1. INTRODUCTION
Inorganic arsenic (iAs) in food and water is a major global health concern. An established carcinogen, chronic arsenic exposure also increases the risk of cardiovascular disease, respiratory disease, neurologic deficits, and diabetes.(Chen et al. 2011; Council 2001; Kuo et al. 2013; Moon et al. 2012; Naujokas et al. 2013; Tyler and Allan 2014; Wu et al. 2014) After ingestion, iAs (arsenate and arsenite) is metabolized into mono- and di-methylated arsenicals (MMA and DMA); DMA has a shorter circulating half-life and is more rapidly excreted through the urine as compared to iAs.(Aposhian and Aposhian 2006; Challenger 1951; Cullen WR 1989; Hayakawa et al. 2005; Naranmandura et al. 2006; Vahter 2002) The urinary distribution of arsenic metabolites across human populations ranges from 10–30% for iAs, 10–20% for MMA and 60–80% for DMA.(Chiou et al. 1997; Del Razo et al. 1997; Hopenhayn-Rich et al. 1996; Navas-Acien et al. 2009; Vahter 2000) Higher percentages of iAs (iAs%) and MMA (MMA%) and lower percentages of DMA (DMA%) in urine are thought to reflect a less efficient arsenic metabolism profile and have been associated with higher risk of cancer, skin lesions and cardiovascular disease.(Chen et al. 2003a; Chen et al. 2003b; Chen et al. 2005; Del Razo et al. 1997; Hsueh et al. 1997; Steinmaus et al. 2006; Wu et al. 2006; Yu et al. 2000) Conversely, higher DMA% and lower MMA% have been associated with diabetes, metabolic syndrome and higher body mass index.(Chen et al. 2012; Del Razo et al. 2011; Kuo et al. 2015; Mendez et al. 2016; Nizam et al. 2013; Wang et al. 2007) Understanding non-modifiable (genetics, sex, life-stage) and modifiable (smoking, alcohol intake, kidney function, body mass index, nutrition) determinants of arsenic metabolism is important given the role of arsenic metabolism in arsenic toxicity.(Balakrishnan et al. 2016; Council 2013; Gribble et al. 2013; Jansen et al. 2016)
Nutritional status is a major susceptibility factor for arsenic-related disease, at least in part through the impact of nutrition on one-carbon metabolism (OCM).(Council 2013) OCM, a network of interrelated biochemical reactions dependent on sufficient intake of vitamin B2 (riboflavin), vitamin B6, folate (vitamin B9) and vitamin B12, plays an essential role in methylation processes throughout the body, including the methylation reactions involved in arsenic metabolism (Figure 1).(Hall and Gamble 2012; Howe et al. 2014) In studies from Bangladesh, both cross-sectional (Gamble et al. 2005) and folic acid supplementation trials demonstrated (Gamble et al. 2006; Peters et al. 2015) that higher folate is associated with increased arsenic methylation efficiency, resulting in higher DMA% and lower iAs% and MMA% in urine and in reduced blood arsenic concentrations. In cross-sectional studies, greater dietary intake of vitamins B12 and B2 (Heck et al. 2007) and higher plasma B12 (Hall et al. 2009a) have been associated with lower iAs% and higher MMA%, in Bangladeshi adults. Further, both epidemiologic (Chung et al. 2006; Pilsner et al. 2009; Zablotska et al. 2008) and experimental studies (Acharyya et al. 2015; Bhattacharjee S 2013) have reported OCM nutrients to be associated with lower risk for arsenic-related disease.
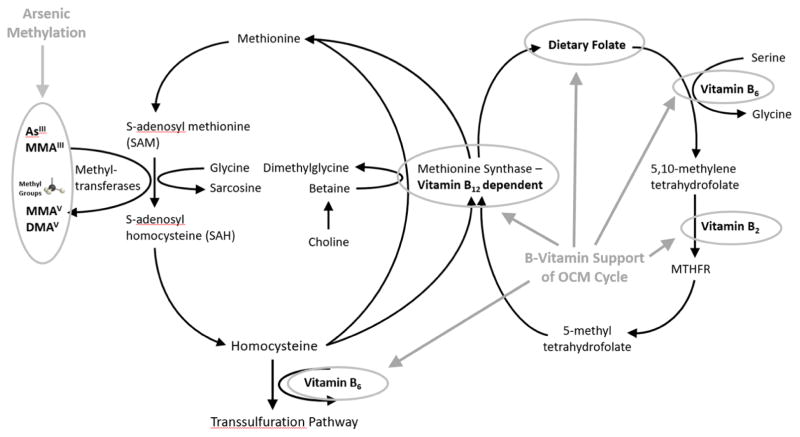
Figure 1. One-Carbon Metabolism, B-vitamins, and Arsenic Metabolism.
Methionine is activated by methionine adenosyltransferase to form S-adenosylmethionine (SAM). SAM provides the methyl group for most methylation reactions in the body (including arsenic methylation reactions), after which adenosylhomocysteine (SAH) is formed as a byproduct. SAH is hydrolyzed to homocysteine, which is then used in the transsulfuration pathway, or is regenerated into methionine via betaine or B12 dependent pathways. Dietary folate is converted to 5-methyl tetrahydrofolate through B6 and B2 dependent reactions, which provides the methyl group for the regeneration of homocysteine to methionine via the B12 pathway.
The generalizability of the OCM findings in Bangladesh to US populations with low-moderate arsenic exposure and different dietary patterns is unclear. We evaluated the association of OCM nutrients with arsenic metabolism biomarkers in the Strong Heart Study (SHS), a population-based cohort study initiated to assess cardiovascular risk factors in American Indian adults residing in Arizona, Oklahoma and North and South Dakota. We used dietary intake estimates of B2, B6, folate and B12 as measures of OCM nutrients and percentages of urinary inorganic arsenic (iAs%) and its methylated metabolites (MMA% and DMA%), as measures of arsenic metabolism. We also modeled the complexity of both arsenic metabolism profiles and nutrition intake through the use of principal component analysis (PCA).
2. METHODS
2.1 Study Population
The SHS recruited 4,549 American Indians from 13 tribes located in Arizona, Oklahoma and North and South Dakota. Eligible participants were men and women aged 45–74 years at the baseline visit in 1989–1991. The overall participation rate was 62%. All participants provided informed consent and study protocols were approved by multiple institutional review boards, community members and The Indian Health Service. In 2016, one of the communities withdrew their consent for participating in future studies, reducing the overall sample size to 3,516. The final version of this manuscript, along with a lay summary, was sent to, and approved by, all remaining communities.
At the baseline visit (1989–1991), a random sample of 50 males and 50 females from each age decade and at each study site (n=722; 508 after excluding the community that withdrew consent) was selected to participate in a self-administered food frequency questionnaire (FFQ), which provided estimated long-term daily average intake of folate and vitamins B2, B6 and B12 in milligrams.(Committee 1989) We excluded 94 participants with missing data on urine arsenic, and 9 participants missing data on education, alcohol intake, smoking status, body mass index (BMI), estimated glomerular filtration rate (eGFR), and urine creatinine, leaving 405 participants for this study. Participants included in this study were similar to the overall study population on most variables of interest, with the exception of being slightly older than the full cohort (Supplemental Material Table S1).
2.2 Data Collection
Baseline visits included bio-specimen collection, a physical exam, and an interview-administered questionnaire. Visits were performed by trained and certified examiners according to a standardized protocol. Details have been described previously.(Lee et al. 1990)
2.2.1 Urine Arsenic Metabolites
Morning spot urine samples were collected during baseline visit in polypropylene tubes, frozen within 1 to 2 hours of collection, shipped buried in dry ice and stored at <−70°C in the Penn Medical Laboratory, MedStar Research Institute, Washington, DC.(Lee et al. 1990) The freezers have been operating under a strict quality control system to guarantee secure sample storage. For arsenic analyses, urine samples were thawed in 2009–2010, and up to 1.0 mL from each urine sample was transferred to a small vial, transported on dry ice to the Trace Element Laboratory at Graz University, Austria and stored at <−70°C until analyses.(Scheer et al. 2012)
Quality control and quality assurance methods for urine arsenic analysis have been described in detail.(Scheer et al. 2012) Urine concentrations of arsenite, arsenate, methylarsonate (MMA) and dimethylarsinate (DMA) were measured using high performance liquid chromatography/inductively coupled plasma-mass spectrometry (HPLC/ICPMS). The limits of detection were 0.1 μg/L for arsenite, arsenate, MMA and DMA. The inter-assay coefficients of variation for arsenite and arsenate, MMA and DMA were 5.6%, 6.3%, 3.5% and 4.4% respectively. Urine arsenobetaine concentrations were measured using cation-exchange HPLC/ICPMS together with other less common arsenic cations, and the low concentration measured (median (IQR): 0.73 (0.49, 1.47) μg/L) confirmed seafood consumption in this population was infrequent. The limit of detection for urine arsenobetaine was 0.1 μg/L and the inter-assay coefficient of variation was 6.9%. No participants in this analysis had arsenic species below the limit of detection.
