Abstract
Microtubule-based transport in cells is powered by a small set of distinct motors, yet timing and destination of transport can be controlled in a cargo-specific manner. The mechanistic basis for this specificity is not understood. To address this question, we analyzed the Drosophila Klarsicht (Klar) protein that regulates distinct microtubule-based transport processes. We find that localization of Klar to its cargoes is crucial for Klar function. Using mutations, we identify functionally important regions of Klar that confer distinct cargo specificity. In ovaries, Klar is present on the nuclear envelope, a localization that requires the C-terminal KASH domain. In early embryos, Klar is attached to lipid droplets, a localization mediated by a novel C-terminal domain encoded by an alternatively spliced exon. In cultured cells, these two domains are sufficient for targeting to the correct intracellular location. Our analysis disentangles Klar's modular organization: we propose that a core region integral to motor regulation is attached to variable domains so that the cell can target regulators with overlapping, yet distinct functions to specific cargoes. Such isoform variation may be a general strategy for adapting a common regulatory mechanism to specifically control motion and positioning of multiple organelles.
INTRODUCTION
Motor-driven transport of organelles is crucial for many cellular processes, for establishing and maintaining cellular polarity, and for development. Microtubule-based motors power, for example, the movement of vesicles in the secretory pathway, promote chromosome segregation at mitosis, deliver axonal vesicles, and mediate localization of cytoplasmic determinants (Bloom and Goldstein, 1998; Hirokawa, 1998; Fischer, 2000; Almenar-Queralt and Goldstein, 2001).
In a single cell, many different cargoes move simultaneously, yet timing and destination of transport have to be controlled independently. Surprisingly, much of this transport is accomplished by only a few general motors (Vale, 2003). Cytoplasmic dynein, for example, functions in processes as diverse as mitosis, axonal transport, Golgi positioning, and mRNA trafficking. Somehow, cells must regulate these motors appropriately for the cargo they carry. Dynein complexes with different sets of light and intermediate chain subunits seem to be recruited to different cargoes (Nurminsky et al., 1998; Tai et al., 2001), but whether varying subunit composition is simply a way of attaching dynein to a variety of cargoes or whether this variation also influences activity and regulation of the motors remains unresolved.
Many cellular cargoes move bidirectionally, switching between plus- and minus-end–directed motion in quick succession (Welte, 2004). Such cargoes seem to have motors for both directions stably attached, so docking of motors is not a major mode of regulation. As yet unknown mechanisms control how frequently cargoes switch between plus- and minus-end motion. These mechanisms must allow control of net transport in a cargo-specific manner. Remarkably, the best understood model systems for bidirectional transport share many general features (Gross, 2004), raising the possibility that the overall regulatory strategy is conserved across many transport processes. When such a general mechanism is adapted for cargo-specific control, the identity of the cargo would need to be somehow linked to the mechanisms that control the activity of the attached motors. Whether there are dedicated molecules that perform such a bridging function between cargo identity and motor complexes is unknown.
Biophysical analysis in Drosophila has identified the Klarsicht (Klar) protein as a crucial factor in the regulation of bidirectional transport of lipid droplets (Welte et al., 1998). Lipid droplets in early embryos move bidirectionally along microtubules, and the balance of plus- and minus-end travel distances changes twice over a 2-h period, resulting in switches in the direction of net transport. In the absence of Klar, travel distances, travel velocities, and stall forces are greatly reduced, for both plus- and minus-end travel. These defects are much more severe than when transacting regulation is impaired (in this or other model systems; De Vos et al., 2003; Gross et al., 2003; Rodionov et al., 2003) and suggest that without Klar the motors for plus- and minus-end motion are active indiscriminately, engaging in a tug-of-war (Welte et al., 1998). Thus, Klar seems to be central for understanding how the activity of opposite-polarity motors is coordinated during bidirectional transport. In this system, Klar controls the minus-end motor cytoplasmic dynein (Gross et al., 2000; Gross et al., 2002) and an as yet unknown plus-end motor.
Lack of Klar also disrupts the developmental regulation of droplet transport. In the wild type, these organelles are initially distributed throughout the periphery of the embryo (syncytial blastoderm, phase I), constantly moving back and forth along microtubules. At the beginning of cellularization (phase II), plus-end travel distances are up-regulated, causing net inward motion; the droplets accumulate basally, near microtubule plus-ends. One hour later (gastrulation, phase III), plus-end travel lengths decrease and droplets redistribute outward, apically. In embryos from klar mutant females (referred to as “klar embryos”), this switch from net inward to net outward motion in phase III fails to occur because the balance of plus- and minus-end motion does not change correctly. Based on these phenotypic analyses, it was proposed that Klar may form a complex between the plus- and minus-end motors, controlling the response to transacting signals and coordinating motor activity (Welte et al., 1998).
Klar is not only a central player in the mechanism of bidirectional transport of lipid droplets but also it controls motor activity for at least two other transport processes. In differentiating photoreceptors of wild-type animals, nuclei migrate first basally and then apically; in the absence of Klar, this switch in direction fails to occur, resulting in mispositioned nuclei (Fischer-Vize and Mosley, 1994; Welte et al., 1998). And in embryonic salivary glands, Klar controls minus-end–directed transport of secretory vesicles and thus modulates the growth of the apical membrane (Myat and Andrew, 2002). Whether nuclei in photoreceptors and vesicles in salivary glands move bidirectionally like lipid droplets is not known, but both the minus-end motor dynein and the plus-end motor kinesin I are important for the correct positioning of photoreceptor nuclei (Whited et al., 2004). Whether Klar controls the motion of additional cargoes has not been determined.
Klar may not only provide insight into motor coordination but also into the problem of cargo specificity. In embryos, the klar defect seems limited to lipid droplets as the distribution of other organelles in these embryos is normal (Welte et al., 1998); in particular, there are no reported defects in nuclear positioning or membrane growth. Understanding Klar might thus provide an inroad into uncovering how a common regulator controls different cargoes in different cells.
Comparing the klar genomic region (Celniker et al., 2002) and the reported klar cDNA (Mosley-Bishop et al., 1999) predicts 19 exons (exon 0 to exon 18; Figure 1A). The only obviously conserved region in the predicted Klar protein (∼250 kDa) is the C-terminal 60-amino acid Klarsicht, ANC-1, Syne-1 homology (KASH) domain (Starr and Han, 2002). KASH domains are present in many proteins, including the actin-binding proteins ANC-1 and Syne-1, and the dystrophin-related Msp300. ANC-1 and Syne-1 are perinuclear and tether nuclei to the actin cytoskeleton (Apel et al., 2000; Starr and Han, 2002). For Syne-1, the KASH domain is both necessary and sufficient to target the protein to the nuclear envelope (Zhang et al., 2001). A Myc-tagged Klar protein expressed ectopically in photoreceptors is also perinuclear (Mosley-Bishop et al., 1999), but the localization of endogenous Klar is unknown, in photoreceptors or any other tissue.
Figure 1.
