autonomy and multidisciplinary team communication and collaboration [57].
Type 3: substance use treatment + other treatments at an HIV facility
Four of 22 studies at HIV facilities described HIV care and substance use integration involving comorbidity treatment or a harm prevention approach. Two studies incorporated hepatitis C virus infection (HCV) care using a multidisciplinary model merging addiction, psychiatry, HIV and HCV treatment at a Centre for AIDS Research Coinfection Clinic [70,71]. Two studies from Canada explored an HIV day health and residential program providing medical care, counselling, social service referrals and a supervised injection program; staff reported greater trust and patient engagement, and patients felt supported in their safe injection habits, while social and structural determinants of health were better met, increasing treatment adherence [33,34].
Model 2: integration of HIV services at substance use facilities
Twenty studies described care for HIV and substance use provided at substance use facilities (Table 4). Our definition of a substance use facility is a drug addiction/misuse/use centre or other healthcare setting primarily aiming to treat or mitigate the effects of drug addiction/misuse/use.
Table 4.
Summary of integrations provided at substance use facilities
| Type | Activity | Author | n = |
|---|---|---|---|
| 1. HIV screening and prevention counselling |
● Prevention counselling only | ● Lee 2015 (Taiwan) | 1 |
| ● Screening and prevention counselling |
● Seewald 2013 (USA), Kmeic 2012 (USA), Conners 2012 (USA), Henry 2010 (USA), Cartter 1990(USA), Gunn 2005 (USA) | 6 | |
| 2. HIV treatment | ● Nurse led intensive care coordination |
● Andersen 2003 (USA) | 1 |
| ● Pharmacological treatment | ● Surah 2012 (Ireland), Sanchez 2012 (Spain), Cooperman 2007 (USA), Achmad 2009 (Indonesia), Berg 2009 (USA), Lucas 2004 (USA), Lucas 2007 (USA), Sorensen 2012 (USA), Tran & Bruce 2015 (Tanzania) | 9 | |
| 3. HIV treatment and other care services |
● Bachireddy 2014 (Ukraine), Selwyn 1993 (USA), Rothman 2007 (USA) | 3 |
Type 1: HIV screening and prevention counselling at a substance use facility
Eight studies integrated HIV prevention counselling and screening at substance use facilities. One abstract described counselling without HIV screening in an MMT program in Taiwan [82]. Six papers described programs integrating prevention counselling with HIV screening activities. One paper provided an overview of state-wide integration of HIV services with MMT programs, indicating efficacy in testing linked to pre- and post-test counselling in substance use treatment facilities [44]. Another found evidence of efficacy of HIV and hepatitis B (HBV) counselling and testing, as well as HBV vaccination, in high risk patients in a non-residential drug-rehabilitation program [51]. Four of six studies from the USA focused on integrating rapid HIV testing into substance use treatment facilities. A study of patient acceptance in an ambulatory detoxification setting in the USA found rapid testing was acceptable to those with alcohol and opioid use [56]. Two studies from American Veterans Health centres that explored nurse led HIV rapid testing implementation found positive staff perceptions and readiness to provide HIV rapid testing. Reported barriers included increased staff workload, staff hesitancy performing medical procedures, and anxiety delivering HIV-positive results, as well as challenges linking patients to off-site HIV care [46,65].
Type 2: HIV treatment at a substance use facility
Ten studies integrated HIV treatment at substance use facilities. One study from the USA described promising results from a nurse-led intensive care coordination protocol, where nurses accompanied participants for HIV treatment and facilitated integration of medical recommendations with substance use treatment [40].
Nine studies explored integrating pharmacological treatment (ART, HAART or DAART) into substance use treatment facilities. One prospective cohort study from Spain assessed HAART effectiveness at a drug use out-patient centre, where active drug users in the program achieved virological suppression rates on par with non-drug using matched subjects [76]. Seven studies explored HIV treatment in MMT programs. In Indonesia, an MMT program offered ART which improved HIV case detection and ART uptake [80]. Two studies in the USA offered HAART and directly observed therapy (DOT) treatment options to current and former opioid users in an MMT program, but with no clear results [41,47]. However, three studies from the USA explored integrating DAART into MMT programs in outpatient substance use treatment facilities with positive results for virological suppression; all faced participant drop out and poor adherence challenges [58,59,68]. In Tanzania, a study at an MMT that dispensed ARTs found evidence that methadone use enhanced client retention and linkage to care [79]. From a staff perspective, a study in Ireland of 30 addiction staff with HIV clinic links explored attitudes 6 months post integration showing positive staff perceptions of patients and of the integration [77].
