Abstract
The cultivation of American elderberry (Sambucus nigra subsp. canadensis) continues to increase as the use of this botanical has expanded. It is well understood that elderberries contain a variety of polyphenols, including anthocyanins, which have purported health benefits. However, information is lacking regarding the impact of environmental, management, and genotypic factors on the quantity and type of polyphenols and anthocyanins produced. Quantification of sixteen polyphenols including eight anthocyanins present in juice from three genotypes of American elderberry grown at two Missouri sites from 2013–2014 was performed. Large variances in anthocyanin and other polyphenol content were observed between the different harvest seasons, locations and genotypes. Although specific phytochemical trends due to those factors were not apparent, a discriminant analysis was able to correctly identify 45 of 48 juice samples by genotype, based on their polyphenol profiles. This type of characterization could be beneficial in elderberry authentication studies and to help develop and document high-quality dietary supplement products with specific phytochemical contents.
Keywords: elderberry, anthocyanin, polyphenol, mass spectrometry, UHPLC, discriminant analysis, solid phase extraction, Sambucus
TOC Graphic
INTRODUCTION
American elderberry [Sambucus nigra L. subsp. canadensis (L.) Bolli] has emerged as a popular crop for producing dietary supplements, natural food colorants, wines, and other commercial products from both its fruit and flowers.1 Although European elderberry (Sambucus nigra L. subsp. nigra) has been studied extensively for its bioactive components and potential human health benefits, less is known about the American elderberry; especially its bioactive components.2–4 Elderberries, including the American subspecies, are a rich source of polyphenols, which are responsible for some of the purported health benefits associated with their antioxidant activity.5 Antioxidants have the ability to scavenge free radicals, reducing the amount of oxidative stress that can cause cardiovascular diseases, cancer, and neurological disorders over a long period of time.6
Multivariate techniques, such as discriminant analysis, are providing new information, such as chemical profiles, location identification, and sample authentication. Multivariate statistical methods, for example, have been used previously to develop a chemical profile of ginseng and to classify commercial ginseng products.7 Ultra-high performance liquid chromatography – quadrupole time of flight (UHPLC-QTOF) mass spectrometry analysis revealed ten compounds that were unique among three ginseng species. Yuk et al. also used this method to determine which species of ginseng were found in various commercial herbal supplements. In a different study, Italian saffron (Crocus sativus L.) samples from five growing locations were analyzed for various flavonoids and several other bioactive compounds using high-performance liquid-chromatography (HPLC).8 Classification to the actual saffron growing location using a discriminant analysis was performed with 88% accuracy.
Several studies have investigated the phytochemical profile of elderberry fruit and its juice,5,9,10 elderberry extracts,11 and elderberry based dietary supplements.12 Previous studies on American elderberry have found significant differences in polyphenol content within vegetative tissues13 and within fruit and fruit juice10,14,15 based on environmental (e.g., location, climate, soil type, precipitation) and crop management factors. This confirms that, in addition to genetics, environmental variations can potentially influence the phytochemical profile of American elderberry. This work investigates the potential effect genotype, growing location and year-to-year factors have on the polyphenol content of American elderberry juice. A complete analysis of all polyphenols is shown for the 2013 and 2014 growing seasons. Partial polyphenol data for 2012 and 2015 are also presented. Discriminant analysis is used to help show that different cultivars of elderberry can be determined based on their polyphenol content. Additional data is presented that shows wide variation in polyphenol content is observed with growing year and location.
MATERIALS AND METHODS
Plant Material
American elderberry fruit for this study was grown at the University of Missouri’s Southwest Research Center near Mt. Vernon (MV) and Missouri State University’s State Fruit Experiment Station at Mountain Grove (MG), both in southern Missouri. Three commercially available elderberry cultivars (Adams II, Bob Gordon, and Wyldewood) were used. Plants were propagated from our own mother plants in late winter, 2011, and transplanted to the sites in June, 2011. Experimental plots contained four plants of the same cultivar, planted 1.2 m apart. At each site, 48 plots were established in four rows, with plots separated 2.4 m within and 3.1 m between rows. The three cultivars were each assigned to 16 of the 48 plots in a completely randomized manner. During the growing seasons, plants were irrigated via drip lines to provide 2.5 to 4.0 cm water per week when rainfall was lacking. Weeds and pests were managed using standard horticultural practices.16 During the establishment year (2011), inflorescences were removed to encourage development of healthy roots and structure.
Ripe fruit for this study was collected during four consecutive growing seasons (2012– 2015). The fruit was collected in late August when the fruit was in full ripeness. The elderberry fruit was transported to lab under refrigeration, where it was stored at −20°C. The fruit was later thawed and de-stemmed by hand. The juice was prepared using fruit that showed uniform ripeness (visually) and approximately the same sized berries. The fruit was hand-pressed using a French press into individual aliquots of juice. All other debris, pulp, and seeds were discarded. The juice was then filtered through a nylon filter (0.22 μm), and 1.5 mL of each replicate was individually aliquotted into 1.5 mL microcentrifuge tubes, and then re-frozen and stored at −20°C until analysis as previously described17.