Molecular lesions in various klar alleles and their effect on Klar protein expression in early embryos. (A) The klar gene spans ∼100 kb in the genome. This map is based on the klar gene annotation available on FlyBase (The FlyBase Consortium, 2003). Predicted exons are labeled from 0 to 18. Translation is predicted to start in exon 2 (cyan line) and stop in exon 18 (red line). The green box in exon 18 indicates the KASH domain. The epitopes of antibodies Klar-N, Klar-M, and Klar-C were mapped to exon 4, 9, and 18, respectively, as indicated by double-headed arrows and the corresponding amino acid number. (B and C) Proteins from phase II embryos of various genotypes were separated by SDS-PAGE and analyzed by Western blotting by using antibody Klar-M (B) or Klar-N (C). Both antibodies detect a major form of Klar above 250 kDa, plus additional bands. Comparison to alleles with N-terminal lesions (e.g., klarB) shows that all these bands are Klar specific. These bands might be breakdown products of a longer form or specific Klar isoforms resulting from alternative splicing/protein processing. Class II alleles express Klar proteins similar in size to the wild type. Class I alleles express truncated protein forms. (D) Molecular lesions in various alleles. The klar gene structure is the same as in A. Arrows point to the approximate position of the molecular lesions of klar alleles (see Table 1 for details). Alleles with nonsense mutations are red, and alleles with chromosomal breaks are blue. Lesions of class I alleles are located 5′ to exon 15, whereas class II alleles disrupt exon 18.
To characterize the intracellular localization of Klar and its function in regulating transport, we developed antibodies against distinct regions of the protein and determined the molecular lesions in a panel of klar alleles. Using a combination of genetics, immunolocalization, and molecular studies, we compared which domains of Klar are necessary for its function in two transport processes: lipid-droplet motion and nuclear migration in photoreceptors. We find that alternatively spliced C-terminal sequences act as targeting domains that are both necessary and sufficient for correct intracellular localization of Klar.
MATERIALS AND METHODS
Fly Stocks
Oregon-R was the wild-type stock. klarmBP, klarmBX18, klarmFN1, klarmBX3, klarmCD4, klarmBX12, klarmBX13 (Fischer-Vize and Mosley, 1994), and klar1 (Welte et al., 1998) have been described previously. klarA, klarB, klarC, and klarD were generated by ethylmethane sulfonate (EMS) mutagenesis and screening for noncomplementation of the klar1 embryonic phenotype. We generated klarYG3 by imprecise excision of P element EP(3)3104 (Rørth, 1996). Df(3L)emcE12 is a large deletion encompassing klar and neighboring genes.
Mapping Molecular Lesions of klar Alleles
Genomic DNA was prepared from 50 flies per genotype and used as template in polymerase chain reaction (PCR) amplification or inverse PCR. For klarA, klarB, klarC, klarD, klarmFN1, klar1, and klarmCD4, PCR products of specific regions were sequenced and compared with parental lines, when available. The breakpoint of klarmBX3 was mapped by inverse PCR (Liao et al., 2000) and sequencing. Breakpoints of klarYG3, klarmBX12, and klarmBX13 were roughly mapped by PCR amplification to identify genomic regions not contiguous in the mutants.
Generation of Antibodies
Three Klar fragments (277–556aa, 875-1169aa, and 1895–2262aa) were expressed as glutathione S-transferase (GST)-fusion proteins in bacteria. Purified proteins were mixed and used to immunize mice at the Princeton University Monoclonal Antibody Facility. Three Klar-positive hybridomas (Klar-N, Klar-M, and Klar-C) were subcloned and analyzed in detail. Antibody epitopes were mapped using various Klar-GST fusion proteins and Western analysis.
Immunostaining, Immunoblotting, and In Situ Hybridization
Embryos, larval tissues, and adult ovaries were heat fixed as described previously (Wieschaus and Nüsslein-Volhard, 1986). To costain embryos for lipid droplets and other markers, the vitelline membrane was removed manually after fixation. Antibodies Klar-N, Klar-M, and Klar-C were used at dilutions 1:5, 1:50, and 1:5, respectively, both for immunostaining and immunoblotting. Rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) was used as secondary antibody. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories) served as tertiary antibody for immunoblotting. Alexa 488- or Alexa 546-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR) was used as tertiary antibody for immunostaining. DNA was labeled by Hoechst 33258, the nuclear envelope by Alexa 594-conjugated wheat germ agglutinin (WGA), and lipid droplets either by Nile Red or BODIPY 493/503 (all reagents from Molecular Probes). In situ hybridization was performed as described previously (Gross et al., 2003). To physically displace lipid droplets within the embryo, dechorionated embryos (suspended in a 0.4% NaCl, 0.03% Triton X-100 solution) were centrifuged for 10 min at 13,200 rpm (Eppendorf Table-top centrifuge 5415D) before fixation.
Images in Figures 3, A–I, and Figure 4D were acquired on a Nikon Eclipse E600 epifluorescence microscope with a Spot Insight QE camera (Diagnostic Instruments, Sterling Heights, MI). All other micrographs were recorded on a Leica TCS SP2 confocal microscope. Images were assembled with Adobe Photoshop and Adobe Illustrator.
Figure 3.
Motion of lipid droplets and of photoreceptor nuclei require partially distinct and partially overlapping regions of Klar. Class I alleles (represented by klar1) disrupt droplet transport, whereas class II alleles (represented by klarmBX12) do not. Both classes disrupt nuclear migration in photoreceptor cells. (A–I) Wild-type, class I, and class II embryos were compared by transmitted light to reveal changes in transparency (A–C, phase II; D–F, phase III) or by staining with Nile Red to detect lipid droplets (G–I, phase III, green). In phase III embryos, lipid droplets remain basally for class I alleles but spread apically in the other genotypes. (J–L) Wild-type, klar1, and klarmBX12 eye imaginal disks were fixed and stained for the neural antigen Elav (green) to reveal photoreceptor nuclei. Distribution of nuclei at the apical side of the disk was documented by confocal microscopy. In the wild type, the nuclei are arranged in a regular pattern apically. For both klar alleles, most nuclei are missing from this apical region, indicating basal mislocalization. Bars, 100 μm (A–I) and 8 μm (J–L).
Figure 4.
Alternate exons of klar. (A) Published klar cDNAs suggest the existence of alternate exons. The figure shows the location of cDNA sequences (from BDGP) relative to the klar genomic region. cDNA clone LD08331 includes an alternative exon 15X (=exon 15 plus 15ext). Exon 15X has an alternative stop codon (red line) distinct from the canonical one in exon 18. cDNA GH05536 contains the alternate exon G at its 5′ end (start codon, cyan line). Bent arrows show the two inferred transcription start sites. (B) Conservation of the 15ext-encoded protein. Klar exon 15 was identified by sequence similarity, the sequences after exon 15 (representing 15ext) were translated, and predicted ORFs were aligned to each other. The first 50aa displayed only weak sequence similarity (our unpublished data), but the C-terminal 66aa displayed significant conservation: 53% identical (red) and 77% similar (yellow) between seven Drosophila species. Conservation extends to the mosquito A. gambiae; a 46aa stretch of the predicted 15X ORF can be aligned with the Drosophila sequences (35% identical, 70% similar). Species used were D. melanogaster, Drosophila simulans, D. yakuba, Drosophila ananassae, D. pseudoobscura, Drosophila virilis, Drosophila mojavensis, and A. gambiae. (C) ORFs from potential exons G from six Drosophila species. Color code and species are as under B. The six protein sequences are 61% identical and 74% similar. (D) A 15ext-specific probe detects cytoplasmically localized RNA in early embryos, from preblastoderm stages (top) to phase II (bottom). This signal is absent from klarYG3 and klarmBP embryos, in which the 5′ promoter is mutant or on a different chromosome than 15X, respectively.