Type 3: HIV treatment + other conditions at a substance use facility
Three studies described integration of HIV and substance use treatment with management of other conditions, at substance use facilities. One study from the Ukraine assessed three integration models addressing substance use, HIV and TB, suggesting greater service integration increases ART access and improved patient quality of life [75]. In the USA, two studies explored primary care provision for HIV-infected drug users at substance use facilities with positive results; however, treatment paradigm differences between substance use and HIV care were reported as challenging to integration [64,66].
Model 3: integration of HIV and substance use services at other facilities
Integration activities took place at five other facility types described in six papers, while one paper described a multi-location macro health systems integration of HIV, substance use and other services (Table 5).
Table 5.
Summary of integrations provided at other facilities
| Location | Type | Author | n = |
|---|---|---|---|
| Syringe access site | • HIV prevention counselling | • Burr 2014 (USA) | 1 |
| STD clinic | • HIV Testing | • Hennessey 2007 (USA) | 1 |
| Emergency dept | • HIV Testing & Prevention Counselling | • Bernstein 2012 (USA) | 1 |
| Mobile | • HIV Treatment | • Altice 2003 (USA), Altice 2004 (USA) | 2 |
| Multiple – patient centered | • HIV Care | • Tato 2000 (Spain) | 1 |
| Systems integration | • HIV + Substance Use + Mental Health + Hepatitis | • Hoffman 2004 (USA) | 1 |
Two studies took place in clinical settings; one STD clinic and one emergency department. Both described HIV testing and prevention counselling amongst substance using patients and reported improved HIV testing uptake [42,53].
Three studies were located at harm reduction sites or mobile locations. One study from the USA explored a nurse-led health promotion program at syringe access sites and described efficacy in reaching large numbers of PWID [43]. Two studies described a mobile health clinic in the USA that provides HIV counselling, case management, drug treatment coordination, health status assessment, and medical care [38,39]. The papers reported improved virological results in patients receiving HAART and following DAART introduction, reported patient preference for mobile site treatment.
One paper described a patient-centered integration in Spain, offered jointly through the AIDS Patient Homecare Program (PADS) and Red Cross drug addiction program, delivered at patients’ preferred location[78]. PADS coordinated medical, substance use and comorbidity care and multiple social supports for patients and their families; reporting that substance use treatment, mainly through methadone, was important in patient success.
One paper described a systems level initiative in the USA, integrating service provision between government bureaus for HIV/AIDS, substance use and communicable diseases [55]. The main program output was a strategic plan offering joint assistance and provider training, coordination and information access, as well as joint procurement processes and contracting services.
Patients’ perspectives
Two papers described patients’ perspectives on integrated HIV and substance use services, regardless of patient entry point. One cross-sectional study from Vietnam explored 510 MMT patient perspectives on integrated and decentralized MMT clinics and reported high preference for integration[83]. In India, a qualitative study explored HIV-positive PWID barriers to ART access and reported complex individual, social and system barriers while calling for supports built into existing treatment options [81].
Measures of effectiveness of integration
32 studies evaluated one or more measures of effectiveness of the integrated intervention, program or model. We define patient outcomes as a change in patients’ health status, knowledge, behaviours or attitudes. We define service delivery outcomes as measures reflecting effectiveness in delivery of the service, program or integration, including staff perceptions (See Table 6).
Table 6.
Types of outcome measures reported
| Types of outcomes reported (n = number of studies reporting outcomes) |
||
|---|---|---|
| Models of integration | Patient outcomes | Service delivery outcomes |
| HIV facility (n = 22; n = 16 reported outcomes) |
|
|
| Substance use facility (n = 20; n = 13 reported outcomes) |
|
|
| Other Facility (n = 7; n = 3 reported outcomes) |
|
|
| Patient Perspectives (n = 2; n = 0 reported outcomes) |
N/A | N/A |
One study reported long term outcomes of a macro integration from an organizational quality perspective [64]. Eight studies within HIV facilities were qualitative, reporting patient or staff perceptions of integration [33,34,48,49,57,73,81,83]. Two studies provided cost analyses, reporting on total integration cost as well as cost per client [51,67]. One study looked at three substance use disorder clinics and found that six months post rapid HIV testing implementation, acceptance rates and HIV testing were higher at the most integrated site [46]. Another study assessed different integration models, and reported Health Quality of Life (HRQoL), Quality Healthcare Index (QHI) composite scores and likelihood of patients’ receiving ART and opioid substitution therapy (OST) found patients receiving integrated care had significantly higher QHI scores compared to those receiving non-collocated services or harm reduction only (71.9% versus 54.8% versus 37.0%, p < 0.001); patients receiving integrated care were significantly more likely to receive ART (49.5% versus 19.2%, p < 0.001) [75].