Chemicals
Water, acetonitrile, and formic acid were all HPLC grade and purchased from Fisher Scientific (Fair Lawn, NJ, USA). Trifluoroacetic acid (TFA; 99%) was obtained from Acros Organics (Morris Plains, NJ, USA). Cyanidin-3-O-glucoside analytical standard (≥ 95%), chlorogenic acid (reference standard), rutin (HPLC grade), neo-chlorogenic acid (analytical standard grade), crypto-chlorogenic acid (analytical standard grade), quercetin 3-rutinoside (analytical standard grade), quercetin 3-glucoside (analytical standard grade), kaempferol 3-rutinoside (analytical standard grade), isorhamnetin 3-rutinoside (analytical standard grade), and isorhamnetin 3-glucoside (analytical standard grade) were purchased from Sigma Aldrich (St. Louis, MO, USA).
Solid Phase Extraction (SPE)
Juice samples were thawed in an oven at 60°C for 5 min. This treatment yielded the same results as thawing the juice at room temperature for 30 min. A modification of the SPE protocol previously used by He and Giusti was used for polyphenol separation.18 200 μL of juice was diluted with 200 μL of water (0.01% TFA) and vortexed. An Oasis C18 SPE cartridge (Waters, Milford, MA, USA) was first washed with 5 mL of water (0.01% TFA). Then the diluted juice sample and an additional 5 mL of water (0.01% TFA) were added to the cartridge. The sample fraction was then collected after 10 mL of methanol (0.01% TFA) was run through the cartridge. The final methanolic fraction was dried under a constant flow of nitrogen gas. Samples were reconstituted in water (2% acetic acid) for HPLC analysis. Anthocyanins were separated from the juice using a previously developed method19. Briefly, mixed mode cation-exchange SPE was used (Oasis MCX 3cc, 60mg, Waters, Milford, MA, USA) and extractions performed using a Supelco Visiprep vacuum manifold.
HPLC-UV Analysis of Polyphenols
The HPLC method was adapted from that that of Kim and Lee20 and is briefly summarized. The HPLC system consisted of a Hitachi L-7100 pump, a Hitachi L-7200 autosampler (20 μL injection), and a Hitachi L-7400 UV detector (detection wavelength 320 nm) with a Synergi™ 4 μm Hydro-RP 80Å (2.0 × 150 mm) column (Phenomenex, Torrance, CA, USA) fitted with a SecurityGuard C18 (ODS) 4.0 × 3.0 mm guard column (Phenomenex). The mobile phase used was: (A) water (2% acetic acid) and (B) 50:50 water/acetonitrile (0.5% acetic acid). The gradient was 2% B initially until 2 min, then linearly ramped to 40% B from 2–52 min, 100% B from 53–58min, and 2% B from 59–63 min. The mobile phase flow rate was 0.6 mL/min and system was run at room temperature. Data were recorded and processed by a Hitachi D-7000 data acquisition package with ConcertChrom software on a microcomputer. Standards of chlorogenic acid and rutin were prepared at 0, 10, 25, 50, 100 and 200 μg/mLand quantification was done by comparing the area under the chromatographic peaks of elderberry samples to the calibration curves. Polyphenols monitored were neo-chlorogenic acid, chlorogenic acid, crypto-chlorogenic acid, quercetin 3-rutinoside, quercetin 3-glucoside, kaempferol 3-rutinoside, isorhamnetin 3-rutinoside, and isorhamnetin 3-glucoside. Comparison of a polyphenol to the chlorogenic acid and rutin standard curves allowed for the determination of an average response factor for each compound. The compound are reports as rutin/chlorogenic acid equivalents.
UHPLC-MS/MS Analysis of Anthocyanins
A previously described UHPLC-electrospray ionization mass spectrometry/mass spectrometry (UHPLC-ESI-MS/MS) method was used for anthocyanin analysis.19 A full analytical method validation was performed including the analysis of limit of quantitation, recovery, matrix- effect, linearity, and intra/inter-day reproducibility. Cyanidin-3-O-glucoside standards were prepared at 1, 10, 50, 100, 250, 500, and 1,000 ng/mL. Quantification was performed using the area under the mass spectral peak for individual multiple reaction monitoring ion channels for each anthocyanin and comparing it to a standard curve. Individual anthocyanins monitored were cyanidin 3-O-coumaroyl-sambubioside-5-glucoside, cyanidin based anthocyanin, cyanidin 3-O-sambubioside-5-glucoside, cyanidin 3-O-coumaroyl-sambubioside, cyanidin 3-O-sophoroside, cyanidin 3-O-rutinoside, cyanidin 3-O-sambubioside, and cyanidin 3-O-glucoside. Multiple reaction monitoring ion chromatograms of each anthocyanin are shown in Figure 1. The individual anthocyanin concentrations are reported as cyanidin 3-O-glucoside equivalents. The anthocyanins are identified by their MS/MS spectra and are consistent with previously published results3,14,21.
Figure 1.