Bioinformatics
We first compared the “full-length” Klar protein sequence from Drosophila melanogaster to the genomic sequences of Drosophila yakuba, Drosophila pseudoobscura, and Anopheles gambiae and found significant sequence similarity at the protein level for all exons in D. yakuba and D. pseudoobscura and many exons in A. gambiae. The relative exon order was the same as in D. melanogaster, suggesting that these species encode a klar gene of similar complexity. For D. yakuba and D. pseudoobscura, the intron between exons 15 and 16 contained sequences related to 15ext and G. For A. gambiae, 15ext, but not G could be found in a similar search. We then searched the genomic sequences of additional Drosophila species available in the trace archive at the National Center for Biotechnology Information for sequences similar at the DNA level to exons 15 + 15ext or G of either D. melanogaster or D. pseudo-obscura. Putative open reading frames (ORFs) were aligned by ClustalW by using MegAlign (DNA Star). Promoter prediction used the McPromoter program at http://genes.mit.edu/McPromoter.html (Ohler et al., 2002).
Red Fluorescent Protein (RFP) Constructs
RFP was amplified from plasmid mRFP1 (Campbell et al., 2002) and cloned into the XhoI and XbaI sites of vector pAC5.1/V5-His B (Invitrogen, Carlsbad, CA). This vector allows high-level, constitutive expression in S2 cells. Various klar regions were amplified either from wild-type genomic DNA or cDNA clone LD08331 and cloned 3′ to the RFP gene, by using XbaI and BstB I sites.
Cell Culture and Transfection
Drosophila S2 cells were maintained as described previously (Nawathean et al., 2005). Cells were transfected by the Cellfectin method (Invitrogen) at ∼80% confluence 1 d after plating on glass coverslips in six-well tissue culture dishes. BODIPY 493/503 and Hoechst 33258 were used to stain lipid droplets and DNA in live cells, respectively. Fluorescence images were taken 36–48 h after transfection.
RESULTS
Klar Is Widely Expressed
To determine where Klar protein is expressed, we generated monoclonal antibodies (Klar-N and Klar-M) that recognize epitopes near the N terminus and in the center of the protein (exon 4 and exon 9, respectively; Figure 1A). Both antibodies detected Klar by Western analysis (see below). Only Klar-M gave a strong signal by immunostaining. Comparison with a klar mutant showed that this signal was Klar specific (Figure 2, A–D). Klar was expressed in cells where it is known to be important for transport: in early embryos during droplet motion and at high levels in eye imaginal discs posterior to the morphogenetic furrow, i.e., in the region of the disk where photoreceptor nuclei migrate apically (Figure 2, A and B). Unexpectedly, Klar also was present in many other cells in which no function for Klar had previously been reported, including in embryos of various stages, other larval imaginal disks, larval brains, and adult ovaries (Figure 2, C and D; our unpublished data). This broad expression suggests that Klar has many as yet unknown roles during Drosophila development and may control a wide range of transport processes. Thus, insight into Klar function has the potential to shed light on the regulation of diverse developmental processes.
Figure 2.
Expression and localization of the Klar protein. (A–D) Early embryos and third instars were fixed and stained with the Klar-M antibody (green). Comparison between the wild type (top) and a klar mutant (klarmBP/Df(3L)emcE12, bottom) shows specificity of staining. klarmBP carries a chromosomal break between the putative klar promoter and the Klar-M epitope in exon 9 (Mosley-Bishop et al., 1999). (A) Phase I embryos. (B) Eye imaginal disks (posterior toward the bottom). Klar signal is highly increased in the posterior of the wild-type disk, probably because klar mRNA is up-regulated in a broad stripe posterior to the morphogenetic furrow (Mosley-Bishop et al., 1999). (C) Wing imaginal disks. (D) Larval brains. (E–G) Wild-type and klar embryos were fixed and stained for Klar (antibody Klar-M, green) and DNA (Hoechst 33258, blue). (E) Punctate Klar staining is evenly distributed in the embryo periphery in phase I and displays basal accumulation 30–40 μm from the surface in phase II; this distribution is similar to that of lipid droplets at these stages. (F) In halo phase II embryos (right), Klar distribution is shifted apically relative to the wild type (left). Lipid droplets are similarly mislocalized in halo embryos. The white brackets indicate the breadth of apical-basal distribution of the majority of Klar dots. (G) Klar distribution in embryos for different klar alleles. For class II alleles (represented by klarmBX12), Klar distribution is similar to the wild type. For class I alleles, Klar protein is either mislocalized (klarmBX3 and klar1) or not detectable (klarD, klarmFN1, and klarB) above background. (H) Wild-type and klarB embryos costained for lipid droplets (BOPIPY 493/503, green) and Klar (antibody Klar-M, red). In many cases, lipid droplets are next to single dots of Klar staining. As fixation conditions that preserved neutral lipids were suboptimal for Klar signal, colocalization of Klar and lipid droplets is likely more extensive than suggested by these images. (I) Centrifugation assay to probe the physical association of Klar and lipid droplets. Centrifuged early embryos were stained for DNA (Hoechst 33258, blue) and either lipid droplets (BODIPY 493/503, green; in the panel WT-Lipid cap) or Klar (antibody Klar-M, green; all other panels). Droplets accumulate on one side of the embryo, in a layer that can be recognized by its distinct appearance in transmitted light (our unpublished data) and by the positioning of nuclei just below it. Klar is highly enriched in the droplet layer for the wild-type and klarmBX12, present throughout the embryo but not recruited to the droplet layer for klarmBX3 and klar1, and undetectable in klarB. Bars, 30 μm (A and E), 80 μm (B–D), 8 μm (F and G), 2 μm (H), and 100 μm (I).
Klar Localizes to Lipid Droplets
Based on the phenotype of klar mutants, it had been proposed that Klar is present on the cargoes it regulates, physically interacting with motors and coordinating their activity (Welte et al., 1998). Klar is indeed expressed at the correct time to directly regulate the motors that power motion of embryonic lipid droplets and of photoreceptor nuclei (Figure 2, A and B). Is it also present at the correct intracellular location to serve such a role? As Klar's function is best characterized for lipid-droplet transport, we asked where Klar localizes relative to this cargo and whether this localization was functionally important.
In wild-type embryos, Klar-M revealed a large number of distinct dots. These Klar dots were evenly distributed in the periphery of phase I embryos (Figure 2E) and underwent basal accumulation during phase II. Because this Klar distribution mimics that of lipid droplets at these stages, Klar may be associated with lipid droplets.