Nineteen intervention studies were included [35–40,42,52,59,61–63,65,66,68,69,76,79,80]; 14 were cohort studies; two had a control group receiving usual care [76,80] one compared against seronegative patients [66] and 11 compared against baseline data [36–40,42,62,63,65,68,79]. One study was a case-control [59]. Four were RCTs [35,52,61,69], one compared a control (usual care group) with two intervention arms [52], one a usual care group against one intervention group [61]. Two papers compared two intervention groups against each other with no usual care control. All studies reported results pointing to positive outcomes from integration, but had high or moderate risk of bias; therefore while outcomes show promising results, the high risk of bias warrants caution in making assumptions on efficacy (Supplemental File 3).
Discussion
This review explores different approaches to integrated HIV and substance use services based on patient entry points, synthesizing integration evidence at HIV, substance use and other facilities, as well as patient perspectives. The extent of integration varies, from combined screening activities to fully integrated screening, treatment and referrals for HIV, substance use and other comorbidities.
These models have advantages and disadvantages (Table 7). A positive finding is that across models the potential to increase HIV and substance use detection as well as provide structure, accountability and support for treatment adherence has been realized in some sites. Also, a single care provider may reduce the likelihood of drug interactions, while integrated services can facilitate communication across providers. When HIV, substance use and other conditions are managed together, enhancing continuity of care, there is evidence that acute episodes may be reduced, translating into reduced costs for patients [38,39]. However, some studies noted challenges to integration. For example, BHIVES achieved promising patient outcomes, but implementation barriers included higher costs, appropriate financing, workforce training, and challenges in combining differing clinical practices. Further, there is evidence that staff at substance use facilities may be hesitant to perform HIV testing, seen as a medical procedure, as well as in communicating positive HIV test results to patients. Integration also requires strong referral links to primary care, mental health and social services to address multiple and diverse patient needs [84]. A criminal justice approach to substance use has, in some regions, created legal barriers limiting pharmacological treatments and harm reduction activities [85]. Punitive approaches make it difficult to see the individual as a patient, preventing or delaying access to care [18,86].
Table 7.
Summary of advantages and disadvantages reported in studies by integration model
| Model 1: HIV facilities | Model 2: substance use facilities | Model 3: other facilities | |
|---|---|---|---|
| Potential advantages |
Substance use screening
Substanceuse treatment
Substance use + others
|
HIV screening
HIV treatment
HIV + others
|
Clinical facilities
Harm reduction and mobile facilities
Patient led location
|
| Potential disadvantages |
Substance use screening
Substance use treatment
Substance use + others
|
HIV screening
HIV Treatment
HIV + others
|
Clinical facilities
Harm reduction, mobile and patient led facilities
|
We identified innovative approaches including integrated HIV and substance use care in mobile, community and residential settings. Such programs offer a people-centered approach allowing greater patient agency in managing substance use, HIV care and psychosocial supports. There is need for responsive, appropriate and convenient programs addressing the often-chaotic lives of people who use drugs [87] and these programs show promise reaching marginalized groups and provide a platform to build trust, educate, provide treatment and encourage adherence. Residential facilities have achieved success using robust harm reduction strategies coupled with housing, medical facilities and psychosocial support [33,34]. Other patient-centered programs utilized intensive nurse-led care to create coordinated protocols and foster provider-patient relationships [40]. These approaches require staff to be treatment advocates, use multidisciplinary approaches and have adequate access to not only mental health and clinical resources but social, transport and housing linkages [40,88,89].
Of note is the evidence describing patient perspectives of systems without integrated services, where patients encounter multiple family, social and system level barriers to care [81,83]. Patients reported fear of discrimination, unmet basic needs and unfriendly hospital environments and procedures, with inadequate counselling and perceived lack of confidentiality [81]. In contrast, studies describing fully integrated harm reduction approaches reported positive patient perceptions, especially of holistic care provision to address unmet social needs [33,34].
Strengths and limitations
We used a wide range of databases and conference archives to increase paper representation from low- and middle-income countries. We also included studies published in languages other than English. However, studies were mostly from the USA, dealing with BUP/NX or the BHIVES initiative. Also, the review included many descriptive papers which, while providing insights into various integrated approaches, could not be used to infer effectiveness. In total 33 studies reported integration measures of effectiveness, four of which were RCTs in which methodological quality varied and overall had high or moderate risk of bias. Our review did not compare between integrated versus non-integrated services outcomes due to lack of literature. Further, due to the broad nature of the review we were unable to account for differences in treatment modalities for different types of substance use that may impact their ability to be effectively integrated into clinical care.