2.2.2 Nutrition Variables
Dietary intake of OCM-related micronutrients was measured during the baseline visit through estimated daily averages of dietary intake of vitamins B2, B6, folate and B12 in the past-year. These variables, as well as total caloric intake, were measured through an interviewer-administered Block 119-item food frequency questionnaire (FFQ). The Block questionnaire is one of the most widely used questionnaires with demonstrated reliability and validity.(Fretts et al. 2012) Each participant was asked how often, on average, a particular food was consumed during the past year using measures of consumption frequency and portion size adjustment.(Fretts et al. 2012)To enhance accuracy of the questionnaire in this cohort, additional questions relating to foods commonly consumed by American Indians were added.(Fretts et al. 2012) Of note, folate was calculated based on dietary intake alone as doses of folic acid supplementation were not available (only whether supplements were taken or not) and mandatory folic acid fortification was not implemented by the date of FFQ administration.
2.2.3 Other Variables
Sociodemographic (age, sex, and education) and life-style (vitamin supplementation use and drinking and smoking status) study variables were ascertained through a standardized questionnaire (separate from the FFQ) by trained and certified interviewers.(Lee et al. 1990) Height and weight measurements for BMI calculation (weight in kilometers divided by height in meters squared) were conducted during the physical exam. Urine creatinine was measured from the spot urine samples collected for arsenic analysis using an automated alkaline picrate methodology.(Lee et al. 1990) eGFR was calculated from creatinine, age and sex using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, as previously described.(Shara et al. 2012)
2.3 Statistical Analysis Methods
To evaluate arsenic metabolism, we computed the relative proportions of inorganic and methylated arsenic metabolites by dividing each metabolite concentration over the sum of those species x 100. In this way, a methylation profile was estimated for each participant, consisting of iAs%, MMA% and DMA%, totaling to 100%. We also evaluated arsenic metabolism using principal component analysis (PCA), as recently proposed.(Balakrishnan et al. 2016; Jansen et al. 2016) PCA was conducted using each arsenic species percentage, then scaling them. PCA is useful in the analysis of arsenic metabolism biomarkers because it removes the inter-dependence of the three biomarkers (iAs%, MMA% and DMA%) allowing for an improved, and potentially more biologically meaningful, interpretation of each metabolite. Previous studies have suggested that the first arsenic metabolism principal component (As metabolism PC1) reflects the ability to produce DMA (i.e., the secondary methylation step) while the second arsenic metabolism principal component (As metabolism PC2) reflects the conversion of iAs to MMA (i.e., the primary methylation step).(Balakrishnan et al. 2016; Jansen et al. 2016) Inorganic arsenic exposure was estimated as the sum of urinary concentrations of iAs, MMA and DMA (ΣAs). ΣAs concentrations were right skewed and log-transformed for the analyses.
The analysis of dietary estimates of OCM nutrients (vitamin B2, vitamin B6, folate and vitamin B12) requires adjustment for total caloric intake in most analyses. Because nutrient and total caloric intake variables were measured from the same questionnaire, their errors are strongly correlated. To cancel out these errors and improve the validity of energy-adjusted nutrients, nutrient density and residual methods have been suggested over simply including caloric intake as a variable in regression models.(Rhee et al. 2014) We used a residual analysis approach and regressed each log-transformed vitamin intake on log-transformed total caloric intake. We then added the mean log-transformed value of each nutrient to the nutrient residuals to create calorie-corrected nutrient variables. Because some OCM nutrients share common dietary sources and they are metabolically inter-dependent, we also used PCA to identify major PCs across log-transformed, calorie-corrected OCM nutrients.
Arsenic methylation patterns were compared across sociodemographic, health, nutrition and behavioral characteristic variable categories using Kruskal-Wallis tests. Pearson correlation coefficients were conducted to estimate univariate correlations between iAs%, MMA%, DMA% and calorie-corrected vitamins (vitamin B2, vitamin B6, folate and vitamin B12) (Figure 2).
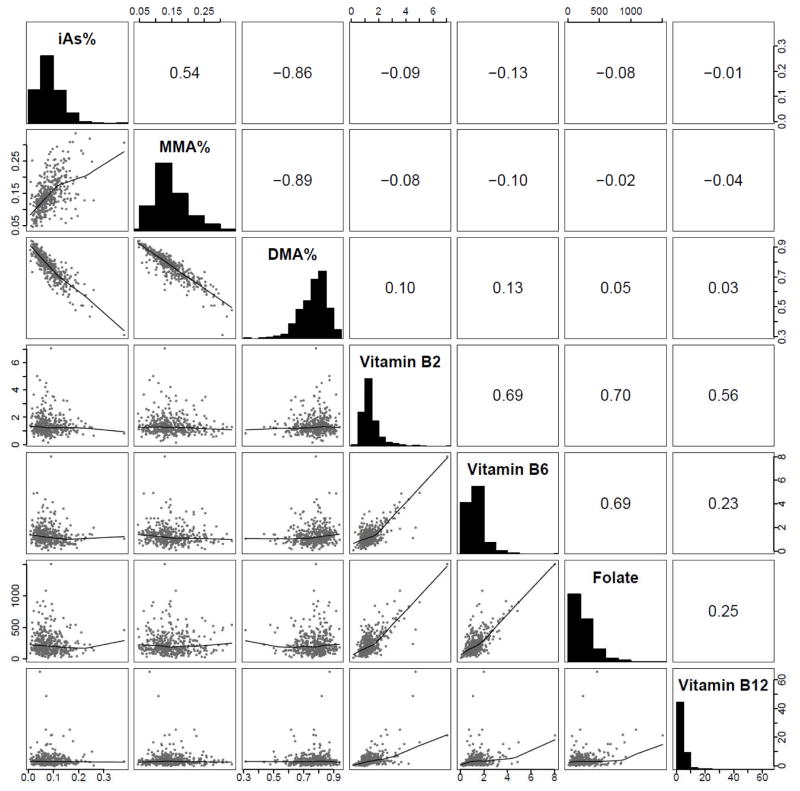
Figure 2. Pearson Correlation Matrix for Arsenic Species Percentages and Calorie-Corrected Nutrition Variables.
Correlations and histograms of arsenic species percentages and calorie-corrected, using a residual analysis method of calorie-adjustment, nutrition variables (B12 outlier not included).
The mean difference (95% CI) of each of arsenic species percentages (iAs%, MMA%, DMA%) and arsenic metabolism principal component (As metabolism PC1 and As metabolism PC2) comparing the two highest to the lowest tertile of each calorie-corrected nutrient variable (vitamin B2, vitamin B6, folate and vitamin B12) was estimated using linear regression models. We used the following progressive adjustments for known arsenic metabolism and nutrition intake determinants in our models evaluating arsenic metabolism both as individual arsenic species’ percentages as well as principal components: model 1 adjusted for ΣAs (log-transformed) and urine creatinine (log-transformed); model 2 further adjusted for age (continuous), sex, study center (Arizona, Oklahoma, North/South Dakota), BMI (continuous), smoking status (never, former, current), alcohol use (never, former, current), and eGFR (continuous); model 3 further adjusted for the other OCM calorie-corrected nutrient variables. Because adjustment for all nutrients at once can be difficult due to possible collinearity, we also used OCM PCs to evaluate the association of arsenic metabolism biomarkers with independent summary variables of calorie-corrected nutrients.
We conducted multiple sensitivity analyses. First, we evaluated the potential impact of outliers in our models using resistant regression with consistent results (data not shown). Second, we evaluated an alternative method of correcting nutrients for total caloric intake (log-transformed) using adjustment in the main regression analysis instead of the residual-based approach, with similar findings (data not shown). Third, we used specific gravity as an alternative to urinary creatinine to account for urine dilution, with consistent results (Supplemental Material Table S2). Main analyses include adjustment for urinary creatinine as it has been shown to affect arsenic metabolism, possibly through the competition for methyl groups in the synthesis of creatine, the precursor to creatinine.(Peters et al. 2015) Urinary creatinine reflects both dietary creatine intake (primarily from meat) and endogenous synthesis of creatine. In addition, we ran regressions with and without a participant with an outlying high (>100μg) value for B12, again with consistent results (data not shown). We also ran regressions with and without participants reported to take folic acid or multivitamin supplements (n=9) with consistent results (data not shown). Further, to address potential concerns regarding DMA from food sources at low levels of arsenic exposure, we stratified by center due to geographical differences in exposure sources (water is a major source in Arizona and North and South Dakota, while in Oklahoma the predominant source is food). Results were similar in both areas of lower exposure (Oklahoma) and higher exposure (Arizona and Dakotas) (Supplemental Material Table S2). Finally, exploratory analyses were conducted to investigate potential pairwise interactions between each OCM nutrient. A joint categorical variable reflecting high (equal to or greater than the median) and low (below the median) intake, as well as quantitative interaction terms, of each OCM nutrient pair, was created to assess these relationships (Supplemental Material Table S3).