We double-stained embryos for Klar and for lipid droplets, by using the neutral lipid-specific dye BODIPY493/503. Fixation conditions that preserved neutral lipids were suboptimal for Klar preservation, but we nevertheless found many cases in which Klar dots were adjacent to lipid droplets (Figure 2H), consistent with the notion that Klar was associated with the droplets.
Because these colocalization data were merely suggestive, we used two independent strategies to alter the intracellular location of lipid droplets. Klar distribution should change in the same manner if Klar is indeed physically attached to the droplets. In a genetic approach, we used embryos deleted for the halo gene. In phase II halo embryos, lipid droplets accumulate apically, below the nuclei, rather than basally; no other organelles are mislocalized (Gross et al., 2003). In such embryos, the distribution of Klar dots was indeed shifted apically (Figure 2F). Because apical Klar accumulation in halo embryos is not as complete as that of lipid droplets (Gross et al., 2003), we propose that a fraction of Klar is associated with other cargoes whose distribution is undisturbed. A second approach takes advantage of the low buoyant density of lipid droplets. For many animal eggs, even mild centrifugation leads to stratification of the visible material, with lipid droplets on one side of the embryo, yolk granules on the other, and nuclei just below the droplet layer (Morgan, 1927, chapter XXII). We verified by direct staining for lipid droplets and DNA that this was also true for early Drosophila embryos (Figure 2I, WT-Lipid cap). By immunostaining, Klar was highly enriched in the droplet layer, just above the nuclei (Figure 2I, WT). In summary, we conclude that a major pool of Klar in early embryos is present on lipid droplets, supporting the notion that Klar is a crucial component of the postulated motor coordination complex, directly regulating the activity of the attached motors.
Mislocalized Klar Proteins Fail to Support Normal Droplet Transport
Is localization of Klar to lipid droplets important for its function? To address this question, we compared the effects of 13 mutant klar alleles that had been isolated in several independent genetic screens (Table 1). We determined whether the mutant Klar proteins could support normal droplet motion and whether they were able to localize correctly.
Table 1.
klar alleles
| Allele | Class | Screen | Mutagen | Molecular lesion | Localization embryo (Klar-M) | Localization ovary (Klar-C) |
|---|---|---|---|---|---|---|
| YG3 | I | Embryo | P excision [1] | ∼11kb deletion around exon 0 | Undetectable | Perinuclear |
| B | I | Embryo | EMS [1] | AA #210 Leu → Ter (exon 3) | Undetectable | Perinuclear |
| C | I | Embryo | EMS [1] | AA #210 Leu → Ter (exon 3) | Undetectable | Perinuclear |
| A | I | Embryo | EMS [1] | AA #221 Trp → Ter (exon 3) | Undetectable | Perinuclear |
| mBP | I | Eye | Hybrid dysgenesis [3] | between exons 4 and 5a | Undetectable | Perinuclear |
| mBX18 | I | Eye | X-rays [3] | between exons 4 and 5b | Undetectable | Perinuclear |
| mFN1 | I | Eye | EMS [3] | AA #1333 Gln → Ter (exon 11) | Undetectable | Perinuclear |
| D | I | Embryo | EMS [1] | AA #1430 Trp → Ter (exon 12) | Undetectable | Perinuclear |
| 1 | I | Embryo | EMS [2] | AA #1555 Gln → Ter (exon 14) | Even | Perinuclear |
| mBX3 | I | Eye | X-rays [3] | After AA #1598 fused to unrelated DNA (exon 14)c | Even | Perinuclear |
| mBX13 | II | Eye | X-rays [3] | Breakpoint between AA #1991 and #2062 (exon 18) | Basal | Undetectable |
| mCD4 | II | Eye | EMS [3] | AA #2203 Gln → Ter (exon 18) | Basal | Cytoplasm |
| mBX12 | II | Eye | X-rays [3] | Breakpoint between AA #2212 and #2226 (exon 18) | Basal | Undetectable |
The 13 klar alleles analyzed fall into two classes according to their effect on Klar staining and on droplet transport in embryos: Class I alleles have aberrant Klar staining and disrupt net apical droplet transport in phase III. Class II alleles have wild-type staining and support apical droplet transport. Both classes disrupt nuclear migration in eye imaginal disks. The table lists the origin of these alleles (type of screen and mutagenesis), their molecular lesions, and the observed immunostaining in early embryos and ovaries. The position of the lesion is given relative to the protein sequence of the originally reported Klar protein (Mosley-Bishop et al., 1999). Although these alleles might carry additional missense mutations in regions we did not sequence, the relative sizes of the mutant proteins on Western blots (Figure 1) are consistent with the notion that the premature stop codons or breakpoints we identified are responsible for the truncations. Ter, translation termination codon.
When the rough molecular mapping of the klarmBP breakpoint (Mosley-Bishop et al., 1999) is compared with the genomic sequence from FlyBase, it should fall between exons 4 and 5. This is consistent with the size of the mutant protein on Klar-N Westerns and the absence of signal on Klar-M Westerns
Rough molecular mapping puts the klarmBX18 lesion close to the klarmBP breakpoint (Mosley-Bishop et al., 1999). Our Western results are also consistent with a lesion between exon 4 and 5
klarmBX3 is due to a chromosomal transposition, originally mapped as Tp(3;3)61C4-7/87E6-F1 (Fischer-Vize and Mosley, 1994). We sequenced the exact breakpoint using inverse PCR; the encoded Klar protein is predicted to be normal up to AA #1598 and then fused to unrelated sequences derived from chromosomal region 70A1-B1. Notation for Klar-M staining: basal, Klar dots accumulate basally in phase II; even, Klar dots throughout peripheral cytoplasm. The Klar-C ovary staining pattern summarizes the major signal; the characteristics of additional Klar dots in the cytoplasm will be described elsewhere. [1], unpublished alleles generated by M. Welte and Y. Guo; [2], (Wieschaus and Nüsslein-Volhard, 1986); [3], (Fischer-Vize and Mosley, 1994)
Bulk movement of droplets can be assessed by changes in embryo transparency. In the wild type, net inward transport in phase II gives rise to a clear periphery (Figure 3A), and net outward movement in phase III turns the periphery opaque (Figure 3D). For all klar alleles tested, droplets accumulated basally in phase II. For 10 of the alleles, the mutant embryos had a transparent periphery in phase III (Figure 3E) because lipid droplets remained basally, around the yolk sac (Figure 3H). We will refer to these alleles with aberrant droplet transport as class I. For the remaining three alleles (class II), lipid droplets spread apically in phase III (Figure 3I), resulting in an opaque periphery just as in the wild type. Thus, class II alleles support normal net droplet transport, whereas class I alleles do not.
Immunolocalization revealed that all 10 class I alleles had aberrant Klar staining: Klar dots were either evenly distributed in the cytoplasm (klarmBX3 and klar1 in Figure 2G) or not detectable (klarmFN1, klarD, and klarB in Figure 2G) (Table 1). For the class II alleles (klarmBX12 in Figure 2G), Klar dots accumulated basally as in the wild type. These results suggest that Klar localizes to lipid droplets in class II, but not in class I alleles, and that if Klar fails to localize to lipid droplets, it cannot carry out its regulatory function.