Implications for research
Our review shows that research on integrated HIV and substance use care has focused on treatment approaches at the meso-level and small-scale clinical integration. There is need for further macro-level evaluations of systemic HIV and substance use service integration. Further, there is need to pursue research exploring meso-level integrations focusing on shared values and the tools necessary to overcome barriers obstructing links between HIV and substance use care.
Overall, there is need for longer term and more robust studies evaluating effectiveness. There is also a lack of long-term outcomes or relevant impacts on HIV and substance use, including reduced transmission rates and overall mortality. The longest follow up period reported in studies comparing outcomes was 12 months. This highlights the need for well-designed, robust studies with clear outcome indicators evaluating and comparing interventions. Also, further qualitative and mixed methods studies are necessary to better explore patient perspectives.
There is a particular need for research on integration approaches using model 3 whereby services are offered at diverse locations. While many studies described HIV testing initiatives, only one described a substance misuse screening initiative. Further research is required on sensitive approaches, such as community based or mobile service delivery, for specific needs of often vulnerable, transient and hard to reach populations. As the findings from studies of model 3 show, people living with HIV and substance use have diverse interactions with the health system and it is important to understand how to screen, refer or treat substance use in a variety of service provision settings.
Although our review included non-English papers and aimed for broad representation, only five studies were from low- or middle- income countries; one from Indonesia, one from Vietnam, one from India, one from Kenya and one from Tanzania, none from Latin America. There is need for studies from areas with a high burden of HIV and PWID, given their greater risk of HIV infection, and from different regions and health systems. However, as the lack of studies may be reflective of laws or policies in different regions placing prohibitions on substance use, it is important to address punitive approaches to substance use that may inhibit innovation and research on PWID.
Conclusions
We identified three models integrating HIV and substance use; services in HIV facilities, substance use facilities and others. Benefits to integration are reported largely in terms of patient outcomes, including how integrated service can better enable patients to uptake and adhere to treatment; there are also demonstrable service outcomes including staff satisfaction with integrated approaches and easier referral to mental health and social services. Despite many countries pursuing a criminal justice approach, the UNAIDS 2016–2021 Strategy identifies the need to commit to service integration for those with drug dependency. The evidence reviewed here shows the need for innovative and holistic responses at the intersection of substance use and HIV services, especially the provision of integrated care at non-traditional sites and amongst vulnerable groups.
Biography
VH: Data extraction, risk of bias assessment, manuscript writing.
FCL, FC, SO, GM, LS, NW, DB, SH, WM: Data extraction, risk of bias assessment, contributed to writing of the manuscript. All authors criteria for authorship read and met: All authors agree with manuscript results and conclusions.
KB, PPiot, MM, PPerel, HLQ: extraction, risk of bias assessment, analysed data, manuscript writing. All authors have read and approved the final version.
Competing interests
None.
To access the supplementary material to this article please see Supplementary Files under Article Tools online.
