Abstract
Study Design
This study was a post-hoc subgroup analysis of prospectively collected data in the Spine Patient Outcomes Research Trial (SPORT).
Objective
Determine the risk factors for and to compare the outcomes of patients undergoing revision disc excision surgery in SPORT.
Summary of background data
Risk factors for reherniation and outcomes after revision surgery have not been well-studied. This information is critical for proper patient counseling and decision making.
Methods
Patients who underwent primary discectomy in the SPORT intervertebral disc herniation cohort were analyzed to determine risk factors for undergoing revision surgery. Risk factors for undergoing revision surgery for reherniation were evaluated using univariate and multivariate analysis. Primary outcome measures consisted of Oswestry Disability Index (ODI), the Sciatica Bothersomeness index (SBI), and the Short Form 36 (SF-36) at six weeks, three months, six months, and yearly to four years.
Results
Of 810 surgical patients patients, 74 (9.1%) received revision surgery for rehernation. Risk factors for reherniation included: younger age (HR 0.96 (0.94–0.99)), lack of a sensory deficit (HR 0.61 (0.37–0.99)) lack of motor deficit (HR 0.54 (0.32–0.91)) and higher baseline ODI score (HR 1.02 (1.01–1.03)). The time adjusted mean improvement from baseline to four years was less for the reherniation group on all outcome measures (BP 39.5 vs. 44.9, p=0.001; PF 37.1 vs. 44.5, p<0.001; ODI 33.9 vs. 38.3, p <0.001; SBI 8.7 vs. 10.5, p<0.001). At four years, only SBI (−9 vs. −11.4, p=0.002) was significantly lower in the reherniation group.
Conclusions
Younger patients with higher baseline disability without neurological deficit are at increased risk of undergoing revision surgery for reherniation. Those considering revision surgery for reherniation will likely improve significantly following surgery, but possibly not as much as with primary discectomy.
Keywords: Disc herniation, disc reherniation, revision disc surgery, SPORT
Introduction
Lumbar discectomy continues to be one of the best options for patients with intervertebral disc herniation (IDH), who have failed non-operative treatment. Trials show that IDH patients improve more quickly with surgery than without and have superior long-term outcomes [1–3]. In a minority of patients who have undergone successful treatment of their herniated disc, their symptoms return due to a recurrent disc herniation. [1–4]. In fact, the single best factor correlated with good clinical outcomes from discectomy is the absence of reherniation [5]. Unfortunately, IDH patients treated surgically are up to 10 times more likely to have a future spine operation compared to the general population, and 62% of reoperations after discectomy can be attributed to reherniation [6, 7]. Overall, reherniation rates of surgically treated IDH patients in recent studies vary from 3%–18% [8, 9].
Several attempts have been made to evaluate risk factors for recurrent IDH. Reherniation predictors include large annular defects as well as patient characteristics such as smoking, frequent lifting, high body mass index, male gender, younger age, diabetes, and postoperative activity [9–14]. However, the results of these prior studies were not consistent, and the relatively small number of patients sustaining a reherniation has limited the power of these studies to evaluate risk factors.
For patients who experience a reherniation, the decision as to whether to undergo a revision surgery is a complex one, with the indications and expected outcomes less well defined than for a primary discectomy. Some studies have concluded that revision surgery outcomes were worse than for primary discectomy [2, 15–17], while other investigations showed no significant outcome difference for revision surgery compared with primary operations [12, 13, 18–21].
In order to better define risk factors for reherniation and outcomes from revision surgery, we analyzed data from the Spine Patient Outcomes Research Trial (SPORT) [22]. We aimed to answer four study questions: 1)What was the reherniation rate for IDH patients treated surgically in SPORT at 8-years? 2) Did patient or herniation characteristics predict recurrence? 3) Were outcomes at four years from revision surgery different than four year outcomes from primary discectomy? 4) Are there differences in baseline characteristics or outcomes between patients who experience early revision surgery (within one year of primary surgery) versus late revision surgery?
Materials and Methods
SPORT included a prospective randomized controlled trial and an observational cohort conducted in the spine practices of 13 institutions in 11 states. SPORT was approved by the human subject committee of each participating institution. Patient follow up was conducted at six weeks, three months, six months, and yearly up to eight years. Funding was received from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (U01-AR45444) and the Office of Research on Women’s Health, the National Institutes of Health; the National Institute for Occupational Safety and Health, and the Centers for Disease Control and Prevention. The Multidisciplinary Clinical Research Center in Musculoskeletal Diseases at Dartmouth is funded by NIAMS (P60-AR048094 and P60-AR062799). These sources took no role in the study other than funding the project.
This study was a post-hoc subgroup analysis of prospectively collected data in the SPORT IDH cohort. All patients had radicular symptoms for at least 6 weeks prior to enrollment. They had cross-sectional imaging demonstrating a herniated disc, and physical exam findings consistent with the herniation on imaging. Inclusion and exclusion criteria have been published previously [22]. All patients included in this study underwent surgery for IDH, namely decompression of the affected nerve root via open microdiscectomy [22].