To confirm this conclusion, we determined how centrifugation affected Klar distribution (Figure 2I). For the class II allele klarmBX12, Klar was highly enriched in the droplet layer, just as for the wild type. The Klar dots present in class I alleles klarmBX3 and klar1 were not recruited to the droplet layer, but they were instead broadly distributed throughout other regions of the embryo. Lack of signal in klarB embryos demonstrated the specificity of Klar staining. Thus, Klar association with lipid droplets is disrupted in class I alleles and maintained in class II alleles.
A Region of Klar Necessary for Droplet Localization
Because Klar proteins encoded by class I alleles fail to localize correctly, the molecular lesions in these alleles should give insight into the mechanism that controls Klar localization. For those alleles in which no distinct Klar dots were detectable, the proteins might simply not be expressed. Alternatively, the mutant proteins might be present, but diffusely throughout the cytoplasm, not concentrated in distinct locations. To distinguish between these possibilities, we monitored Klar expression in early embryos by Western analysis. In the wild-type and for class II alleles, Klar-M detected several high-molecular-weight bands, with a major band above 250 kDa (Figure 1B). In class I mutants, this Klar band was absent (klarmBP, klarmBX18, klarB, and klarYG3 in Figure 1B), shifted to lower molecular weight (klar1, klarD, and klarmFN1), or grossly increased in intensity (klarmBX3). Several of the alleles (klarD and klarmFN1) in which immunostaining did not reveal localization still expressed protein. These findings were corroborated by Western analysis with Klar-N. This antibody detected the same major form of Klar above 250 kDa (Figure 1C, wild-type lane), and the apparent size of this major Klar band changed similarly in various mutants. The fact that both antibodies recognized multiple Klar-specific bands (as they are all absent in several of the mutant klar alleles) might indicate that embryos express several distinct forms of Klar.
Guided by the apparent size of proteins on Westerns and by previous rough mapping of the chromosomal breakpoints in some alleles (Mosley-Bishop et al., 1999), we sequenced candidate sections of the klar genomic region for the mutant alleles to identify the underlying molecular lesions (Table 1). Allele klarYG3 had a deletion of the putative promoter region. In all other cases, we identified lesions (nonsense mutations or chromosomal breaks) predicted to result in C-terminally truncated Klar proteins (Figure 1D and Table 1).
Class II alleles have lesions in exon 18; they are wild-type in Klar distribution and net droplet transport. Class I alleles have more N-terminal lesions, before and within exon 14, and encode truncated proteins that neither localize correctly nor support normal transport. We conclude that a C-terminal region of Klar (between the beginning of exon 14 and the middle of exon 18) is necessary to localize Klar to lipid droplets.
Droplet Localization May Require the Alternatively Spliced Exon 15X
Why do lesions in exon 18 not disrupt droplet transport? One possibility is that droplets are associated with a Klar isoform that lacks exon 18. Two klar cDNAs (LD08331 and LD24441; Stapleton et al., 2002) characterized by the Berkeley Drosophila genome project (BDGP) suggest that such an isoform exists. These cDNAs have an alternative to exon 15 at their 3′ ends: this exon “15X” is identical to exon 15 in its 5′ sequences but contains 640 additional bases from the start of intron 15 (Figure 4A). We refer to this putative extension as 15ext (exon 15X = exon 15 + 15ext). Translation of 15X would result in an alternative C terminus of Klar, because 15ext contains an in-frame stop codon. Because both cDNAs end 17 bases downstream of a potential PolyA signal (AATAAA), they may represent the genuine 3′ end of certain klar messages.
Klar messages that contain exon 15X are indeed expressed. In situ hybridization with a 15ext-specific probe detected messages in wild-type embryos, but not in embryos of two klar alleles for which chromosomal breaks should prevent transcription of 15X (Figure 4D). The sequence of the predicted translation product of 15ext is significantly conserved (Figure 4B) between fly species representing an evolutionary divergence of 250 Mya (Gaunt and Miles, 2002). A Klar isoform encoded by messages ending with exon 15X would not be disrupted by class II alleles and thus may be the form of Klar that regulates lipid-droplet transport.
A Cell Culture Model for Klar Localization
Expression of Klar is likely even more elaborate. Both alternative splicing of other exons and use of distinct promoters contribute to the generation of multiple Klar isoforms (Guo and Welte, unpublished data; see below). We examined whether it might be possible to recreate Klar's localization to lipid droplets in a simpler experimental system.
We found that Drosophila S2 cells often contain numerous conspicuous particles that specifically stained with the neutral lipid-specific dye BODIPY493/503 (Figure 5B, top left) and therefore represent lipid droplets. S2 cell lipid droplets looked perfectly round and varied greatly in size, even within a single cell, unlike in early embryos where droplets are consistently ∼0.5 μm in diameter.
Figure 5.
Localization of RFP-Klar fusions in S2 cells. (A) Klar-RFP constructs analyzed in cultured cells. Different portions of Klar (indicated by the exon number) were fused to the C terminus of RFP. These fusions were expressed in S2 cells and their intracellular distribution was determined. (B) Distribution of various RFP constructs (red) in S2 cells relative to lipid droplets (green, BODIPY 493/503) and DNA (blue, Hoechst 33258). Even untransfected cells have abundant lipid droplets (top left). RFP alone and RFP-13-14 were present diffusely in the cytoplasm (middle and right top). RFP-13-14-15–15ext (middle) as well as RFP-15ext (left two bottom panels) localized to the surface of lipid droplets. RFP-KASH was enriched perinuclearly, presumably on the nuclear envelope (bottom right). Images taken at different magnification; all bars = 8 μm.
To test for Klar localization, we generated a set of constructs in which Klar fragments were fused in frame to RFP (Figure 5A). We expressed these constructs in S2 cells and monitored their intracellular distribution by fluorescence microscopy (Figure 5B).
RFP by itself was present diffusely throughout the cytoplasm. In contrast, a fusion construct containing the Klar region from exon 13 to the end of 15ext localized specifically to the periphery of lipid droplets, as shown by double labeling with BODIY493/503. All RFP-fusion constructs that contained 15ext localized to lipid droplets, whereas two constructs without 15ext were present throughout the cytoplasm. In particular, the Klar fragment encoded by 15ext was sufficient to target to lipid droplets. The cell culture and embryo data combined suggest that it is this region of Klar (the lipid droplet [LD] domain) that is responsible for associating Klar with lipid droplets.
Nuclear Migration Requires Distinct Functions of Klar
If targeting via the LD domain is so exquisitely specific, how does Klar associate with other cargoes? Next to droplet transport, Klar's best-characterized function is its role in the migration of nuclei in developing photoreceptors. To identify the regions of Klar necessary for this process, we examined how this migration was affected by our collection of klar alleles. In wild-type eye imaginal disks (Figure 3J), photoreceptor nuclei were uniformly positioned in clusters near the apical surface, whereas in disks of klar mutant animals nuclei were missing from this focal plane (Figure 3, K and L) and were displaced basally (Fischer-Vize and Mosley, 1994; Welte et al., 1998). Nuclei were mispositioned for all 13 alleles tested, from both class I and class II (Figure 3 and Table 1). Thus, the functions of Klar in droplet transport and nuclear migration are overlapping, but not identical.