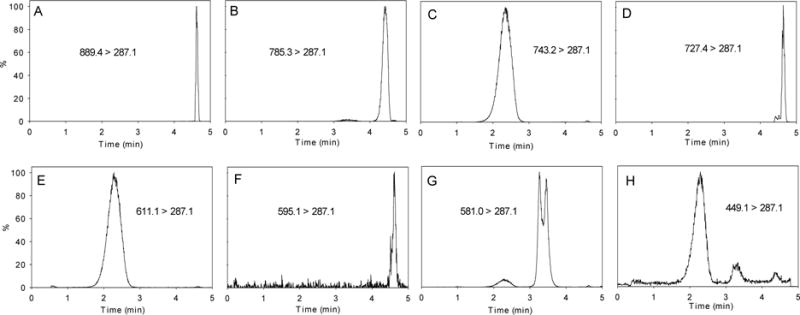
UHPLC-MS/MS multiple reaction monitoring (MRM) ion chromatograms of the eight anthocyanins analyzed: (A) cyanidin-3-O-coumaroyl-sambubioside-5-glucoside, (B) cyanidin based anthocyanin, (C) cyanidin-3-O-sambubioside-5-glucoside, (D) cyanidin-3-O-coumaroyl-sambubioside, (E) cyanidin-3-O-sophoroside, (F) cyanidin-3-O-rutinoside, (G) cyanidin-3-O-sambubioside and (H) cyanidin-3-O-glucoside. The numbers shown represent the MRM transitions monitored for each anthocyanin and are shown again at the top of Table 2.
Discriminant Analysis
Anthocyanin and polyphenol data were normalized to the same concentration units and represented as individual aliquots. Discriminant analysis (DA) was performed using the XLSTAT data analysis software in Microsoft Excel, version 14.5.3 (Microsoft, Santa Rosa, CA, USA) at a tolerance of 0.0001, p-value <0.05 based on the concentration of the eight anthocyanins and eight polyphenols of individual juice aliquots during the course of the study. Box’s and Wilks’ Lambda tests were used to assess if the covariance matrices and means vectors between the genotypes were significantly different, respectively. A confusion matrix using cross-validation, which is routinely constructed as part of DA, was used to test the ability of the method to properly group the samples based on genotype. These results are summarized in the Supplementary Material as Tables S1 and S2.
RESULTS AND DISCUSSION
Major Polyphenols and Anthocyanins Identified
The three most concentrated polyphenols identified in the three genotypes of American elderberry juice were quercetin 3-rutinoside, isorhamnetin 3-rutinoside, and chlorogenic acid (Table 1). Triplicate analyses were performed on each juice sample. Also indicated in the first of column of the table is the number of juice samples for each location and genotype. Quercetin 3-rutinoside had the highest average content among polyphenols for each genotype, year and growing location, except for Bob Gordon at Mountain Grove (MG) in 2012. The most concentrated anthocyanins identified were cyanidin 3-O-coumaroyl-sambubioside-5-glucoside, cyanidin 3-O-sambubioside-5-glucoside, and cyanidin 3-O-sophoroside (Table 2). These findings agree well with a previous study investigating the individual polyphenol content of fruit from ten American elderberry genotypes.5 3-O-coumaroyl-sambubioside-5-glucoside was present in relatively higher concentrations in the Bob Gordon compared to the Wyldewood and Adams II at both locations over the course of the study. Bob Gordon was also a much richer source of cyanidin 3-O-sophoroside than the other two genotypes. Polyphenol concentration ranges in this study were similar to analytes tested by Lee and Finn.5
Table 1.
Average polyphenol content of three elderberry genotypes at two different Missouri growing locations from 2012, 2013 and 2014 (μg/mL± standard error).