2.3.1 Sample Size Justification
Our sample size was fixed as our analysis was based on previously collected data. Therefore, we conducted a minimally detectable effect estimate for each of our main outcomes (iAs%, MMA% and DMA%). We used a sample size of 405 and 80% power for each calculation. For iAs%, using a mean of 8.4 and standard deviation of 4.7, we computed the minimally detectable difference to be 0.66. For MMA%, using a mean of 14.7 and standard deviation of 5.3, we computed the minimally detectable difference to be 0.70. For DMA%, using a mean of 76.9 and standard deviation of 8.8, we computed the minimally detectable difference to be 1.23.
3. RESULTS
3.1 Participant Characteristics
Median (IQR) percentages for arsenic metabolism biomarkers were 7.1 (5.2–10.7)% for iAs%, 13.7 (11.3–17.7)% for MMA%, and 78.2 (71.6–83.3)% for DMA% (Table 1). Median (IQR) for arsenic species concentrations were 0.74 (0.33, 1.58) μg/L for iAs, 1.36 (0.71, 2.65) μg/L for MMA and 7.59 (4.69, 13.52) μg/L for DMA. Median (IQR) daily intake for OCM nutrients were 1.4 (0.9–2.0) mg for vitamin B2, 1.2 (0.8–1.9) mg for vitamin B6, 207 (120.4–336.7) μg for folate, and 3.0 (1.7–5.7) μg for vitamin B12. Several characteristics were associated with higher iAs%, higher MMA% and lower DMA%, including being male, lower BMI, current smoker and current alcohol drinker. Characteristics associated with just higher iAs% included being younger, having eGFR > 60 mL/min/1.73 m2 and having lower intake of B6 and folate. Compared to tertiles 1 and 3, participants in the 2nd tertile of urine creatinine had lower iAs% and higher DMA% (Table 1). Pearson correlation coefficients were moderately positive for iAs% and MMA% (0.54), and strongly negative between both iAs% and MMA% with DMA% (−0.86 and −0.89, respectively) (Figure 2). All OCM nutrition variables were positively correlated with each other both before and after correcting for caloric intake. Calorie-corrected nutrient correlations ranged from 0.23 between vitamin B12 and vitamin B6 to 0.70 between vitamin B2 and folate. Correlations between OCM nutrients were negative with both MMA% and iAs% (ranging from −0.01 for iAs% and vitamin B12 to −0.13 for iAs% and vitamin B6); and positive with DMA% (ranging from 0.03 with vitamin B12 to 0.13 with vitamin B6)(Figure 2).
Table 1.
Participant Characteristics by Arsenic Metabolism (N=405)
| N | iAs% median (IQR) | P-valuea | MMA% median (IQR) | P-valuea | DMA% median (IQR) | P-valuea | |
|---|---|---|---|---|---|---|---|
| Overall | 405 | 7.1 (5.2, 10.7) | 13.7 (11.3, 17.7) | 78.2 (71.6, 83.3) | |||
| Age (years) | |||||||
| <55 | 159 | 8.0 (5.8, 11.7) | 13.3 (11.3, 18.0) | 77.8 (70.6, 82.1) | |||
| 55 to <65 | 128 | 6.9 (5.3, 10.6) | 13.5 (11.2, 17.5) | 79.5 (71.0, 83.5) | |||
| ≥65 | 118 | 6.6 (4.4, 9.5) | 0.007 | 14.6 (11.3, 17.4) | 0.92 | 77.9 (73.1, 83.7) | 0.46 |
| Sex | |||||||
| Male | 181 | 9.2 (6.3, 12.7) | 16.2 (13.0, 19.5) | 75.1 (68.6, 80.2) | |||
| Female | 224 | 6.4 (4.6, 9.0) | <0.001 | 12.6 (9.6, 15.1) | <0.001 | 80.9 (75.7, 84.9) | <0.001 |
| Σ As (μg/L) | |||||||
| 0.70 – <7.10 | 135 | 7.0 (5.0, 10.5) | 13.8 (11.3, 17.2) | 78.8 (73.1, 82.4) | |||
| 7.10 – 14.0 | 136 | 6.9 (4.6, 10.5) | 14.2 (10.6, 17.9) | 78.7 (71.7, 84.3) | |||
| > 14.0 – 87.2 | 134 | 8.0 (5.6, 11.5) | 0.07 | 13.3 (11.5, 18.2) | 0.87 | 77.3 (70.4, 82.6) | 0.23 |
| BMI (kg/m3) | |||||||
| <25 | 72 | 10.3 (7.7, 12.4) | 18.0 (13.2, 21.8) | 71.6 (65.6, 77.3) | |||
| 25 to <30 | 141 | 7.1 (5.1, 10.6) | 14.2 (11.8, 17.9) | 77.9 (72.4, 82.5) | |||
| ≥30 | 192 | 6.5 (4.6, 9.7) | <0.001 | 12.8 (10.1, 15.9) | <0.001 | 80.6 (75.0, 84.7) | <0.001 |
| Smoking Status | |||||||
| Never | 114 | 6.1 (4.3, 9.1) | 12.8 (9.4, 15.6) | 80.3 (75.0, 86.1) | |||
| Former | 138 | 6.8 (4.8, 9.9) | 13.5 (11.7, 17.7) | 79.1 (72.6, 83.7) | |||
| Current | 153 | 9.3 (6.4, 12.6) | <0.001 | 14.7 (12.3, 18.6) | <0.001 | 76.1 (68.8, 80.8) | <0.001 |
| Alcohol | |||||||
| Never | 66 | 5.9 (4.0, 9.2) | 12.8 (9.7, 15.9) | 80.4 (73.6, 85.5) | |||
| Former | 188 | 6.9 (4.6, 10.3) | 13.3 (10.5, 17.2) | 79.4 (73.3, 84.0) | |||
| Current | 151 | 8.8 (6.4, 12.3) | <0.001 | 14.7 (12.6, 18.8) | <0.001 | 76.2 (68.8, 81.0) | <0.001 |
| eGFR (mL/min) | |||||||
| ≤60 | 367 | 5.5 (3.8, 8.3) | 13.3 (9.6, 16.8) | 80.1 (75.1, 85.2) | |||
| >60 | 38 | 7.5 (5.4, 10.9) | 0.001 | 13.7 (11.3, 18.0) | 0.55 | 78.1 (71.2, 82.7) | 0.06 |
| Urine Creatinine (mg/dL) | |||||||
| <0.95 | 136 | 8.5 (5.9, 11.9) | 13.4 (11.4, 16.7) | 78.0 (71.2, 81.9) | |||
| 0.95 – 1.50 | 134 | 6.4 (4.4, 9.3) | 13.6 (10.5, 16.9) | 79.4 (73.6, 85.0) | |||
| >1.50 | 135 | 7.1 (5.0, 11.4) | <0.001 | 14.7 (11.9, 19.0) | 0.12 | 78.2 (69.4, 83.1) | 0.02 |
| Total Caloric Intake (kcal) | |||||||
| <1,230 | 135 | 7.5 (5.1, 10.5) | 13.4 (11.3, 16.5) | 78.7 (72.1, 83.3) | |||
| 1,230–1,968 | 135 | 8.1 (5.4, 11.9) | 14.2 (10.8, 19.8) | 77.0 (69.5, 83.4) | |||
| >1,968 | 135 | 6.7 (5.0, 10.5) | 0.14 | 13.7 (11.5, 17.3) | 0.36 | 79.3 (72.5, 83.5) | 0.24 |
| Vitamin B2 (mg)b | |||||||
| <1.07 | 139 | 8.3 (5.4, 11.5) | 14.1 (11.5, 18.8) | 77.2 (69.9, 81.9) | |||
| 1.07–1.76 | 132 | 7.0 (4.9, 10.8) | 13.3 (10.9, 17.8) | 78.4 (72.0, 83.8) | |||
| >1.76 | 134 | 6.9 (5.4, 10.1) | 0.12 | 13.7 (10.8, 17.1) | 0.39 | 79.0 (73.6, 83.5) | 0.11 |
| Vitamin B6 (mg)b | |||||||
| <0.93 | 135 | 8.5 (5.6, 12.9) | 13.6 (11.5, 18.4) | 76.9 (70.5, 82.1) | |||
| 0.93–1.68 | 135 | 7.7 (5.1, 11.4) | 14.3 (11.4, 18.5) | 77.8 (69.7, 82.6) | |||
| >1.68 | 135 | 6.7 (4.9, 9.3) | 0.005 | 13.2 (10.2, 16.9) | 0.05 | 80.2 (74.8, 84.2) | 0.005 |
| Folate (μg)b | |||||||
| <144.1 | 135 | 8.5 (5.7, 12.8) | 14.1 (12.3, 18.0) | 76.9 (69.9, 81.4) | |||
| 144.1–285.2 | 135 | 6.9 (4.9, 10.5) | 13.2 (10.5, 17.7) | 79.2 (72.1, 84.4) | |||
| >285.2 | 135 | 6.9 (5.0, 10.2) | 0.03 | 13.7 (10.0, 17.9) | 0.22 | 79.3 (71.8, 83.7) | 0.04 |
| Vitamin B12 (μg)b | |||||||
| <2.20 | 136 | 8.0 (5.3, 11.5) | 14.2 (11.2, 18.7) | 77.3 (70.7, 82.2) | |||
| 2.20–4.41 | 134 | 6.8 (4.9, 10.3) | 13.3 (11.2, 17.1) | 79.5 (72.6, 83.6) | |||
| >4.41 | 135 | 7.2 (5.3, 11.2) | 0.15 | 13.9 (11.3, 17.7) | 0.53 | 78..2 (71.3, 83.5) | 0.21 |
ΣAs, total urinary inorganic arsenic; BMI, body mass index; eGFR, estimated glomerular filtration rate
Kruskal-Wallis tests were used to compare methylation medians across variable categories
Vitamins are displayed as crude values, not calorie-adjusted
Variance in arsenic methylation patterns were summarized in two principal components. Arsenic metabolism principal component 1 (As PC1) explained 84.6% of the variance in arsenic species biomarkers and reflected higher DMA% and lower iAs% and MMA% (Table 2). Arsenic metabolism principal component (As PC2) explained 15.4% of the variance and reflected higher MMA% and lower iAs%, independent of DMA%. Variance in OCM nutrients was summarized in four principal components (Table 2). OCM PC1 explained 60.1% of the variance in OCM nutrients and reflected higher intake of all four B-vitamins. OCM PC2 explained 22.7% of the variance and reflected higher vitamin B12 intake and lower intake of vitamin B6 and folate. OCM PC3 explained 10.3% of the variance and reflected higher folate and B2 intake and lower intake of vitamins B6 and vitamin B12. OCM PC4 explained 6.9% of variance and reflected higher intake of vitamin B12 and folate and lower intake of the other nutrients.