References
- [1].Magodoro IM, Esterhuizen TM, Chivese T.. A cross-sectional, facility based study of comorbid non-communicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes. 2016;9(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014;14:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. Aids. 2016;30(2):273–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kaplan RC, Hanna DB, Kizer JR. Recent Insights Into Cardiovascular Disease (CVD) risk among HIV-infected adults. Curr HIV/AIDS Rep. 2016;13(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].UNAIDS UNAIDS Report on the Global AIDS Epidemic. 2012. Available from: http://www.unaids.org/sites/default/files/media_asset/20121120_UNAIDS_Global_Report_2012_with_annexes_en_1.pdf
- [6].Ottersen O, Dasgupta J, Blouin C, Buss P, Chongsuvivatwong V, Frenk J, et al. The political origins of health inequity: prospects for change. Lancet. 2014;383(9917):630–67. [DOI] [PubMed] [Google Scholar]
- [7].Piot P, Abdool Karim SS, Hecht R, Legido-Quigley H, Buse K, Stover J, et al. Defeating AIDS - advancing global health. Lancet. 2015;386(9989):171–218. [DOI] [PubMed] [Google Scholar]
- [8].UNAIDS The gap report. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. Available from: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf [Google Scholar]
- [9].UNODC World Drug Report: 2014. Vienna; 2014. Available from: https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf [Google Scholar]
- [10].Oesterle S, Hill KG, Hawkins JD, Guo J, Catalano RF, Abbott RD. Adolescent heavy episodic drinking trajectories and health in young adulthood. J Stud Alcohol. 2007;65(2):204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].WHO , Global status report on alcohol and health. Geneva; 2014. Available from: http://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf [Google Scholar]
- [12].WHO Maximizing positive synergies between health systems and Global Health Initiatives. Report on the expert consultation on positive synergies between health systems and GHIs. Geneva; 2008. Available from: http://www.who.int/healthsystems/hs_&_ghi.pdf [Google Scholar]
- [13].Brady MA, Hooper PJ, Ottesen EA. Projected benefits from integrating NTD programs in sub-Saharan Africa. Trends Parasitol. 2006;22(7):285–91. [DOI] [PubMed] [Google Scholar]
- [14].Shigayeva A, Coker RJ. Communicable disease control programmes and health systems: an analytical approach to sustainability. Health Policy Plan. 2014;30:1–18. [DOI] [PubMed] [Google Scholar]
- [15].UNGASS Resolution adopted by the general assembly. United Nations; 1998. Available from: http://www.un.org/documents/ga/res/20sp/a20spr02.htm
- [16].UNGASS Resolution adopted by the general assembly on 19 April 2016. United Nations; 2016. Available from: https://documents-dds-ny.un.org/doc/UNDOC/GEN/N16/110/24/PDF/N1611024.pdf?OpenElement
- [17].UN General Assembly Political declaration on HIV and AIDS: on the fast-track to accelerate the fight against HIV and to end the AIDS epidemic by 2030. New York: United Nations; 2016. Available from: http://www.hlm2016aids.unaids.org/wp-content/uploads/2016/06/2016-political-declaration-HIV-AIDS_en.pdf [Google Scholar]
- [18].Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, et al. Public health and international drug policy. Lancet. 2016;387(10026):1427–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mathers B, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–28. [DOI] [PubMed] [Google Scholar]
- [20].Mathers B, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. [DOI] [PubMed] [Google Scholar]
- [21].Reid S. Injection drug use, unsafe medical injections, and HIV in Africa: a systematic review. Harm Reduct J. 2009;6(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Malta M, Strathdee SA, Magnanini MMF, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103(8):1242–57. [DOI] [PubMed] [Google Scholar]
- [23].Azar M, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112(3):178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fisher J, Bang H, Kapiga S. The association between HIV infection and alcohol use: a systematic review and meta-analysis of African studies. Sex Transm Dis. 2007;34(11):856–63. [DOI] [PubMed] [Google Scholar]
- [25].Kalichman S, Simbayi LC, Kaufman M, Cain D, Jooste S. Alcohol user and sexual risks for HIV/AIDS in Sub-Saharan Africa: systematic Review of Empirical Findings. Prevention. 2007;8(2):141–51. [DOI] [PubMed] [Google Scholar]
- [26].Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. Integration of targeted health interventions into health systems: a conceptual framework for analysis. Health Policy Plan. 2010;25:104–11. [DOI] [PubMed] [Google Scholar]
- [27].Groene O, Garcia-Barbero M. Integrated care: a position paper of the WHO European office for integrated health care services. Int J Integr Care. 2001;1:1–10. [PMC free article] [PubMed] [Google Scholar]