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Neo- chlorogenic acid |
Chlorogenic acid |
Crytpto- chlorogenic acid |
Quercetin 3- rutinoside |
Isoquercetin | Kaempferol 3- rutinoside |
Isorhamnetin3- rutinoside |
Isorhamnetin 3-glucoside |
|
|
Adams II
| ||||||||
| 2012 MG (n=12) | 16 ± 3 | 26 ± 8 | 4 ± 1 | 289 ± 48 | 39 ± 3 | 14 ± 2 | 250 ± 31 | 15 ± 5 |
| 2013 MG (n=8) | 76 ± 11 | 181 ± 25 | 2.0 ± 0.3 | 792 ± 143 | 26 ± 4 | 49 ± 7 | 220 ± 41 | 11 ± 3 |
| 2014 MG (n=5) | 7 ± 2 | 28 ± 4 | 3.0±0.2 | 47 ± 13 | 13 ± 3 | 6 ± 1 | 35 ± 9 | 9 ±1 |
| 2012 MV (n=12) | 27 ± 5 | 47 ± 11 | 3.0 ± 0.8 | 244 ± 35 | 25 ± 2 | 13 ± 2 | 199 ± 21 | 8 ± 1 |
| 2013 MV (n=14) | 50 ± 6 | 138 ± 20 | 1.3 ± 0.2 | 212 ± 38 | 13 ± 2 | 17 ± 2 | 87 ± 19 | 7 ± 2 |
| 2014 MV (n=16) | 116 ± 11 | 309 ± 36 | 10 ± 1 | 650 ± 44 | 54 ± 4 | 49 ± 4 | 191 ± 12 | 14 ± 1 |
|
Bob Gordon | ||||||||
| 2012 MG (n=14) | 16 ± 3 | 21 ± 4 | 1.6 ± 0.4 | 129 ± 22 | 25 ± 3 | 15 ± 2 | 143 ± 22 | 7 ± 1 |
| 2013 MG (n=12) | 81 ± 10 | 129 ± 14 | 2.1 ± 0.4 | 699 ± 116 | 24 ± 3 | 75 ± 12 | 272 ± 49 | 10 ± 4 |
| 2014 MG (n=9) | 79 ± 18 | 98 ± 24 | 4 ± 1 | 214 ± 90 | 16 ± 4 | 21 ± 8 | 72 ± 22 | 4.6 ± 0.5 |
| 2012 MV (n=12) | 43 ± 5 | 56 ± 8 | 2.4 ± 0.5 | 256 ± 24 | 27 ± 3 | 24 ± 3 | 144 ± 18 | 5.1 ± 0.6 |
| 2013 MV (n=16) | 51 ± 5 | 61 ± 8 | 1.9 ± 0.2 | 272 ± 44 | 12 ± 1 | 30 ± 4 | 73 ± 17 | 8 ± 3 |
| 2014 MV (n=16) | 66 ± 5 | 70 ± 7 | 9 ± 1 | 501 ± 32 | 36 ± 2 | 45 ± 3 | 145 ± 12 | 13 ± 1 |
|
Wyldewood | ||||||||
| 2012 MG (n=16) | 8 ± 3 | 18 ± 5 | 3.3 ± 0.5 | 235 ± 30 | 37 ± 3 | 17 ± 2 | 190 ± 22 | 12 ± 3 |
| 2013 MG (n=14) | 71 ± 8 | 152 ± 17 | 3.9 ± 0.5 | 692 ± 142 | 20 ± 3 | 40 ± 7 | 176 ± 39 | 9 ± 2 |
| 2014 MG (n=10) | 74 ± 10 | 137 ± 24 | 6 ± 1 | 208 ± 108 | 18 ± 6 | 15 ± 5 | 107 ± 38 | 6 ± 1 |
| 2012 MV (n=15) | 32 ± 6 | 75 ± 17 | 1.6 ± 0.3 | 619 ± 80 | 27 ± 2 | 29 ± 3 | 127 ± 14 | 8 ± 2 |
| 2013 MV (n=7) | 59 ± 12 | 142 ± 30 | 3.0 ± 0.5 | 563 ± 83 | 15.7 ± 1 | 37 ± 4 | 100 ± 14 | 8 ± 3 |
| 2014 MV (n=16) | 71 ± 9 | 183 ± 22 | 11 ± 1 | 1,140 ± 107 | 53 ± 4 | 62 ± 5 | 300 ± 24 | 20 ± 2 |
MG: Mountain Grove, Missouri
MV: Mt. Vernon, Missouri
Table 2.
Average individual anthocyanin content of three elderberry genotypes at two different Missouri growing locations from 2013, 2014 and 2015 in μg/mL cyanidin-3-O-glucoside equivalents ± standard error (parent ion m/z→daughter ion m/z).
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| cyanidin 3-O-coumaroyl-sambubioside-5-glucoside (889.4 → 287.1) | cyanidin based anthocyanin (785.3 → 287.1) | cyanidin 3-O-sambubioside- 5-glucoside (743.2 → 287.1) | cyanidin 3-O-coumaroyl-sambubioside (727.4 → 287.1) | cyanidin 3-O-sophoroside (611.1 → 287.1) | cyanidin 3-O-rutinoside (595.1 → 287.1) | cyanidin 3-O-sambubioside (581.0 → 287.1) | cyanidin 3-O-glucoside (449.1 → 287.1) | |
|
Adams II
| ||||||||
| 2014 MG (n=5) | 8 ± 1 | 0.10 ± 0.04 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.06 ± 0.01 | 0.10 ± 0.04 | 0.29 ± 0.05 |
| 2015 MG (n=15) | 0.44 ± 0.07 | nd | 0.14 ± 0.03 | 0.29 ± 0.06 | 0.13 ± 0.03 | nd* | nd | nd |
| 2013 MV (n=11) | 28 ± 1 | 2.05 ± 0.05 | 18.5 ± 0.1 | 4.4 ± 0.4 | 15.9 ± 0.2 | 0.97 ± 0.02 | 3.9 ± 0.3 | 2.1 ± 0.1 |
| 2014 MV (n=16) | 21 ± 1 | 0.88 ± 0.06 | 5.4 ± 0.4 | 3.1 ± 0.2 | 5.8 ± 0.5 | 0.30 ± 0.02 | 1.9± 0.2 | 1.06 ± 0.08 |
| 2015 MV (n=4) | 3.5 ± 0.8 | 0.21 ± 0.08 | 1.3 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | nd | nd | nd |
|
Bob Gordon | ||||||||
| 2014 MG (n=9) | 30 ± 1 | 0.87 ± 0.08 | 8.4 ± 0.8 | 2.9 ± 0.3 | 14 ± 1 | 0.57 ± 0.09 | 3.0 ± 0.6 | 2.5 ± 0.2 |
| 2015 MG (n=13) | 5.8 ± 0.5 | 0.35 ± 0.04 | 3.6 ± 0.5 | 3.2 ± 0.5 | 5.7 ± 0.7 | 0.15 ± 0.02 | 0.28 ± 0.5 | nd |
| 2013 MV (n=14) | 56 ± 3 | 3.8 ± 0.3 | 41 ± 4 | 7.8 ± 0.4 | 69 ± 7 | 1.28 ± 0.04 | 8.1 ± 0.7 | 4.7 ± 0.3 |
| 2014 MV (n=16) | 19.2 ± 0.8 | 0.74 ± 0.04 | 5.1 ± 0.3 | 2.2 ± 0.2 | 8.9 ± 0.6 | 0.30 ± 0.02 | 1.4 ± 0.11 | 0.89 ± 0.04 |