Table 2.
Summary of Principal Components for Arsenic Species and Calorie-Adjusted Nutrition Variables
| As Metabolism PC1 | As Metabolism PC2 | OCM PC1 | OCM PC3 | OCM PC3 | OCM PC4 | |
|---|---|---|---|---|---|---|
| Variance explained (%) | 84.6 | 15.4 | 60.1 | 22.7 | 10.3 | 6.9 |
| Standard deviation | 1.59 | 0.68 | 1.55 | 0.95 | 0.64 | 0.52 |
| Weights for iAs% | −0.55 | −0.73 | -- | -- | -- | -- |
| Weight for MMA% | −0.56 | 0.69 | -- | -- | -- | -- |
| Weight for DMA% | 0.63 | −0.03 | -- | -- | -- | -- |
| Weight for Vitamin B2 | -- | -- | 0.57 | 0.13 | 0.47 | −0.66 |
| Weight for Vitamin B6 | -- | -- | 0.53 | −0.26 | −0.79 | −0.16 |
| Weight for Folate | -- | -- | 0.52 | −0.46 | 0.37 | 0.62 |
| Weight for Vitamin B12 | -- | -- | 0.36 | 0.84 | −0.12 | 0.39 |
As, arsenic; PC, principal component; OCM, one-carbon metabolism
3.2 Association of One-Carbon Metabolism Nutrients and Arsenic Metabolism Biomarkers
OCM nutrients, specifically vitamins B6 and B2, were associated with more efficient arsenic methylation profiles. In fully adjusted models (Table 3, Model 2), higher intake of vitamins B2 and B6 were associated with lower iAs%, lower MMA% and higher DMA%. Compared to tertile 1, participants in tertile 3 of vitamin B2 intake had 1.00 (95% CI: −2.00, 0.00)% lower iAs%, 1.36 (95% CI: −2.48, −0.23)% lower MMA% and 2.36 (95% CI: 0.54, 4.18)% higher DMA%. Correspondingly, participants in tertile 3 of vitamin B6 intake had 1.36 (95% CI: −2.35, −0.37)% lower iAs%, 1.57 (95% CI: −2.69, −0.45)% lower MMA% and 2.93 (95% CI: 1.13, 4.74)% higher DMA%. These associations, with the exception of vitamin B2 and iAs%, remained statistically significant and similar in magnitude after further adjustment for all OCM nutrients (Table 3, Model 3).
Table 3.
Mean Difference (95% CI) in iAs%, MMA% and DMA% by Calorie-Adjusted Nutrition Tertiles (N=405)
| Model | Model 1a | Model 2b | Model 3c |
|---|---|---|---|
| Vitamin B2 (mg) | |||
|
| |||
| iAs% | |||
| <1.073 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1.073 – 1.43 | −0.09 (−1.19, 1.01) | −0.21 (−1.20, 0.79) | −0.29 (−1.35, 0.77) |
| >1.43 | −0.90 (−2.00, 0.20) | −1.00 (−2.00, 0.00) | −0.68 (−1.89, 0.53) |
| MMA% | |||
| <1.073 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1.073 – 1.43 | −0.47 (−1.75, 0.81) | −0.59 (−1.71, 0.53) | −0.69 (−1.88, 0.50) |
| >1.43 | −0.96 (−2.24, 0.33) | −1.36 (−2.48, −0.23) | −1.58 (−2.94, −0.22) |
| DMA% | |||
| <1.073 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1.073 – 1.43 | 0.57 (−1.53, 2.67) | 0.80 (−1.01, 2.61) | 0.98 (−0.99, 2.90) |
| >1.43 | 1.86 (−0.25, 3.97) | 2.36 (0.54, 4.18) | 2.26 (0.06, 4.46) |
|
| |||
| Vitamin B6 (mg) | |||
|
| |||
| iAs% | |||
| <0.936 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 0.936 – 1.4241 | −0.21 (−1.29, 0.88) | 0.08 (−0.90, 1.06) | 0.11 (−0.69, 1.39) |
| >1.4241 | −1.92 (−3.01, −0.84) | −1.36 (−2.35, −0.37) | −1.18 (−2.32, −0.03) |
| MMA% | |||
| <0.936 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 0.936 – 1.4241 | −1.28 (−2.55, −0.02) | −0.78 (−1.89, 0.34) | −0.85 (−2.00, 0.30) |
| >1.4241 | −2.29 (−3.56, −1.03) | −1.57 (−2.69, −0.45) | −1.80 (−3.09, −0.51) |
| DMA% | |||
| <0.936 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 0.936 – 1.4241 | 1.49 (−0.58, 3.56) | 0.69 (−1.10, 2.49) | 0.74 (−1.12, 2.60) |
| >1.4241 | 4.21 (2.14, 6.28) | 2.93 (1.13, 4.74) | 2.98 (0.89, 5.07) |
|
| |||
| Folate (μg) | |||
|
| |||
| iAs% | |||
| <149 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 149 – 266 | −0.67 (−1.77, 0.42) | −0.40 (−1.40, 0.60) | −0.06 (−1.10, 0.98) |
| >266 | −1.31 (−2.41, −0.21) | −0.80 (−1.79, 0.20) | 0.14 (−1.07, 1.34) |
| MMA% | |||
| <149 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 149 – 266 | −1.50 (−2.82, −0.27) | −0.93 (−2.06, 0.20) | −0.29 (−1.46, 0.88) |
| >266 | −0.70 (−1.97, 0.58) | −0.18 (−1.30, 0.93) | 1.27 (−0.08, 2.62) |
| DMA% | |||
| <149 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 149 – 266 | 2.22 (0.13, 4.32) | 1.33 (−0.50, 3.16) | 0.34 (−1.55, 2.23) |
| >266 | 2.01 (−0.09, 4.10) | 0.98 (−0.83, 2.79) | −1.41 (−3.59, 0.78) |
|
| |||
| Vitamin B12 (μg) | |||
|
| |||
| iAs% | |||
| <2.20 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2.20 – 3.93 | 0.36 (−0.74, 1.46) | 0.52 (−0.47, 1.51) | 0.74 (−0.31, 1.78) |
| >3.93 | −0.27 (−1.37, 0.83) | −0.55 (−1.54, 0.44) | −0.06 (−1.16, 1.04) |
| MMA% | |||
| <2.20 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2.20 – 3.93 | −0.51 (−1.80, 0.77) | −0.44 (−1.57, 0.68) | 0.15 (−1.03, 1.32) |
| >3.93 | 0.01 (−1.28, 1.29) | −0.37 (−1.49, 0.76) | 0.54 (−0.69, 1.78) |
| DMA% | |||
| <2.20 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2.20 – 3.93 | 0.15 (−1.96, 2.26) | −0.08 (−1.90, 1.74) | −0.89 (−2.79, 1.01) |
| >3.93 | 0.26 (−1.85, 2.38) | 0.92 (−0.90, 2.74) | −0.48 (−2.48, 1.51) |
Adjusted for total log arsenic and log urinary creatinine
Adjusted for total log arsenic, log urinary creatinine age, sex, center, smoking, alcohol, eGFR, BMI
Adjusted for total log arsenic, log urinary creatinine age, sex, center, smoking, alcohol, eGFR, BMI, all b-vitamins of interest
In fully adjusted models without adjustment for other OCM nutrients (Table 3, Model 2), folate showed a similar association with arsenic metabolism biomarkers as vitamins B2 and B6, although they were weaker, not statistically significant, and stronger for the second tertile than the third. For example, participants in tertile 2 of folate intake compared to tertile 1 had 0.93 (95% CI: −2.06, 0.20) lower MMA% but participants in tertile 3 of folate intake compared to tertile 1 had only 0.18 (95% CI: −1.30, 0.93) lower MMA%. With further adjustment for other OCM nutrients, the association with tertile 2 of folate was attenuated, whereas tertile 3 reversed direction for all arsenic metabolism biomarkers, although the associations remained not-statistically significant. Again, using MMA% as an example, compared to tertile 1, tertile 2 was associated with 0.29 (95% CI: −1.46, 0.88) lower MMA% and tertile 3 was as associated with 1.27 (95% CI: −0.08, 2.62) greater MMA%. The corresponding associations with vitamin B12 were weaker and not statistically significant in any model.