The KASH Domain Targets Klar to the Nuclear Envelope
Nuclear migration requires in particular exon 18 sequences that are not important for droplet transport. The three class II alleles truncate the open reading frame in exon 18 before or within the KASH domain. Because KASH domains of other proteins are implicated in targeting to the nuclear envelope (Zhang et al., 2001), we hypothesized that it is this domain that targets Klar to nuclei.
By Klar-M immunostaining, Klar localization relative to nuclei was difficult to analyze because many cells had abundant Klar dots both throughout the cytoplasm and near nuclei, possibly because several different forms of Klar are present, associated with different cargoes. To specifically reveal Klar forms with exon 18 sequences, we generated antibody Klar-C, which recognizes an epitope just N-terminal to the KASH domain (Figure 6C).
Figure 6.
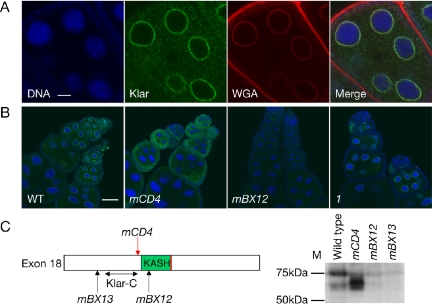
Klar localizes to the nuclear envelope in a KASH-dependent manner. (A) Klar distribution in ovaries. Wild-type ovaries were fixed and stained by Hoechst 33258 for DNA (blue), antibody Klar-C for Klar (green), and WGA for the nuclear envelope (red). Klar protein localized to the nuclear envelope in nurse cells. (B) Klar distribution in various klar mutants. In class II alleles, Klar was either mislocalized throughout the cytoplasm of nurse cells and oocytes (klarmCD4) or not detectable (klarmBX12). For the class I allele klar1, Klar distribution was similar to the wild type. Klar and DNA were detected as for (A). (C) Molecular lesions in class II alleles (left) and their consequence for Klar protein expression by Western analysis (right). klarmCD4 contains a nonsense mutation just before the KASH domain; klarmBX13 and klarmBX12 have chromosomal breaks before or within the KASH domain. A double-headed arrow indicates the approximate location of the Klar-C epitope. By Western analysis, Klar-C detects a 75-kDa band in wild-type ovaries, a shorter form in klarmCD4, and no signal above background for klarmBX12 and klarmBX13. Bars, 8 μm (A) and 40 μm (B).
By immunostaining, Klar-C revealed striking association of Klar with nuclei in several tissues, including the larval and adult gut, larval salivary glands, and adult ovaries. To examine this perinuclear Klar localization in detail, we focused on ovaries because of the large size of the polytene nurse cell nuclei. We detected no Klar-C signal in early embryos, supporting our conclusion that Klar isoforms at this developmental stage lack exon 18 sequences.
In wild-type ovaries, Klar-C revealed strong perinuclear signal in nurse cells, oocytes, and follicle cells. Klar was present in or on the nuclear envelope because Klar signal colocalized with the nuclear envelope marker wheat germ agglutinin (Figure 6A). Klar was distributed unevenly over the nuclear envelope, enriched in multiple distinct foci.
Klar-C signal was specific because no staining was detected in two class II alleles (klarmBX12, Figure 6B; and klarmBX13, not shown). For the class II allele klarmCD4, Klar protein was mislocalized throughout the nurse-cell and oocyte cytoplasm (Figure 6B). This result strongly suggests that the KASH domain is necessary for localization to the nuclear envelope as this allele has a premature stop codon just before the KASH domain (Table 1 and Figure 6C). When we expressed an RFP-KASH fusion in S2 cells (Figure 5B), the protein was enriched perinuclearly, demonstrating that this domain also is sufficient for targeting to the nuclear envelope. We conclude that Klar association with nuclei is mediated by the KASH domain.
Two Separate Promoters Drive Expression of Distinct Klar Isoforms
Surprisingly, we detected perinuclear Klar-C signal in ovaries of flies homozygous for the promoter deletion allele klarYG3 (Table 1). This observation suggested that cells might generate Klar variants not only by alternative splicing but also by using different promoters. To map the approximate position of the promoter responsible for Klar expression in ovaries, we examined Klar-C signal for all our klar alleles (Table 1). Because all class I alleles displayed perinuclear signal (klar1 is shown in Figure 6B), this promoter must be located 3′ to exon 14 (the location of the chromosomal break in klarmBX3). Certain cDNA clones from BDGP (e.g., GH05536 and GM02433) start with a small, alternative exon (G) located within intron 15 (Figure 4A). Promoter prediction programs indicate a high probability for a promoter 130 bases 5′ to G, and the open reading frame in G is highly conserved in other Drosophila species (Figure 4C). We suggest that it is a klar message starting with exon G that gives rise to the perinuclear Klar isoform in ovaries.
Western analysis with Klar-C detected a protein of apparent 75 kDa in wild-type (Figure 6C) and class I ovaries (our unpublished data), consistent with a Klar protein of 62 kDa if cDNA GH05536 is translated. In alleles klarmBX12 and klarmBX13, no corresponding band was detectable, and klarmCD4 ovaries had a band of slightly lower molecular weight, consistent with the lack of the KASH domain predicted from DNA sequencing (Figure 6C).
DISCUSSION
In this article, we provide the first mechanistic insight into Klar, a multifunctional regulator of microtubule-based transport. In different cells, Klar localizes to distinct cargoes, due to alternatively expressed C-terminal–targeting modules. The newly identified LD domain is necessary and sufficient to target Klar to the surface of lipid droplets. The KASH domain is necessary and sufficient to target Klar to the nuclear envelope. Localization is crucial for Klar function and dictates which transport process Klar regulates in a given cell. Because Klar is widely expressed and likely localizes to additional cargoes, understanding Klar-based transport should shed light on a whole range of developmental processes.
Functionally Distinct Klar Isoforms
The single klar gene gives rise to at least three messages and three Klar protein isoforms (Figure 7). A proximal promoter before exon 0 and a distal promoter before exon G are ∼80 kb apart. The originally published klar cDNA (exon 0–18) encodes isoform α that can partially rescue the klar nuclear migration defect in photoreceptors (Mosley-Bishop et al., 1999). The message for the droplet-specific isoform β likely starts at the proximal promoter and ends with exon 15X that encodes the LD domain. Isoform γ prominent in ovaries is encoded by a message transcribed from the distal promoter. We have not noted any gross defects in ovaries when this isoform is disrupted by class II alleles. Other isoforms may exist (BDGP; Guo and Welte, unpublished data). A comprehensive description of all Klar isoforms and of their expression pattern will be important for understanding the full role Klar plays in transport regulation.
Figure 7.