| 2015 MV (n=15) | 10.5 ± 0.6 | 0.7 ± 0.3 | 6.2 ± 0.3 | 4.8 ± 0.4 | 14.7 ± 0.7 | 0.29 ± 0.05 | 0.9 ± 0.3 | nd |
|
Wyldewood | ||||||||
| 2014 MG (n=10) | 21 ± 1 | 0.69 ± 0.06 | 7.3 ± 0.5 | 2.1 ± 0.2 | 8.5 ± 0.8 | 0.37 ± 0.03 | 2.3 ± 0.3 | 2.2 ± 0.3 |
| 2015 MG (n=9) | 3.7 ± 0.5 | 0.19 ± 0.03 | 1.6 ± 0.3 | 2.3 ± 0.4 | 1.8 ± 0.4 | nd | nd | nd |
| 2013 MV (n=11) | 32 ± 3 | 2.2 ± 0.2 | 22 ± 2 | 4.0 ± 0.3 | 16 ± 1 | 1.01 ± 0.04 | 2.6 ± 0.2 | 1.54 ± 0.06 |
| 2014 MV (n=16) | 17.0 ±0.7 | 0.67 ±0.04 | 5.8 ± 0.4 | 1.9 ± 0.1 | 5.4 ± 0.5 | 0.27 ±0.2 | 1.5 ±0.1 | 0.99 ±0.08 |
| 2015 MV (n=0) | n/a** | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
nd: not detected, concentration was below the detection limit
n/a: samples were unavailable for analysis
MG: Mountain Grove, Missouri
MV: Mt. Vernon, Missouri
Influence of Growing Year
Changes in polyphenol content among the three cultivars during the four different growing years were very apparent. At Mt. Vernon (MV), anthocyanin content was 2–8 times higher in 2013 than 2014 for all genotypes. For example, Bob Gordon had almost triple the content of cyanidin 3-O-coumaroyl-sambubioside-5-glucoside and roughly eight times the cyanidin 3-O-sambubioside-5-glucoside and cyanidin 3-O-sophoroside content in 2013 compared to 2014. The same trend was observed at Mountain Grove between growing years, where anthocyanin content was significantly lower in 2015 than in 2014. The cyanidin 3-O-coumaroyl-sambubioside-5-glucoside, cyanidin 3-O-sambubioside-5-glucoside and cyanidin 3-O-sophoroside content were all approximately five fold lower in the Wyldewood genotype in 2015 than in 2014 at Mountain Grove. As shown in Table 1, the quercetin 3-rutinoside content of Adams II elderberry juice ranged from 47–792 μg/mL and the isorhamnetin 3-rutinoside concentration ranged from 35–250 μg/mLas a function of growing year. These changes in component concentrations suggests that different environmental and management variables can have a significant impact on the anthocyanin and polyphenol content of American elderberry juice.
Effects of Genotype and Growing Location
The average concentration of several polyphenols varied between genotypes at the same location, during the same season. For instance, Bob Gordon had about double the concentration of cyanidin 3-O-coumaroyl-sambubioside-5-glucoside (56 μg/mL) compared with Adams II (28 μg/mL) at Mt. Vernon in 2013 (Table 2). In 2014 at Mt. Vernon, Wyldewood had an average quercetin 3-rutinoside concentration of 1140 μg/mL which was roughly double that of Adams II (650 μg/mL) and Bob Gordon (501 μg/mL) that year (Table 1). In a previous study, it was shown that the total phenolic and total monomeric anthocyanin contents were dramatically affected by the genotype, growing location, and year.15
It appears that all of the genotypes have similarities in their polyphenol profiles, with the same analytes generally being the most abundant among the genotypes. However, we observed substantial differences in the concentrations of polyphenols among year, genotype and growing location. Other elderberry field studies have reached similar conclusions, but the precise roles of specific climate, soil, and crop management factors in producing consistent levels and profiles of specific polyphenols and anthocyanins have not been elucidated.9,10,15 A better understanding of these factors is important as the popularity of elderberry as a dietary supplement continues to grow. Elderberry dietary supplements generally include a quantity of elderberry extract, but do not factor in the concentrations of specific phytochemicals present. This may result in different amounts of specific polyphenols in dietary supplement products, even those made by the same processor and having the same or similar labels. Furthermore, in order to investigate the efficacy and function of specific phytochemicals in clinical trials, the content of specific anthocyanins being administered should be analyzed.