When modeling arsenic metabolism using PCA, tertile 3 of vitamins B2 and B6 intake were associated with higher As metabolism PC1, reflecting higher DMA% and lower iAs% and MMA% (Table 4). No significant associations were observed with any of the OCM nutrients and As metabolism PC2. In models using OCM PCs instead of the original nutrient variables, OCM PC1, reflecting higher intake of all OCM nutrients, was negatively associated with iAs% and MMA% and positively associated with DMA% and As metabolism PC1 (Table 4). OCM PC2, representing mostly high vitamin B12, possibly reflecting meat intake, was not associated with arsenic methylation profiles. OCM PC3, reflecting higher intake of vitamin B2 and folate with lower vitamin B6, was positively associated with higher iAs% and MMA% and negatively associated with DMA% and As metabolism PC1, although none of the associations were statistically significant. OCM PC4, reflecting high folate and vitamin B12 and low vitamin B6 and B2, was positively associated with iAs% and MMA%, and negatively with DMA% and As metabolism PC1, however, only the association with MMA% was statistically significant. In analyses evaluating the joint effect of OCM nutrients in pairs, we found an independent additive association between intake of vitamins B6 and B2 with arsenic metabolism biomarkers. Mean DMA% was 2.76 (95% C:I 0.88, 4.65)% higher for participants with both vitamins B6 and B2 above (high) versus below (low) the median, 2.02 (95% CI: −0.10, 4.14)% higher for participants with only high B6 and 1.08 (95%CI −1.09, 3.24)% higher for participants with only high B2 (Supplemental Material Table S3). The joint association was not different from additive (p-value for interaction 0.83). For vitamin B6 and folate, compared to participants with low intake of both vitamins, high folate intake with low B6 was associated with 1.39 (95% CI: 0.19, 2.58)% higher iAs% and 2.58 (95% CI: −4.77, −0.93)% lower DMA% while high folate intake with high vitamin B6 was associated with 1.03 (95% CI: −2.01, −0.05) lower iAs% and 1.65 (95% CI: −0.14, 3.44)% higher DMA%. The p-value for interaction, supporting an antagonistic interaction, between folate and B6 was 0.01 for iAs% and 0.001 for DMA% (no interaction was found for MMA%).
Table 4.
Mean Differencea (95% CI) in iAs%, MMA%, DMA% and Arsenic Principal Components by Nutrition Tertiles and OCM Principal Components
| As Metabolism PC1 | As Metabolism PC2 | iAs% | MMA% | DMA% | |
|---|---|---|---|---|---|
| Calorie-Corrected Nutrition Tertiles | |||||
| Vitamin B2 (mg) | |||||
| <1.073 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 1.073 – 1.43 | 0.14 (−0.18, 0.47) | −0.05 (−0.20, 0.11) | −0.21 (−1.20, 0.79) | −0.59 (−1.71, 0.53) | 0.80 (−1.01, 2.61) |
| >1.43 | 0.43 (0.10, 0.75) | −0.03 (−0.18, 0.13) | −1.00 (−2.00, 0.00) | −1.36 (−2.48, −0.23) | 2.36 (0.54, 4.18) |
| Vitamin B6 (mg) | |||||
| <0.936 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 0.936 – 1.4241 | 0.12 (−0.20, 0.45) | −0.11 (−0.27, 0.04) | 0.08 (−0.90, 1.06) | −0.78 (−1.89, 0.34) | 0.69 (−1.10, 2.49) |
| >1.4241 | 0.53 (0.20, 0.86) | 0.00 (−0.15, 0.16) | −1.36 (−2.35, −0.37) | −1.57 (−2.69, −0.45) | 2.93 (1.13, 4.74) |
| Folate (μg) | |||||
| <149 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 149 – 266 | 0.24 (−0.09, 0.57) | −0.06 (−0.22, 0.09) | −0.40 (−1.40, 0.60) | −0.93 (−2.06, 0.20) | 1.33 (−0.50, 3.16) |
| >266 | 0.18 (−0.15, 0.51) | 0.10 (−0.06, 0.25) | −0.80 (−1.79, 0.20) | −0.18 (−1.30, 0.93) | 0.98 (−0.83, 2.79) |
| Vitamin B12 (μg) | |||||
| <2.20 | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) | 0.00 (ref) |
| 2.20 – 3.93 | −0.02 (−0.35, 0.31) | −0.14 (−0.29, 0.02) | 0.52 (−0.47, 1.51) | −0.44 (−1.57, 0.68) | −0.08 (−1.90, 1.74) |
| >3.93 | 0.17 (−0.16, 0.50) | 0.04 (−0.12, 0.19) | −0.55 (−1.54, 0.44) | −0.37 (−1.49, 0.76) | 0.92 (−0.90, 2.74) |
| One-Carbon Metabolism Principal Components | |||||
| OCM PC1 | 0.13 (0.04, 0.21) | 0.00 (−0.04, 0.04) | −0.33 (−0.59, −0.07) | −0.38 − (0.67, −0.08) | 0.71 (0.23, 1.18) |
| OCM PC2 | 0.03 (−0.11, 0.17) | −0.02 (−0.09, 0.04) | 0.00 (−0.43, 0.43) | −0.17 (−0.65, 0.32) | 0.17 (−0.62, 0.95) |
| OCM PC3 | −0.14 (−0.35, 0.07) | 0.02 (−0.08, 0.12) | 0.29 (−0.34, 0.92) | 0.48 (−0.23, 1.19) | −0.77 (−1.91, 0.38) |
| OCM PC4 | −0.21 (−0.46, 0.05) | 0.07 (−0.04, 0.20) | 0.27 (−0.51, 1.05) | 0.89 (0.01, 1.77) | −1.16 (−2.58, 0.27) |
As, arsenic; PC, principal component; OCM, one-carbon metabolism
Results adjusted for total log arsenic, log urinary creatinine, age, sex, center, smoking, alcohol, eGFR, BMI
4. DISCUSSION
Dietary intake of OCM nutrients was associated with urinary arsenic methylation patterns in a population of rural American Indian adult men and women exposed to low-moderate levels of inorganic arsenic from drinking water and food. In general, higher intake of B-vitamins, in particular B2 and B6, was associated with lower percentages of iAs and MMA and higher percentages of DMA, a profile suggested to reflect enhanced arsenic metabolism. These associations persisted for vitamins B2 and B6 after adjustment for sociodemographic factors, smoking, alcohol intake, BMI, and kidney function, as well as all OCM nutrients. The joint association for vitamins B2 and B6 was independent and not different from additive. For vitamin B12, a vitamin with a relatively high intake in the study population, there was no association with arsenic metabolism biomarkers, a finding that is consistent with results from other populations with generally adequate vitamin B12 intake.(Gamble et al. 2005; Hall et al. 2009b) The association between folate intake and arsenic metabolism in the main analyses was not clear. In joint analyses, an antagonistic association was found between folate and vitamin B6, with higher folate being associated with higher DMA% and lower iAs% only in the presence of high vitamin B6. However, these results should be interpreted with caution given the high correlation between B6 and folate.