Predicted Klar isoforms. The diagram shows the likely extent of the three Klar protein isoforms α, β, and γ (klar gene structure as in Figure 1). Isoforms carry C-terminal targeting domains: KASH (α and γ) or LD (β). Isoform α (predicted to be 250 kDa) can partially rescue the klar nuclear migration defect in photoreceptors. Isoform β is associated with lipid droplets and is expressed from the proximal promoter. It seems likely that it contains most, if not all exons from 2 to 15X because it is affected by point mutations in exons 3, 11, 12, and 14 and is recognized by the exon 9-specific Klar-M antibody, and because cDNA LD08331 contains all exons from 6 to 15X. If so, its molecular weight is 202 kDa. Isoform γ (62 kDa) is present perinuclearly in ovaries and likely contains exons G, 16, 17, and 18 (based on perinuclear ovary staining in class I alleles and the sequence of cDNA GH05539).
Cargo-targeting Modules of Klar
The 60aa KASH domain of Klar is sufficient for perinuclear localization of a heterologous protein in cell culture and necessary for Klar's localization to the nuclear envelope in vivo. This function of the KASH domain is evolutionary conserved. For human Syne-1, the KASH domain directs nuclear envelope localization in COS-7 cells and C2C12 myoblasts (Zhang et al., 2001). For ANC-1, a 346aa region encompassing the KASH domain localizes to the nuclear envelope (Starr and Han, 2002). And in Drosophila photoreceptor cells, an epitope-tagged Klar protein containing the KASH domain (corresponding to isoform α) localizes perinuclearly, in a lamin-dependent manner (Patterson et al., 2004). KASH domains are thought to bind via UNC-84/SUN to nuclear lamins (Starr and Han, 2003).
The 112aa LD domain of Klar is sufficient to recruit a heterologous protein to the surface of lipid droplets in cultured cells, and Klar fragments without LD are diffusely cytoplasmic. In embryos, Klar proteins truncated N-terminal to LD fail to localize to droplets. Together, the LD domain of Klar seems to be both necessary and sufficient for droplet localization. Lipid droplets are specific organelles with their own distinct set of proteins (Londos et al., 1999; Zweytick et al., 2000; Brown, 2001), but how these proteins are targeted is not well understood. The small size of the Klar-LD domain makes it a good model to dissect which features of the protein mediate localization.
Implications for Klar Function
Previous studies of the klar mutant phenotype had identified Klar as a key regulator for bidirectional motion of lipid droplets (Welte et al., 1998). Our analysis now shows that Klar is present at the correct time and the correct place to act as coordinator. Klar function depends on its localization: Klar proteins not localized to droplets fail to support normal droplet motion, and a Klar protein that lacks just the KASH domain is not sufficient for proper migration of photoreceptor nuclei.
In embryos, Klar is concentrated in a spot next to droplets, similar to the distribution of dynein (Gross et al., 2000), consistent with the model that Klar helps form a coordination complex that includes the plus- and minus-end motors (Welte et al., 1998). RFP-LD in S2 cells, in contrast, is present all over the droplet surface; there may be no motors bound to these droplets, or the tested Klar constructs may lack domains necessary to form complexes.
Isoforms α and β are important for regulating the motors powering nuclear migration and droplet transport, respectively. Klar regions common to both isoforms likely mediate shared functions, such as motor coordination. To this core region, the cell can attach variable domains via alternative splicing. These variable components encompass not only the cargo-targeting domains but also additional regions that differ between α and β. Variable regions might fine-tune Klar for particular tasks or might provide docking sites for specific transacting regulators, so that different cargoes could use the same motors and the same coordination machinery, yet still be regulated independently (Welte, 2004). Our analysis has disentangled this elaborate organization of Klar and sets the stage for determining the detailed roles of these modules.
Dissecting Klar function will likely illuminate many transport processes throughout Drosophila development. We detect Klar expression in specific tissues throughout embryogenesis, in larval stages and in adults. Because many Klar dots have no close association with either lipid droplets or nuclei, there are likely undiscovered Klar cargoes. The antibodies we have developed should make it possible to identify these cargoes, by using colocalization or fractionation approaches.
A General Regulatory Strategy
We propose the following framework how cells are able to use a single regulator to control multiple transport processes. The various Klar isoforms are composed of distinct modules: 1) a shared core region important for motor coordination, possibly by physically interacting with the motors; 2) one of several cargo-targeting domains that recognize the identity of the cargo; and 3) variable accessory regions. A shared core makes conserved contacts with the motor complexes and allows cells to reuse the same coordination machinery in different transport processes. Cargo-targeting domains deliver Klar to the correct location. And variable regions fine-tune the biochemical properties of Klar to the particular transport process. In this model, Klar serves as a crucial bridge between the identity of the cargo and motor complexes. The molecular and genetic tools we generated for Klar lay the groundwork for testing this model and for dissecting the mechanisms of Klar-based transport.
This regulatory strategy we discovered for Klar may be a general solution to the problem of how to employ a core regulator in a cargo-specific manner. For example, mammalian Syne-1 can localize to the Golgi, anchors nuclei to actin, and functions with the microtubule motor kinesin II in cytokinesis (Fan and Beck, 2004). Multiple transcripts, using three different transcription start sites, are predicted to encode at least four distinct protein isoforms (Gough et al., 2003) that contain either the KASH domain, Golgi binding sites, or both. For the C. elegans protein ANC-1, it is unknown whether different bands recognized by Western analysis represent functionally distinct isoforms (Starr and Han, 2002). However, ANC-1–dependent anchoring of nuclei to actin requires the nuclear envelope protein UNC-84/SUN, whereas anchoring of mitochondria does not, consistent with the possibility that different ANC-1 isoforms interact with cargo-specific partners. The Drosophila dystrophin-related gene Msp300 produces at least two alternatively spliced transcripts, one that encodes the KASH domain and one that does not (Rosenberg-Hasson et al., 1996). Klar may therefore provide a general paradigm how a single core regulator can be deployed in many different processes yet be controlled independently.
Acknowledgments
We thank J. A. Fischer, the Bloomington Stock Center (Bloomington, IN), and the Szeged Stock Center for fly stocks. We thank A. Jain for DNA sequencing, S. Cotton for Western analysis, P. Nawathean for help with cell culture experiments, M. Rosbash for use of the cell culture facility, and M. Marlow for generating the anti-Klar hybridomas. We are grateful to K. White, E. Wieschaus, N. Simister, J. Gindhart, H.-A. Müller, S. Gross, and E. Rubinstein for comments on the manuscript. This work was supported by a Basil O'Connor Starter Scholar Research Award (March of Dimes Birth Defects Foundation), a Kimmel Scholar Award (Sidney Kimmel Foundation for Cancer Research), and National Institute of General Medical Sciences grant GM-64687 (to M.A.W.). S. J. was supported by a summer research fellowship from the Howard Hughes Medical Institute. Confocal microscopy was made possible by National Institutes of Health Shared Instrumentation Grant S10RR16780.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0920) on January 12, 2005.
Abbreviations used: BDGP, Berkeley Drosophila genome project; LD, lipid-droplet domain of Klar.