Discriminant Analysis
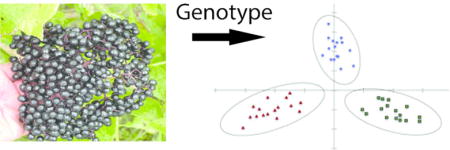
A common statistical analysis approach would be to perform ANOVA between the averages of the different groups and identify significant differences. Although this can provide useful information, with such a large data set it would be difficult to propose conclusions from this analysis, other than to point out where significant differences appear. The goal of discriminant analysis is to be able to perform groupings based on a set of traits, in this case the polyphenol contents of individual elderberry juice samples. It appears that nearly random differences in polyphenol content are observed for the different genotypes between the locations and years based on their average values, but a discriminant analysis can elucidate patterns that may otherwise be missed performing univariate statistics. It would be advantageous to be able to distinguish elderberry genotypes based on their phytochemical profile, and concurrently, to be able to predict the expected phytochemical profile of a product based on genotype regardless of where or how the fruit was grown. Thus, a discriminant analysis was performed for all genotypes in 2014 at the Mt. Vernon location (Figure 2). The reason this data set was chosen is two-fold. First, it isolates growing location and year, which have been shown to significantly impact the phytochemical content. Second, it appears to have the smallest difference in average values between the same anthocyanins among genotypes. Significant differences (p<0.01) were identified for cyanidin 3-O-coumaroyl-sambubioside-5-glucoside, cyanidin 3-O-sophoroside, quercetin 3-rutinoside, isorhamnetin 3-rutinoside, and chlorogenic acid. The discriminant analysis uses all the data for the individual polyphenol content, the individual anthocyanin content, and replicate measurements. Growing year and location were the only constraints. The discriminant analysis was able to successfully group the individual juice samples by genotype (Figure 2). A cross-validation was performed to further test the ability of this statistical method to discriminate the juice samples; this method was able to correctly identify 45 out of 48 samples (94%) based on their individual anthocyanin and polyphenol profile (Table 3). All 16 of the juice samples from Wyldewood genotype were correctly identified. Fourteen of the juice samples from the Adams II genotype were correctly identified, while two samples were incorrectly identified as Wyldewood. Fifteen Bob Gordon samples were properly identified and only one was incorrectly identified as Adams II. This illustrates that although the average concentrations of anthocyanins and polyphenols can be similar, it appears that each of these genotypes has a specific and statistically distinguishable profile.
Figure 2.
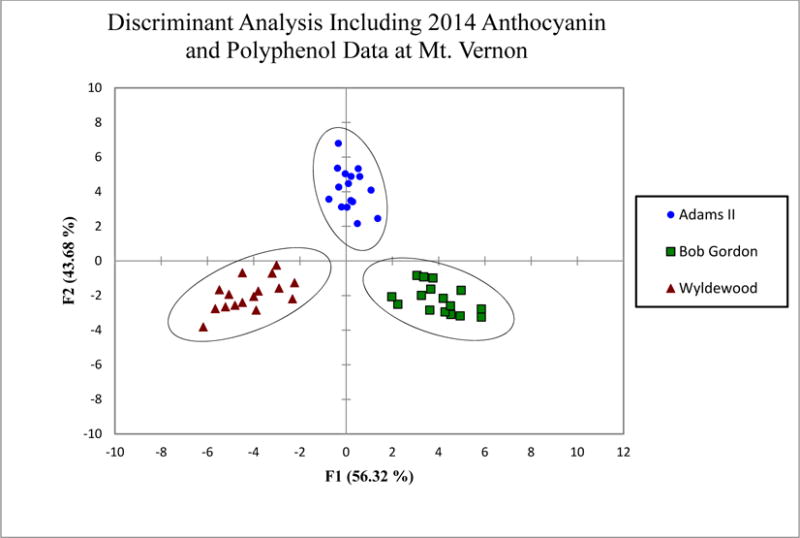
Discriminant analysis of 48 American elderberry juice samples (n=16 from each genotype) from Mt. Vernon in 2014 based on the anthocyanin and polyphenol content of each individual juice aliquot.
Table 3.
Cross validation results from confusion matrix from the 2014 discriminant analysis at Mt. Vernon.
| From\To | Adams II | Bob Gordon | Wyldewood | Total | % correct |
|---|---|---|---|---|---|
| Adams II | 14 | 0 | 2 | 16 | 87.50% |
| Bob Gordon | 1 | 15 | 0 | 16 | 93.75% |
| Wyldewood | 0 | 0 | 16 | 16 | 100.00% |
|
| |||||
| Total | 15 | 15 | 18 | 48 | 93.75% |
Discriminant analyses were also performed on the juice data from Mt. Vernon in 2013 (Figure 3A) and Mountain Grove in 2014 (Figure 3B). Although each of these groups had a smaller sample population, the discriminant analysis was successfully able to group the juice samples by genotype based on the anthocyanin and polyphenol content of these samples. However, the confidence ellipses surrounding the groups were larger and the confusion matrix cross-validation results were slightly lower at approximately 86% for both data sets (Data not shown). The 2014 Mt. Vernon data set is the most comprehensive and thus is the best representation of the discriminant analysis. Although the other discriminant analyses are not as conclusive as the 2014 Mt. Vernon, they illustrate reproducibility of the method over the course of growing seasons and locations.