OCM is critical in the biosynthesis of purines and thymidylate as well as the generation of methyl groups.(Ralph Carmel 2001) OCM facilitates the generation of S-adenosylmethionine (SAM) which serves as a methyl donor for numerous substrates. These substrates are essential to multiple biological processes including cellular signaling, DNA methylation, the synthesis of proteins, lipids, hormones and carbohydrates, and arsenic metabolism.(Ralph Carmel 2001) A product of SAM-dependent methylation reactions, s-adenosylhomocysteine, is hydrolized to homosysteine (Hcys), which can be remethylated to form methionine and activated to regenerate SAM.(Ralph Carmel 2001) OCM functioning, and adequate methyl group availability, is dependent on essential nutrients including folate, vitamin B12, vitamin B6, and vitamin B2 (Figure 1).
Numerous studies conducted in Bangladesh have characterized the relationship between OCM nutrients and arsenic metabolism in populations exposed to high levels of arsenic. Randomized controlled trials (RCTs) (Gamble et al. 2006; Peters et al. 2015) have focused on folic acid given its key role in the recruitment of methyl groups, which are required for methylation reactions in the OCM pathway.(Peters et al. 2015) Results from cross-sectional studies (Gamble et al. 2005; Hall et al. 2007; Li et al. 2008) have also shown associations between higher plasma folate and enhanced arsenic metabolism, consistent with increases in urinary DMA% and decreases in iAs% and MMA% seen in RCTs. Collectively, these studies provide strong evidence for the temporality of these associations. Cross-sectional dietary intake studies of folate and arsenic metabolism from Bangladesh(Heck et al. 2007), Mexico(Lopez-Carrillo et al. 2016) and the U.S.(Steinmaus et al. 2005), however, have been inconsistent.
Evaluation of OCM nutrients beyond folate, including vitamins B2, B6 and B12, all of which play important roles in OCM (Figure 1), has not been as extensive, lacking evidence from RCTs. Higher vitamin B12 (plasma(Hall et al. 2009a) and dietary intake (Heck et al. 2007; Lopez-Carrillo et al. 2016)) was associated with lower iAs% and higher MMA% in some studies but not in studies with a lower prevalence of B12-deficiency.(Gamble et al. 2005; Gamble et al. 2006; Hall et al. 2009b) These results have led to the hypothesis that increases in vitamin B12 may promote the first step of arsenic methylation, leading to higher MMA% and lower iAs%.(Hall et al. 2009a; Howe et al. 2014) The study reporting plasma B12 levels being associated with lower iAs% and higher MMA%, by design, over-selected participants having moderate- to severe-B12 deficiency. The predomination of the methylation of iAs to MMA over MMA to DMA may be specific to under-nourished populations exposed to high levels of arsenic, due to a competition for available methyl groups.(Howe et al. 2014) In our population, characterized by adequate or even high vitamin B12 intake, we found no association with arsenic metabolism. This may be because vitamin B12 truly does not affect arsenic metabolism in populations where vitamin B12 intake is high. However, it is also possible our lack of significant associations for vitamin B12 was due to our study being underpowered to detect an effect size of that magnitude.
Three dietary intake studies, one conducted in the U.S.(Steinmaus et al. 2005), one conducted in women from Mexico (Lopez-Carrillo et al. 2016) and one conducted in Bangladesh,(Heck et al. 2007) reported null results for vitamin B6. The Bangladeshi dietary intake study, however, found similar associations between vitamin B2 and arsenic metabolism as had been reported for vitamin B12; higher vitamin B2 intake was associated with lower iAs% but higher MMA%.(Heck et al. 2007) Associations for vitamin B2 were null in the other two dietary intake studies.(Steinmaus et al. 2005)
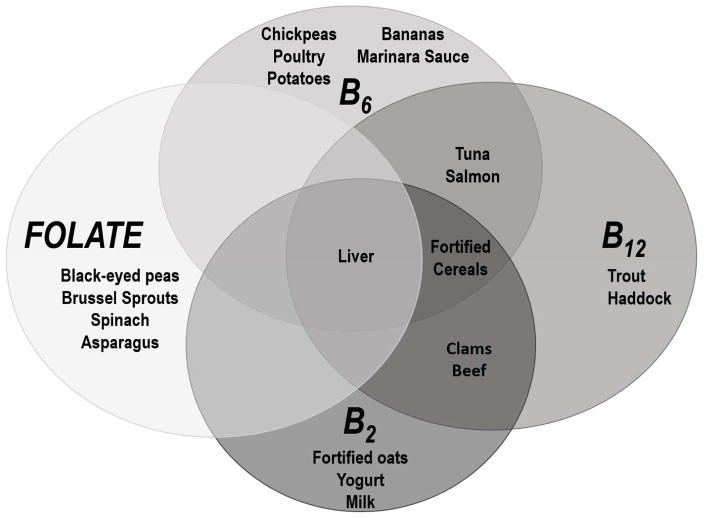
Our analysis on the association between OCM nutrients and arsenic metabolism fills in gaps in our understanding of this relationship in a cohort relevant to the general U.S. population. The SHS population is better nourished (38% of participants from the Bangladeshi dietary intake study were underweight compared to just one participant in our study) and exposed to lower levels of arsenic than the populations studied in Bangladesh. Further, dietary patterns differ substantially between the two populations: the SHS diet generally includes fewer fresh fruits and vegetables (significant sources of vitamin B6 and folate) (Figure 3) and more meat/protein (significant sources of B2 and B12 and intake and has been associated with arsenic metabolism (Heck et al. 2007; Steinmaus et al. 2005)) than typical Bangladeshi diets.(Berg et al. 2012; Fretts et al. 2012) This is reflected in our participants’ higher levels of vitamins B12 and B2 and lower levels of folate and vitamin B6 than in Bangladesh (Supplemental Material Table S4). Participants in the dietary intake study conducted among Mexican women had OCM nutrient intake patterns more similar to those in the Bangladeshi dietary intake study.(Heck et al. 2007; Lopez-Carrillo et al. 2016)
Figure 3. Major dietary sources of one-carbon metabolism (OCM) nutrients.
Foods listed under OCM nutrients are considered to be high dietary sources of that nutrient (provide 20% or more of the daily value).(NIH 2016a) Foods fortified with folic acid are not included in the figure because U.S. folic acid fortification occurred after the SHS baseline visit.
To our knowledge, only one other study has evaluated OCM nutrients and arsenic metabolism in a U.S. population.(Steinmaus et al. 2005) This study, conducted in individuals from Western Nevada and Kings County, California, exposed to arsenic levels in drinking water generally above 10 μg/L(Steinmaus et al. 2003), found no association between dietary intake of OCM nutrients and arsenic metabolism; however, the study was small (N=87), some OCM nutrients were not available (no evaluation of vitamin B12), and the majority of the population was male (75%) with a large percentage having a history of bladder cancer (26%).(Steinmaus et al. 2005)
In our study, the strongest associations between vitamin intake and arsenic metabolism were for the least extensively studied OCM nutrients, vitamins B2 and B6. Greater intake of vitamins B2 and B6 were associated with higher DMA% and lower iAs% and MMA%. Our PCA results support these findings with greater intake of vitamins B2 and B6 also being associated with higher As metabolism PC1, which reflects lower iAs% and MMA% and higher DMA%. Because metabolism of arsenic first involves the conversion of iAs to MMA and then the conversion of MMA to DMA, As metabolism PC1 may represent overall methylation to DMA, or the secondary arsenic methylation index.
The magnitude of increases in DMA% and decreases in MMA% for participants in the third versus first tertile of intake for B2 and B6 were significant but modest (ranging from 1.5 to 3 percentage point differences). Translating this into clinical relevance, increasing intake of vitamin B6 by 1.06 mg, for example, reflecting the increase in intake needed to move those at the median of the first tertile to the median of the third tertile of vitamin B6 intake, could result in MMA% 1–2 percentages lower. Although on the individual level, this may appear small, on the population level it could translate to significant effects on health outcomes. For example, a case-control study from Chile reported a 1.11 (1.06, 1.17) and 1.05 (1.00, 1.10) greater odds of skin cancer and bladder cancer, respectively, for each 1% increase in MMA%.(Melak et al. 2014) Further, in a Bangladesh-based trial, folate deficient participants received folic acid supplementation for 12 weeks. This resulted in a decline in MMA% in urine from 13% to 10% yet a 22% reduction in blood MMA levels and 14% reductions in participants’ total blood arsenic.(Gamble et al. 2006; Gamble et al. 2007)
Further, results from our pairwise interactions suggest there is an additive independent association for high intake of vitamins B2 and B6 with enhanced arsenic metabolism. Our findings add evidence that vitamins B2 and B6, less studied B-vitamins involved in OCM, may also affect arsenic metabolism in the same direction as has been reported for folate; and perhaps, play a stronger role in arsenic metabolism than folate in well-nourished protein-sufficient populations. This finding is also supported by the OCM PC analysis. OCM PC1, reflecting higher intake of all four vitamins, was associated with lower iAs% and MMA% and higher DMA% and As metabolism PC1. However, OCM PC4, reflecting low B2 and B6 intake and high folate and B12 intake, was significantly associated with higher MMA% and non-significantly associated with higher iAs% and lower DMA%. Thus, despite high folate and B12 intake, low intake of B2 and B6 (e.g., a diet with low intake of dairy, poultry and fruit but high intake of green vegetables and certain fish) resulted in lower arsenic metabolism efficiency. This result for PC4 is consistent with the joint analysis for folate and vitamin B6, where high folate was associated with lower arsenic metabolism efficiency in the presence of low vitamin B6 but with higher arsenic metabolism efficiency with high B6 intake. These findings suggest that high folate alone may not enhance arsenic metabolism in this population. Although we cannot disregard that these findings could be related to measurement error of folate nutritional status in the absence of plasma folate data, additional research is needed to evaluate if this pattern is consistent in other populations. Overall, these results suggest that, as it has been reported for plasma folate in undernourished and low-protein populations, increasing levels of OCM nutrients, specifically B2 and B6, can enhance arsenic methylation capacity in a well-nourished protein-sufficient population exposed to low-moderate arsenic.