- [28].Briggs C, Garner P. Strategies for integrating primary health services in middle- and low-income countries at the point of delivery. Cochrane Database Syst Rev. 2006;2:CD003318. [DOI] [PubMed] [Google Scholar]
- [29].WHO Integrated health services – what and why? Technical Brief No. 1. 2008. Available from: http://www.who.int/healthsystems/technical_brief_final.pdf
- [30].Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. A systematic review of the evidence on integration of targeted health interventions into health systems. Health Policy Plan. 2010;25:1–14. [DOI] [PubMed] [Google Scholar]
- [31].Curry N, Ham C. Clinical and service integration: the route to improved outcomes. Sugarman J, editor London: The Kings Fund; 2010. Available from: https://www.kingsfund.org.uk/sites/files/kf/Clinical-and-service-integration-Natasha-Curry-Chris-Ham-22-November-2010.pdf [Google Scholar]
- [32].Thomas J, Harden A, Oakley A, Oliver S, Sutcliffe K, Rees R, et al. Integrating qualitative research with trials in systematic reviews. Bmj. 2004;328(7446):1010–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krusi A, Small W, Wood E, Kerr T. AIDS Care: psychological and socio-medical aspects of AIDS/HIV. AIDS Care. 2009;21(5):638–44. [DOI] [PubMed] [Google Scholar]
- [34].McNeil R, Dilley LB, Guirguis-Younger M, Hwang SW, Small W. Impact of supervised drug consumption services on access to and engagement with care at a palliative and supportive care facility for people living with HIV/AIDS: a qualitative study. J Int AIDS Soc. 2014;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aharonovich E, Greenstein E, O’Leary A, Johnston B, Seol SG, Hasin DS. HealthCall: technology-based extension of Motivational Interviewing to reduce non-injection drug use in HIV primary care patients: a pilot study. AIDS Care. 2012;24(12):1461–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aharonovich E, Hatzenbuehler ML, Johnston B, O’Leary A, Morgenstern J, Wainberg ML, et al. A low-cost, sustainable intervention for drinking reduction in the HIV primary care setting. AIDS Care. 2006;18(6):561–68. [DOI] [PubMed] [Google Scholar]
- [37].Altice F, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients recieving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Altice F, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Cid. 2004;38(Supplment 5):S376–S387. [DOI] [PubMed] [Google Scholar]
- [39].Altice F, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. J Urban Health. 2003;80(3):416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Andersen M, Paliwoda J, Kaczynski R, Schoener E, Harris C, Madeja C, et al. Integrating medical and substance abuse treatment for addicts living with HIV/AIDS: evidence-based nursing practice model. Am J Drug Alcohol Abuse. 2003;29(4):847–59. [DOI] [PubMed] [Google Scholar]
- [41].Berg K, Mouriz J, Li X, Duggan E, Goldberg U, Arnsten JH. Rationale, design and sample characteristics of a randomized controlled trial of directly observed antiretrovial therapy delivered in methadone clinics. Contemp Clin Trials. 2009;30:481–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bernstein E, Heeren T, Winter M, Ashong D, Bliss C, Madico G, et al. Long-term follow-up after voluntary human immunodeficiency virus/sexually transmitted infection counseling, point-of-service testing, and referral to substance abuse treatment from the emergency department. Acad Emerg Med. 2012;19(4):386–95. [DOI] [PubMed] [Google Scholar]
- [43].Burr C, Storm DS, Hoyt MJ, Dutton L, Berezny L, Allread V, et al. Integrating health and prevention services in syringe access programs: a strategy to address unmet needs in a high-risk population. Public Health Rep. 2014;120(Supplement 1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cartter M, Petersen LR, Savage RB, Donagher J, Hadler JL. Short communication providing HIV counseling and testing services in methadone maintenance programs. Aids. 1990;4:463–65. [DOI] [PubMed] [Google Scholar]
- [45].Cheever L, Kresina TF, Cajina A, Lubran R. A model federal collaborative to increase patient accessto buprenorphine treatment in HIV primary care. Acquir Immune Defic Syndrome. 2011;56(Supplment 1):S3–S6. [DOI] [PubMed] [Google Scholar]
- [46].Conners E, Hagedorn HJ, Butler JN, Felmet K, Hoang T, Wilson P, et al. Evaluation the implementation of nurse-initated HIV rapid testing in three Veterans Health Administration substance use disorder clinics. Int J STD AIDS. 2012;23:799. [DOI] [PubMed] [Google Scholar]
- [47].Cooperman N, Parsons JT, Chabon B, Berg KM, Arnsten JH. The development and feasibility of an intervention to improve HAART adherence among HIV-positive patients receiving primary care in methadon clinics. J HIV AIDS Soc Serv. 2007;6(1–2):101–20. [Google Scholar]