Figure 3.
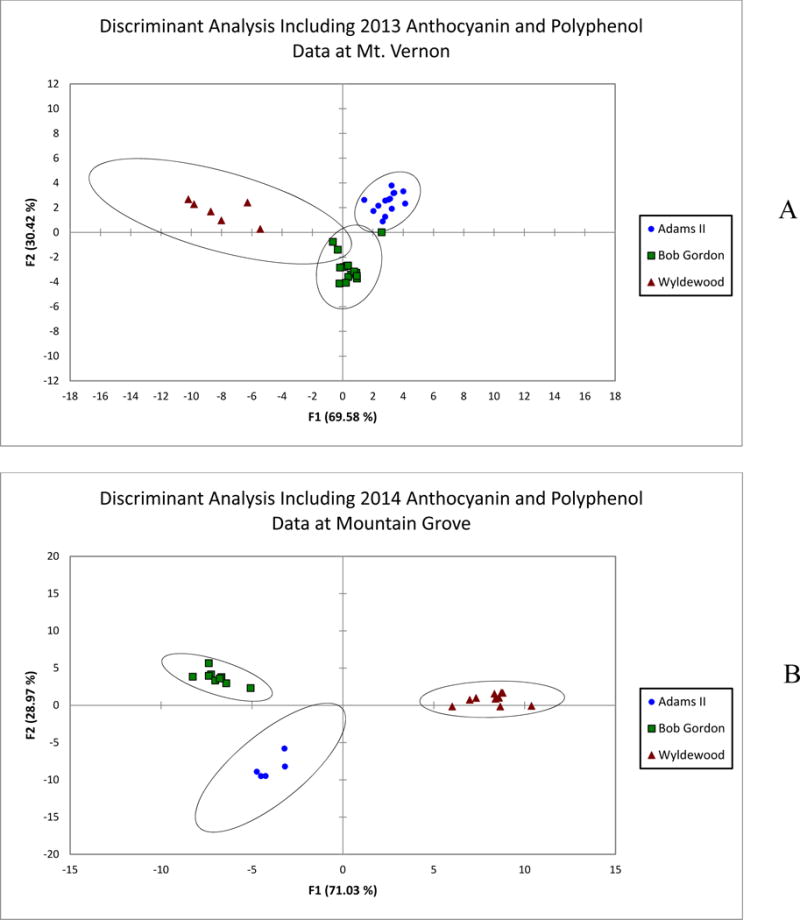
Discriminant analysis of American elderberry juice samples from Mt. Vernon in 2013 (A) and Mountain Grove in 2014 (B) based on the anthocyanin and polyphenol content of each individual juice aliquot.
Large deviations were present when measuring polyphenols from different field replications of juice samples from the same genotype, location and year. This suggests that even more minor factors among plots, such as fruit ripeness, pest and weed control, and soil variability can influence the overall magnitude of the anthocyanins and polyphenols present. However, when analyzing individual juice aliquots based on their polyphenol concentration, the discriminant analysis can distinguish the genotype of a juice. It is important to gain insight into American elderberry’s phytochemical profile as it continues to become more popular in the dietary supplement and commercial industry. This type of analysis could be crucial to standardizing elderberry-based dietary supplements, or when determining if such supplements have been adulterated with other fruits or fillers. It could also help improve consistency between lots and batches of dietary supplements with a standardized amount of specific anthocyanins. The three genotypes studied herein are commercially available American elderberry cultivars. Adams II, Bob Gordon, and Wyldewood were derived from indigenous germplasm originating in New York, Missouri, and Oklahoma, respectively.14 The disparate geographical origins of these cultivars may have generated subtle but unique genetic factors that influence metabolite composition, unique enough to be distinguished by discriminate analysis.
Supplementary Material
Acknowledgments
This publication was made possible by Grant Number P50AT006273 from the National Center for Complementary and Alternative Medicines (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health. This work was also partially funded through the Center for Agroforestry, University of Missouri under cooperative agreements with the USDA-ARS Dale Bumpers Small Farm Research Center, Booneville, AR. The tremendous assistance of colleagues at Missouri State University, including John D. Avery, Jr. and Martin Kaps, is gratefully acknowledged.
Abbreviations
- C18
octadecyl carbon chain
- DA
discriminant analysis
- ESI
electrospray ionization
- HPLC
high-performance liquid chromatography
- MCX
mixed-mode cation-exchange
- QTOF
quadrupole time-of-flight mass spectrometr
- SPE
solid phase extraction
- TFA
trifluoroacetic acid
- UHPLC-MS/MS
ultra-high performance liquid chromatography/tandem mass spectrometry
Footnotes
However, no copyright claim is made to original U.S. Government works, or works produced by employees of any Commonwealth realm Crown government in the course of their duties.