In independent linear regressions analyses for folate, the stronger association with MMA% and DMA% for participants in the second tertile of folate rather than the third tertile, and the reversing of direction in tertile 3 after adjustment for other nutrients, is difficult to interpret. It could be related to measurement error from the difficulty of dietary folate measurement. We did not calculate dietary folate equivalents (DFEs), which are used to incorporate all sources of folate intake, in addition to accounting for the higher bioavailability of folic acid from supplements and fortified foods compared with naturally occurring folate in foods.(Bailey 1998; Park et al. 2013) However, sensitivity analyses excluding participants reporting vitamin supplementation use yielded consistent results, and mandatory fortification of foods with folic acid was not implemented in the United States until 1998 (almost ten years after data collection). It is also possible the patterns seen for folate and arsenic metabolism in our analysis are not consistent with other studies due to interactions with intake of other vitamins, unmeasured confounding and/or oxidation of food folates during cooking. Studies in well-nourished populations exposed to a broad range of folate through food fortification and supplementation use, with measurements of both folate intake and biomarkers, are necessary to assess the dose-response relationship of folate intake with arsenic metabolism.
Limitations of our study include relying on self-reported dietary data from the FFQ, a dietary assessment tool that is associated with underestimates of intake, particularly energy and protein.(Mossavar-Rahmani et al. 2015; Prentice et al. 2011) Further, self-reporting intake over a full year may introduce measurement error due to recall bias. Confirmation of findings through evaluation of nutrition biomarkers would have enhanced analyses and study interpretation. Still, correlations between dietary intake estimates and plasma biomarkers of vitamin B12 and folate reported in a recent feeding study (r=0.63 for Vitamin B12 and r=0.66 for DFE) support the strength of intake estimates to reflect internal vitamin levels.(Lampe et al. 2017) In addition, data were lacking on other nutrients involved in OCM, such as methionine and choline, which would have allowed a more comprehensive understanding of the full impact of OCM and arsenic metabolism. Further, B-vitamin intake was based solely on diet, lacking information on supplements. Although we were able to confirm that seafood intake in this population was low, we were unable to adjust for other food sources of inorganic arsenic that may confound the findings, such as rice intake. Finally, since there are gene variants, for example, the common single nucleotide polymorphism in the gene for MTHFR, the 667 C→ T mutation, that lead to lower blood folate levels, the extent to which B-vitamin intake reflects internal levels of those vitamins, at least in part, is genetically determined. Additional research to evaluate the role of OCM related genetic variants and its impact on these relationships in this population would be useful.
5. CONCLUSIONS
Our study provides evidence that even at low levels of arsenic exposure and in well-nourished populations, OCM nutrient intake may affect the efficiency of arsenic metabolism. Further, our findings suggest vitamins B6 and B2, two previously understudied OCM vitamins, may play a stronger role in arsenic metabolism than folate in well-nourished protein-sufficient populations.
Supplementary Material
HIGHLIGHTS.
Greater intake of one-carbon metabolism (OCM) vitamins B6 and B2, was associated with arsenic metabolism (ASM) efficiency
In principal component (PC) analyses, the PC reflecting greater OCM vitamin intake was also associated with enhanced ASM
High vitamin B6 and B2 intake had an additive effect on ASM efficiency
High folate with low B6 was associated with reduced ASM, but high intake of both vitamins was associated with enhanced ASM
Abbreviations
- As PC 1
Arsenic Metabolism Principal Component 1
- As PC 2
Arsenic Metabolism Principal Component 2
- BMI
Body Mass Index
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- DFE
Dietary Folate Equivalent
- DMA
Dimethylarsenate
- eGFR
Estimate Glomerular Filtration Rate
- FFQ
Food Frequency Questionnaire
- HPLC/ICPMS
High Performance Liquid Chromatography/Inductively Coupled Plasma-Mass Spectrometry
- iAs
Inorganic Arsenic
- MMA
Monomethylarsonate
- OCM
One-Carbon Metabolism
- OCM PC 1
One-Carbon Metabolism Principal Component 1
- OCM PC 2
One-Carbon Metabolism Principal Component 2
- OCM PC 3
One-Carbon Metabolism Principal Component 3
- OCM PC4
One-Carbon Metabolism Principal Component 4
- PCA
Principal Component Analysis
- RCT
Randomized Controlled Trial
- ΣAs
Total arsenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharyya N, Deb B, Chattopadhyay S, Maiti S. Arsenic-induced antioxidant depletion, oxidative DNA breakage, and tissue damages are prevented by the combined action of folate and vitamin b12. Biol Trace Elem Res. 2015;168:122–132. doi: 10.1007/s12011-015-0324-5. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Aposhian MM. Arsenic toxicology: Five questions. Chem Res Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- Bailey LB. Dietary reference intakes for folate: The debut of dietary folate equivalents. Nutr Rev. 1998;56:294–299. doi: 10.1111/j.1753-4887.1998.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Balakrishnan P, Vaidya D, Franceschini N, Voruganti VS, Gribble MO, Haack K, et al. Association of cardiometabolic genes with arsenic metabolism biomarkers in american indian communities: The strong heart family study (shfs) Environ Health Perspect. 2016 doi: 10.1289/EHP251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, Daley CM, Nazir N, Kinlacheeny JB, Ashley A, Ahluwalia JS, et al. Physical activity and fruit and vegetable intake among american indians. J Community Health. 2012;37:65–71. doi: 10.1007/s10900-011-9417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee SSC, Pal S. Additive beneficial effect of folic acid and vitamin b12 co-administration on arsenic induced oxidative damage in cardiac tissue in vivo. Asian J Pharm Clin Res. 2013;6:64–69. [Google Scholar]
- Challenger F. Biological methylation. Adv Enzymol Relat Subj Biochem. 1951;12:429–491. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- Chen JW, Chen HY, Li WF, Liou SH, Chen CJ, Wu JH, et al. The association between total urinary arsenic concentration and renal dysfunction in a community-based population from central taiwan. Chemosphere. 2011;84:17–24. doi: 10.1016/j.chemosphere.2011.02.091. [DOI] [PubMed] [Google Scholar]
- Chen JW, Wang SL, Wang YH, Sun CW, Huang YL, Chen CJ, et al. Arsenic methylation, gsto1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern taiwan. Chemosphere. 2012;88:432–438. doi: 10.1016/j.chemosphere.2012.02.059. [DOI] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, et al. Arsenic methylation and skin cancer risk in southwestern taiwan. J Occup Environ Med. 2003a;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Hsueh YM, Smith TJ, Ryan LM, et al. Arsenic methylation and bladder cancer risk in taiwan. Cancer Causes Control. 2003b;14:303–310. doi: 10.1023/a:1023905900171. [DOI] [PubMed] [Google Scholar]
- Chen YC, Su HJ, Guo YL, Houseman EA, Christiani DC. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control. 2005;16:75–81. doi: 10.1007/s10552-004-2235-1. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione s-transferase m1 and t1 among current arsenic-exposed residents in taiwan. Mutat Res. 1997;386:197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- Chung JS, Haque R, Guha Mazumder DN, Moore LE, Ghosh N, Samanta S, et al. Blood concentrations of methionine, selenium, beta-carotene, and other micronutrients in a case-control study of arsenic-induced skin lesions in west bengal, india. Environ Res. 2006;101:230–237. doi: 10.1016/j.envres.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Committee SHSS. The strong heart study: Cardiovascular disease in american indians, manual 1989 [Google Scholar]
- Council NR. Arsenic in drinking water, 2001 update. Washington DC: 2001. [Google Scholar]
- Council NR. Critical aspects of epa’s iris assessment of inorganic arsenic: Interim report. Washington, DC: National Academies Press; 2013. [Google Scholar]