References
- Almenar-Queralt, A., and Goldstein, L. S. (2001). Linkers, packages and pathways: new concepts in axonal transport. Curr. Opin. Neurobiol. 11, 550-557. [DOI] [PubMed] [Google Scholar]
- Apel, E. D., Lewis, R. M., Grady, R. M., and Sanes, J. R. (2000). Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 275, 31986-31995. [DOI] [PubMed] [Google Scholar]
- Bloom, G. S., and Goldstein, L. S. (1998). Cruising along microtubule highways: how membranes move through the secretory pathway. J. Cell Biol. 140, 1277-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. (2001). Lipid droplets: proteins floating on a pool of fat. Curr. Biol. 11, R446-R449. [DOI] [PubMed] [Google Scholar]
- Campbell, R. E., Tour, O., Palmer, A. E., Steinbach, P. A., Baird, G. S., Zacharias, D. A., and Tsien, R. Y. (2002). A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celniker, S. E., et al. (2002). Finishing a whole-genome shotgun: release 3 of the Drosophila melanogaster euchromatic genome sequence. Genome. Biol. 3, RESEARCH0079. [DOI] [PMC free article] [PubMed]
- De Vos, K. J., Sable, J., Miller, K. E., and Sheetz, M. P. (2003). Expression of Phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol. Biol. Cell 14, 3636-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J., and Beck, K. A. (2004). A role for the spectrin superfamily member Syne-1 and kinesin II in cytokinesis. J. Cell Sci. 117, 619-629. [DOI] [PubMed] [Google Scholar]
- Fischer, J. A. (2000). Molecular motors and developmental asymmetry. Curr. Opin. Genet. Dev. 10, 489-496. [DOI] [PubMed] [Google Scholar]
- Fischer-Vize, J. A., and Mosley, K. L. (1994). Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development 120, 2609-2618. [DOI] [PubMed] [Google Scholar]
- Gaunt, M. W., and Miles, M. A. (2002). An insect molecular clock dates the origin of the insects and accords with paleontological and biogeographic landmarks. Mol. Biol. Evol. 19, 748-761. [DOI] [PubMed] [Google Scholar]
- Gough, L. L., Fan, J., Chu, S., Winnick, S., and Beck, K. A. (2003). Golgi localization of Syne-1. Mol. Biol. Cell 14, 2410-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S., Welte, M., Block, S., and Wieschaus, E. (2000). Dynein-mediated cargo transport in vivo: a switch controls travel distance. J. Cell Biol. 148, 945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, S. P. (2004). Hither and yon: a review of bi-directional microtubule-based transport. Phys. Biol. 1, R1-R11. [DOI] [PubMed] [Google Scholar]
- Gross, S. P., Guo, Y., Martinez, J. E., and Welte, M. A. (2003). A determinant for directionality of organelle transport in Drosophila embryos. Curr. Biol. 13, 1660-1668. [DOI] [PubMed] [Google Scholar]
- Gross, S. P., Welte, M. A., Block, S. M., and Wieschaus, E. F. (2002). Coordination of opposite-polarity microtubule motors. J. Cell Biol. 156, 715-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa, N. (1998). Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519-526. [DOI] [PubMed] [Google Scholar]
- Liao, G. C., Rehm, E. J., and Rubin, G. M. (2000). Insertion site preferences of the P transposable element in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97, 3347-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos, C., Brasaemle, D. L., Schultz, C. J., Segrest, J. P., and Kimmel, A. R. (1999). Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin. Cell Dev. Biol. 10, 51-58. [DOI] [PubMed] [Google Scholar]
- Morgan, T. H. (1927). Experimental Embryology, New York: Columbia University Press.
- Mosley-Bishop, K. L., Li, Q., Patterson, K., and Fischer, J. A. (1999). Molecular analysis of the klarsicht gene and its role in nuclear migration within the differentiating cells of the Drosophila eye. Curr. Biol. 9, 1211-1220. [DOI] [PubMed] [Google Scholar]
- Myat, M. M., and Andrew, D. J. (2002). Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell 111, 879-891. [DOI] [PubMed] [Google Scholar]
- Nawathean, P., Menet, J. S., and Rosbash, M. (2005). Assaying the Drosophila negative feedback loop with RNA interference in S2 cells. In: Circadian Rhythm: Methods in Enzymology, Vol. 393, Yong, M. W. (ed.) Academic Press, 608-620. [DOI] [PubMed] [Google Scholar]
- Nurminsky, D. I., Nurminskaya, M. V., Benevolenskaya, E. V., Shevelyov, Y. Y., Hartl, D. L., and Gvozdev, V. A. (1998). Cytoplasmic dynein intermediate-chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol. Cell. Biol. 18, 6816-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohler, U., Liao, G. C., Niemann, H., and Rubin, G. M. (2002). Computational analysis of core promoters in the Drosophila genome. Genome Biol. 3, RESEARCH 0087. [DOI] [PMC free article] [PubMed]
- Patterson, K., Molofsky, A. B., Robinson, C., Acosta, S., Cater, C., and Fischer, J. A. (2004). The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol. Biol. Cell 15, 600-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov, V., Yi, J., Kashina, A., Oladipo, A., and Gross, S. P. (2003). Switching between microtubule- and actin-based transport systems in melanophores is controlled by cAMP levels. Curr. Biol. 13, 1837-1847. [DOI] [PubMed] [Google Scholar]
- Rørth, P. (1996). A modular misexpression screen in Drosophila detecting tissue-specific phenotypes. Proc. Natl. Acad. Sci. USA 93, 12418-12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Hasson, Y., Renert-Pasca, M., and Volk, T. (1996). A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech. Dev. 60, 83-94. [DOI] [PubMed] [Google Scholar]
- Stapleton, M., et al. (2002). The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome. Res. 12, 1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr, D. A., and Han, M. (2002). Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406-409. [DOI] [PubMed] [Google Scholar]
- Starr, D. A., and Han, M. (2003). ANChors away: an actin based mechanism of nuclear positioning. J. Cell Sci. 116, 211-216. [DOI] [PubMed] [Google Scholar]
- Tai, A. W., Chuang, J. Z., and Sung, C. H. (2001). Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J. Cell Biol. 153, 1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The FlyBase Consortium. (2003). The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 31, 172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale, R. D. (2003). The molecular motor toolbox for intracellular transport. Cell 112, 467-480. [DOI] [PubMed] [Google Scholar]
- Welte, M. A. (2004). Bidirectional Transport along Microtubules. Curr. Biol. 14, R525-537. [DOI] [PubMed] [Google Scholar]
- Welte, M. A., Gross, S. P., Postner, M., Block, S. M., and Wieschaus, E. F. (1998). Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547-557. [DOI] [PubMed] [Google Scholar]
- Whited, J. L., Cassell, A., Brouillette, M., and Garrity, P. A. (2004). Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development 131, 4677-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus, E., and Nüsslein-Volhard, C. (1986). Looking at embryos. In: Drosophila - A Practical Approach, ed. D. B. Roberts, Oxford: IRL Press, 199-227.
- Zhang, Q., Skepper, J. N., Yang, F., Davies, J. D., Hegyi, L., Roberts, R. G., Weissberg, P. L., Ellis, J. A., and Shanahan, C. M. (2001). Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 114, 4485-4498. [DOI] [PubMed] [Google Scholar]
- Zweytick, D., Athenstaedt, K., and Daum, G. (2000). Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta 1469, 101-120. [DOI] [PubMed] [Google Scholar]