- [48].Drainoni M, Farrell C, Sorensen-Alawad A, Palmisano JN, Chaisson C, Walley AY. Patient perspectives of an integrated program of medical care and substance use treatment. AIDS Patient Care STDS. 2014;28(2):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Egan J, Netherland J, Gass J, Finkelstein R, Weiss L. Patient perspectives on buprenorphine/naloxone treatment in the context of HIV care. J Acquir Immune Defic Syndr. 2011;56(Supplment 1):S46–S53. [DOI] [PubMed] [Google Scholar]
- [50].Finkelstein R, Netherland J, Sylla L, Gourevitch MN, Cajina A, Cheever L. Policy implications of integrating buprenorphine/naloxone treatment and HIV care. J Acquir Immune Defic Syndr. 2011;56(Supplement 1):S98–104. [DOI] [PubMed] [Google Scholar]
- [51].Gunn R., Lee MA, Callahan DB, Gonzales P, Murray PJ, Margolis HS. Integrating hepatitis, STD, and HIV services into a drug rehabilitation program. Am J Prev Med. 2005;29(1):27–33. [DOI] [PubMed] [Google Scholar]
- [52].Hasin D, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, et al. Reducing heavy drinking in HIV primary care: a randomized trial of a brief intervention, with and without technological enhancement. Addiction. 2013;108:1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hennessy R, Weisfuse I, Schlanger K. Does integrating viral hepatitis services into a public STD clinic attract injection drug users for care?. Public Health Rep. 2007;122 Suppl 2:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Henry S, Hagedorn HJ, Feld JE, Golden JF, Horns H, Knapp HE, et al. A formative evaluation of organizational readiness to implement nurse-initiated HIV rapid testing in two veterans health administration substance use disorder clinics. J HIV AIDS Soc Serv. 2010;9(1):7–26. [Google Scholar]
- [55].Hoffman H, Castro-Donlan CA, Johnson VM, Church DR. The Massachusetts HIV, hepatitis, addiction services integration (HHASI) experience: responding to the comprehensive needs of individuals with co-occurring risks and conditions. Public Health Rep. 2004;119:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kmiec J. Acceptance of rapid HIV testing in an ambulatory detoxification setting. In 23rd Annual Meeting and Symposium of the American Academy of Addiction Psychiatry; 2012; Aventura, Florida, USA: Available from: http://www.turkpsikiyatri.org/arsiv/AAAM_2012_Program_Complete_Final.pdf [Google Scholar]
- [57].Lombard F, Proescholdbell RJ, Cooper K, Musselwhite L, Quinlivan EB. Adaptations across clinical sites of an integrated treatment model for persons with HIV and substance abuse. AIDS Patient Care STDS. 2009;23(8):631–38. [DOI] [PubMed] [Google Scholar]
- [58].Lucas G, Mullen BA, McCaul ME, Weidle PJ, Hader S, Moore RD. Adherence, drug use, and treatment failure in a methadone-clinic-based program of directly administered antiretroviral therapy. AIDS Patient Care STDS. 2007;21(8):564–74. [DOI] [PubMed] [Google Scholar]
- [59].Lucas G, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38(Suppl 5):S409–13. [DOI] [PubMed] [Google Scholar]
- [60].O’Neill L, Savage S. Substance abuse assessment and brief treatment in HIV clinic settings. In Association for Medical Education and Research in Substance Abuse Conference; 2007; Washington, DC. [Google Scholar]
- [61].Papas R, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction. 2011;106:2156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Parsons J, Rosof E, Punzalan JC, Di Maria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: results of a pilot project. AIDS Patient Care STDS. 2005;19(1):31–39. [DOI] [PubMed] [Google Scholar]
- [63].Proeschold-Bell R, Heine A, Pence BW, McAdam K, Quinlivan EB. A cross site comparative effectiveness study of an integrated HIV and substance use treatment program. AIDS Patient Care STDS. 2010;24(10):651–58. [DOI] [PubMed] [Google Scholar]
- [64].Rotham J, Rudnick D, Slifer M, Agins B, Heiner K, Birkhead G. Co-located substance use treatment and HIV prevention and primary care services, New York State, 1990-2002: a model for effective service delivery to a high-risk population. J Urban Health. 2007;84(2):226–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Seewald R, Bruce RD, Elam R, Tio R, Lorenz S, Friedmann P, et al. Effectiveness and feasibility study of routine HIV rapid testing in an urban methadone maintenance treatment program. Am J Drug Alcohol Abuse. 2013;39(4):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Selwyn P., Budner NS, Wasserman WC, Arno PS. Utilization of on-site primary care services by HIV-seropositive and seronegative drug users in a methadone maintenance program. Public Health Records. 1993;108(4):492–500. [PMC free article] [PubMed] [Google Scholar]