- Cullen WRRK. Arsenic speciation in the environment. Chemical Reviews. 1989;89:713–764. [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Vargas H, Albores A, Gonsebatt ME, Montero R, et al. Altered profile of urinary arsenic metabolites in adults with chronic arsenicism. A pilot study. Arch Toxicol. 1997;71:211–217. doi: 10.1007/s002040050378. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Valenzuela OL, Castellanos EH, Sanchez-Pena LC, Currier JM, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the zimapan and lagunera regions in mexico. Environ Health. 2011;10:73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SA, Mete M, et al. Associations of processed meat and unprocessed red meat intake with incident diabetes: The strong heart family study. Am J Clin Nutr. 2012;95:752–758. doi: 10.3945/ajcn.111.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in bangladesh. Environ Health Perspect. 2005;113:1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Ahsan H, Pilsner JR, Ilievski V, Slavkovich V, et al. Folate and arsenic metabolism: A double-blind, placebo-controlled folic acid-supplementation trial in bangladesh. Am J Clin Nutr. 2006;84:1093–1101. doi: 10.1093/ajcn/84.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble MV, Liu X, Slavkovich V, Pilsner JR, Ilievski V, Factor-Litvak P, et al. Folic acid supplementation lowers blood arsenic. Am J Clin Nutr. 2007;86:1202–1209. doi: 10.1093/ajcn/86.4.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Body composition and arsenic metabolism: A cross-sectional analysis in the strong heart study. Environ Health. 2013;12:107. doi: 10.1186/1476-069X-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, et al. Determinants of arsenic metabolism: Blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–1509. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Liu X, Slavkovich V, Ilievski V, Mi Z, Alam S, et al. Influence of cobalamin on arsenic metabolism in bangladesh. Environ Health Perspect. 2009a;117:1724–1729. doi: 10.1289/ehp.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Liu X, Slavkovich V, Ilievski V, Pilsner JR, Alam S, et al. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in bangladesh. Environ Health Perspect. 2009b;117:825–831. doi: 10.1289/ehp.0800164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Gamble MV. Nutritional manipulation of one-carbon metabolism: Effects on arsenic methylation and toxicity. J Toxicol. 2012;2012:595307. doi: 10.1155/2012/595307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: Arsenic-glutathione complexes are substrates for human arsenic methyltransferase cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, et al. Consumption of folate-related nutrients and metabolism of arsenic in bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG, Niedzwiecki MM, Hall MN, Liu X, Ilievski V, Slavkovich V, et al. Folate and cobalamin modify associations between s-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed bangladeshi adults. J Nutr. 2014;144:690–697. doi: 10.3945/jn.113.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YM, Chiou HY, Huang YL, Wu WL, Huang CC, Yang MH, et al. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:589–596. [PubMed] [Google Scholar]
- Jansen RJ, Argos M, Tong L, Li J, Rakibuz-Zaman M, Islam MT, et al. Determinants and consequences of arsenic metabolism efficiency among 4,794 individuals: Demographics, lifestyle, genetics, and toxicity. Cancer Epidemiol Biomarkers Prev. 2016;25:381–390. doi: 10.1158/1055-9965.EPI-15-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13:831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care. 2015;38:620–627. doi: 10.2337/dc14-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe JW, Huang Y, Neuhouser ML, Tinker LF, Song X, Schoeller DA, et al. Dietary biomarker evaluation in a controlled feeding study in women from the women’s health initiative cohort. Am J Clin Nutr. 2017;105:466–475. doi: 10.3945/ajcn.116.144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The strong heart study. A study of cardiovascular disease in american indians: Design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- Li L, Ekstrom EC, Goessler W, Lonnerdal B, Nermell B, Yunus M, et al. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant bangladeshi women. Environ Health Perspect. 2008;116:315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Carrillo L, Gamboa-Loira B, Becerra W, Hernandez-Alcaraz C, Hernandez-Ramirez RU, Gandolfi AJ, et al. Dietary micronutrient intake and its relationship with arsenic metabolism in mexican women. Environ Res. 2016;151:445–450. doi: 10.1016/j.envres.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, Perez L, et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern chile. Toxicol Appl Pharmacol. 2014;274:225–231. doi: 10.1016/j.taap.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, Gonzalez-Horta C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Ceron RH, Morales DV, et al. Chronic exposure to arsenic and markers of cardiometabolic risk: A cross-sectional study in chihuahua, mexico. Environ Health Perspect. 2016;124:104–111. doi: 10.1289/ehp.1408742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: An updated systematic review. Curr Atheroscler Rep. 2012;14:542–555. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossavar-Rahmani Y, Shaw PA, Wong WW, Sotres-Alvarez D, Gellman MD, Van Horn L, et al. Applying recovery biomarkers to calibrate self-report measures of energy and protein in the hispanic community health study/study of latinos. Am J Epidemiol. 2015;181:996–1007. doi: 10.1093/aje/kwu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranmandura H, Suzuki N, Suzuki KT. Trivalent arsenicals are bound to proteins during reductive methylation. Chem Res Toxicol. 2006;19:1010–1018. doi: 10.1021/tx060053f. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, et al. Urine arsenic concentrations and species excretion patterns in american indian communities over a 10-year period: The strong heart study. Environ Health Perspect. 2009;117:1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. Folate dietary supplement fact sheet. U.S. Department of Health & Human Services; 2016a. [Google Scholar]
- NIH. Riboflavin fact sheet for health professionals. U.S. Department of Health & Human Services; 2016b. [Google Scholar]
- NIH. Vitamin b12 dietary supplement fact sheet. U.S. Department of Health & Human Services; 2016c. [Google Scholar]
- NIH. Vitamin b6 dietary supplement fact sheet. US Department of Health and Human Services; 2016d. [Google Scholar]
- Nizam S, Kato M, Yatsuya H, Khalequzzaman M, Ohnuma S, Naito H, et al. Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in bangladesh. Int J Environ Res Public Health. 2013;10:1006–1019. doi: 10.3390/ijerph10031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Vollset SE, Melse-Boonstra A, Chajes V, Ueland PM, Slimani N. Dietary intake and biological measurement of folate: A qualitative review of validation studies. Mol Nutr Food Res. 2013;57:562–581. doi: 10.1002/mnfr.201200105. [DOI] [PubMed] [Google Scholar]
- Peters BA, Hall MN, Liu X, Parvez F, Sanchez TR, van Geen A, et al. Folic acid and creatine as therapeutic approaches to lower blood arsenic: A randomized controlled trial. Environ Health Perspect. 2015;123:1294–1301. doi: 10.1289/ehp.1409396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D, et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect. 2009;117:254–260. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174:591–603. doi: 10.1093/aje/kwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph Carmel DWJ, editor. Homocysteine in health and disease. Cambridge University Press; 2001. [Google Scholar]
- Rhee JJ, Cho E, Willett WC. Energy adjustment of nutrient intakes is preferable to adjustment using body weight and physical activity in epidemiological analyses. Public Health Nutr. 2014;17:1054–1060. doi: 10.1017/S1368980013001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. Arsenic species and selected metals in human urine: Validation of hplc/icpms and icpms procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4:406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shara NM, Wang H, Mete M, Al-Balha YR, Azalddin N, Lee ET, et al. Estimated gfr and incident cardiovascular disease events in american indians: The strong heart study. Am J Kidney Dis. 2012;60:795–803. doi: 10.1053/j.ajkd.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Bates MN, Smith AH. Case-control study of bladder cancer and drinking water arsenic in the western united states. Am J Epidemiol. 2003;158:1193–1201. doi: 10.1093/aje/kwg281. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a u.S. Population. Environ Health Perspect. 2005;113:1153–1159. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, Rey OA, et al. Arsenic methylation and bladder cancer risk in case-control studies in argentina and the united states. J Occup Environ Med. 2006;48:478–488. doi: 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett. 2000;112–113:209–217. doi: 10.1016/s0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Wang SL, Chang FH, Liou SH, Wang HJ, Li WF, Hsieh DP. Inorganic arsenic exposure and its relation to metabolic syndrome in an industrial area of taiwan. Environ Int. 2007;33:805–811. doi: 10.1016/j.envint.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Wu F, Molinaro P, Chen Y. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Health Rep. 2014;1:148–162. doi: 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Hsueh YM, Hong CT, Su CL, Chang SF, et al. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol Appl Pharmacol. 2006;216:168–175. doi: 10.1016/j.taap.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Yu RC, Hsu KH, Chen CJ, Froines JR. Arsenic methylation capacity and skin cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1259–1262. [PubMed] [Google Scholar]
- Zablotska LB, Chen Y, Graziano JH, Parvez F, van Geen A, Howe GR, et al. Protective effects of b vitamins and antioxidants on the risk of arsenic-related skin lesions in bangladesh. Environ Health Perspect. 2008;116:1056–1062. doi: 10.1289/ehp.10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.