References
- 1.Charlebois D, Byers PL, Finn CE, Thomas AL. Elderberry: Botany, horticulture, potential. Hort Rev. 2010;37:213–280. [Google Scholar]
- 2.Barak V, Halperin T, Kalickman I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur Cytokine Netw. 2001;12:290–296. [PubMed] [Google Scholar]
- 3.Veberic R, Jakopic J, Stampar F, Schmitzer V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins, and selected polyphenols. Food Chem. 2009;114:511–515. [Google Scholar]
- 4.Zakay-Rones Z, Thom E, Wollan T, Wadstein J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J Int Med Res. 2004;32:132–140. doi: 10.1177/147323000403200205. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Finn CE. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. Nigra) cultivars. J Sci Food Agric. 2007;87:2675. doi: 10.1002/jsfa.3029. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuk J, Patel DN, Isaac G, Smith K, Wrona M, Olivos HJ, Yu K. Chemical profiling of ginseng species and ginseng herbal products using UPLC/QTOF-MS. J Braz Chem Soc. 2016;27:1483. [Google Scholar]
- 8.D’Archivio AA, Giannitto A, Maggi MA, Ruggieri F. Geographical classification of Italian saffron (Crocus sativus L.) based on chemical constituents determined by high-performance liquid-chromatography and by using linear-discriminant analysis. Food Chem. 2016;212:110–116. doi: 10.1016/j.foodchem.2016.05.149. [DOI] [PubMed] [Google Scholar]
- 9.Mudge E, Applequist WL, Finley J, Lister P, Townesmith AK, Walker KM, Brown PN. Validation of select flavonols and chlorogenic acid content of elderberry collected throughout the eastern United States. J Food Compost Anal. 2016;47:52–59. doi: 10.1016/j.jfca.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas AL, Byers PL, Gu S, Avery JD, Jr, Kaps M, Datta A, Fernando L, Grossi P, Rottinghaus GE. Occurrence of polyphenols, organic acids, and sugars among diverse elderberry genotypes grown in three Missouri (USA) locations. Acta Hort. 2015;1061:47–154. doi: 10.17660/ActaHortic.2015.1061.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duymuş HG, Göger F, Hüsner Can Başer K. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014;155:112–119. doi: 10.1016/j.foodchem.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Vlachojannis C, Zimmermann BF, Chrubasik-Hausmann S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother Res. 2015;29:561–565. doi: 10.1002/ptr.5284. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AL, Byers PL, Finn CE, Chen YC, Rottinghaus GE, Malone AM, Applequist WL. Occurrence of rutin and chlorogenic acid in elderberry leaf, flower, and stem in response to genotype, environment, and season. Acta Hort. 2008;765:197–206. [Google Scholar]
- 14.Finn CE, Thomas AL, Byers PL, Serçe S. Evaluation of American (Sambucus canadensis) and European (S. nigra) elderberry genotypes grown in diverse environments and implications for cultivar development. Hort Science. 2008;43:1391. [Google Scholar]
- 15.Thomas AL, Perkins-Veazie P, Byers PL, Finn CE, Lee J. A comparison of fruit characteristics among diverse elderberry genotypes grown in Missouri and Oregon. J Berry Res. 2013;3:159–168. doi: 10.3233/JBR-130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byers PL, Thomas AL, Cernusca MM, Godsey LD, Gold MA. Growing and marketing elderberries in Missouri. University of Missouri Center for Agroforestry; 2014. (Agroforestry in Action Publication # AF1016-2014). [Google Scholar]
- 17.Johnson MC, Thomas AL, Greenlief CM. Impact of frozen storage on the anthocyanin and polyphenol contents of American elderberry fruit juice. J Agric Food Chem. 63:5653–5659. doi: 10.1021/acs.jafc.5b01702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Giusti MM. High-purity isolation of anthocyanins mixtures from fruits and vegetables – A novel solid-phase extraction method using mixed mode cation-exchange chromatography. J Chromatogr A. 2011:1218–7922. doi: 10.1016/j.chroma.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Johnson MC, Lu P, Fritsche KL, Thomas AL, Cai Z, Greenlief CM. Determination of anthocyanins and total polyphenols in a variety of elderberry juices by UPLC/MS and other methods. Acta Hort. 2015;1061:43–51. doi: 10.17660/ActaHortic.2015.1061.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Lee CY. In: Unit I1.2: Extractions and isolation of polyphenolics, in Handbook of Analytical Food Chemistry. Wrolstad RE, editor. Wiley; New York: 2005. pp. 471–482. [Google Scholar]
- 21.Turker N, Aksay S, Ekiz HI. 2004. Effects of storage temperature on the stability of anthocyanins of a fermented black carrot (Daucus carota var L.) beverage: shalgam. J Agric Food Chem. 2015;52:3813. doi: 10.1021/jf049863s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