- [67].Schackman B, Leff JA, Botsko M, Fiellin DA, Altice FL, Korthuis PT, et al. The cost of integrated HIV care and buprenorphine/naloxone treatment: results of a cross-site evaluation. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S76–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sorensen J, Haug NA, Larios S, Gruber VA, Tulsky J, Powelson E, et al. Directly administered antiretroviral therapy: pilot study of a structural intervention in a methadone maintenance. J Subst Abuse Treat. 2012;43:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sullivan L, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, et al. A trial of integrated buprenophine/naloxone and HIV clinical care. Cid. 2006;43(Supplment 4):S184–90. [DOI] [PubMed] [Google Scholar]
- [70].Taylor L. Delivering care to injection drug users coinfected with HIV and hepatitis C virus. Cid. 2005;40(Supplment 5):S355–S361. [DOI] [PubMed] [Google Scholar]
- [71].Taylor L, Maynard MA, Friedmann PD, Macleod CJ, Rich JD, Flanigan TP, et al. Buprenorphine for human immunodeficiency virus/hepatitis C virus-coinfected patients: does it serve as a bridge to hepatitis C virus therapy? J Addict Med. 2012;6:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Turner B, Laine C, Lin Y-T, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch Intern Med. 2005;165:1769–76. [DOI] [PubMed] [Google Scholar]
- [73].Weiss L, Egan JE, Botsko M, Netherland J, Fiellin DA, Finkelstein R. The BHIVES Collaborative: organization and evaluation of a multisite demonstration of integrated buprenorphine/naloxone and HIV treatment. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S7–S13. [DOI] [PubMed] [Google Scholar]
- [74].Weiss L, Netherland J, Egan JE, Flanigan TP, Fiellin DA, Finkelstein R, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S68–S75. [DOI] [PubMed] [Google Scholar]
- [75].Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend. 2014;134:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sanchez G, Llibre JM, Torrens M, Sanvisens A, Mateu G, Knobel H, et al. Effectiveess of antiretroviral therapy in HIV-1-infected active drug users attended in a drug abuse outpatient treatment facility providing a multidsciplinary care strategy. Curr HIV Res. 2012;10(4):356–63. [DOI] [PubMed] [Google Scholar]
- [77].Surah S, Kinahan J, Keating S, Mulcahy F, Keenan E, Lyons F. Assessment of addiction staff attitudes 6 months post integration of HIV and addiction services. In 18th Annual Conference of the British HIV Association; 2012; Birmingham, UK: Available from: http://www.bhiva.org/documents/Conferences/2012Birmingham/Abstracts2012.pdf [Google Scholar]
- [78].Tato J, Nicolás I, Saborido T, Lacasta S, Rodríguez N, Tomás X, et al. Drogodependencias-SIDA: intervención socio-sanitaria en el ámbito domiciliario (1990-1998). Addiciones. 2000;12(4):461–66. [Google Scholar]
- [79].Tran O, Bruce RD, Masao F, Ubuguyu O, Sabuni N, Mbwambo J, et al. Linkage to care among methadone clients living with HIV in Dar es Salaam Tanzania. J Acquir Immune Defic Syndr. 2015;69:e43–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Achmad Y., Istiqomah AN, Iskandar S, Wisaksana R, van Crevel R, Hidayat T. Integration of methadone maintenance treatment and HIV care for injecting drug users: a cohort study in Bandung, Indonesia. Indones J Intern Med. 2009;41(Supplment 1):23–27. [PubMed] [Google Scholar]
- [81].Chakrapani V, Velayudham J, Shunmugam M, Newman PA, Dubrow R. Barriers to antiretroviral treatment access for injecting drug users living with HIV in Chennai, South India. AIDS Care. 2014;26(7):835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee T., Chawarski MC, Peng C, Hung C, Metzger D. Implementation of drug and HIV risk counseling in MMT programs in Taiwan. Drug Alcohol Depend. 2015;146:e173. [Google Scholar]
- [83].Tran B, Ohinmaa A, Duong AT, Nguyen LT, Vu PX, Mills S, et al. Cost-effectiveness of integrating methadone maintenance and antiretroviral treatment for HIV-positive drug users in Vietnam’s injection-driver HIV epidemics. Drug Alcohol Depend. 2012;125:260–66. [DOI] [PubMed] [Google Scholar]
- [84].Durvasula R, Miller T. Substance abuse treatment in persons with HIV/AIDS: challenges in managing triple diagnosis. Behav Med. 2014;40(2):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Altice F, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wolf D, Carrieri M, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–66. [DOI] [PubMed] [Google Scholar]
- [87].Meyer J, Althoff A, Altice F. Optimizing care for HIV-infected people who use drugs: evidence-based approaches to overcoming healthcare disparities. Cid. 2013;57(9):1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Soto T, Bell J, Pillen M. Literature on Integrated HIV care: a review. AIDS Care. 2004;16 Suppl 1:43–55. [DOI] [PubMed] [Google Scholar]
- [89].Zaller N, Gillani F, Rich J. A model of integrated primary care for HIV-positive patients with underlying substance use and mental illness. AIDS Care. 2007;19(9):1128–33. [DOI] [PubMed] [Google Scholar]