Any patient who underwent a second operation to address a recurrent disc herniation at the same level and side as the index surgery was included in the reherniation subgroup. Patients may have experienced a reherniation that was treated non-operatively, however these patients were not included in the reherniation group since it was not possible to conclusively identify a reherniation in the absence of repeat surgery.
The early reherniation group included patients who underwent revision surgery within one year of the primary surgery, with the late reherniation group including those who underwent revision surgery more than one-year after the primary surgery. If patients experienced more than one recurrent herniation, only the initial revision surgery was included in this analysis.
The outcome measures used in this analysis included the Oswestery Disability Index (ODI), the Sciatica Bothersomness index (SB), and the Short Form 36 (SF-36) [23–27]. The SF-36 consists of several subscales including the Bodily Pain Index, (BP), Physical Function Index (PF) and Mental Component Summary (MCS). Patient satisfaction, opioid use and operative complications were also included as secondary outcome measures.
We combined the randomized and observational cohorts in SPORT and performed an as-treated analysis including data out to eight years following primary discectomy [1]. Patient, herniation, and perioperative characteristics were compared between the reherniation and no reherniation groups using Chi-square tests for categorical variables and t tests for continuous variables. To analyze the risk factors for reherniation, a Cox proportional hazards model was used to determine independent predictors of reherniation. Variables that were significant at the p < 0.10 level in the univariate analysis were included as potential predictors in the final multivariable regression model. Final selection for the model was done using the stepwise method, in which we sequentially entered the most significant variable and then removed variables that did not maintain significance at p < 0.05. Age and sex were included in the model by default. Primary outcome analyses compared the no-reherniation and reherniation groups using changes from baseline at each follow-up point, with a mixed-effects longitudinal regression model including a random individual effect to account for correlation between repeated measurements within individuals.
Follow-up times were measured from the time of primary surgery for no reherniation visits, and from the time of revision surgery for reherniation patients. Baseline scores were those recorded at the visit immediately preceding primary surgery for both the no reherniation and reherniation patients. This procedure has the effect of including all changes from baseline prior to reherniation surgery in the estimates of the no-reherniation (primary surgery) treatment effect and all changes after reherniation surgery in the estimates of the reherniation surgery treatment effect. The maximum follow-up time used for outcomes from reherniation was limited to four years in order to sufficiently power the outcomes analysis.
The analyses were adjusted for age, sex, race, marital status, compensation, smoking status, asymmetric sensory decrease, asymmetric motor weakness, work lifting demand, herniation location, working status, stomach comorbidity, depression, diabetes, other comorbidity, self-rated health trend, duration of most recent episode, treatment preference, baseline score (for SF-36, ODI, and Sciatica Bothersomeness Index), and medical center. Across the four years of follow-up, overall comparisons of the “area under the curve” between groups were made using a Wald Test. Computations were performed using SAS procedure PROC MIXED for continuous data and PROC GENMOD for binary outcome (SAS version 9.2, SAS Institute Inc., Cary, NC). Statistical significance was defined as p < 0.05 based on a two-sided hypothesis test with no adjustments made for multiple comparisons.
Results
There were 1244 IDH patients enrolled in SPORT, 820 patients received surgical treatment, and 810 were available for at least one follow-up through 8-years. The rate of reoperation due to reherniation in SPORT at 8 years was 9.1% (74/810, Table 1). More than a third of reoperations for reherniation were early (37.8%: 28/74), occurring within one year of the primary surgery. The majority (92%: 68/74) underwent revision discectomy, while 15% (11/74) underwent a fusion as well. Eight patients had two reoperations for reherniation, however only the first reherniation was included in the analyses.
Table 1.
Reherniation surgery rates by time period for combined Randomized and Observational cohort*
| Time period | Total, N = 810† (no. [%]) |
|---|---|
| 1-year | 28 (4%) |
| 2-year | 40 (5%) |
| 3-year | 44 (6%) |
| 4-year | 50 (6%) |
| 5-year | 54 (7%) |
| 6-year | 60 (8%) |
| 7-year | 67 (8%) |
| 8-year | 74 (9%) |
One-, 2-, 3-, 4-, 5-, 6-, 7-, and 8-year reherniation surgery rates are Kaplan-Meier estimates. Numbers and percentages are based on the first reherniation surgery if more than one reherniation surgery. Surgical procedures include any additional spine surgery, not just reoperation at the same level.
A total of 820 patients had surgery. Surgical information was available for 810 patients.
Univariate analyses demonstrated that patients with reherniation surgery were significantly younger (37 years vs. 41 years, p=0.003), more likely to lift at work (73% vs. 60%, p=0.047), had worse baseline symptoms (BP 18.3 vs. 24.0, p=0.01; PF 25.3 vs. 33.3, p=0.006; ODI 60.4 vs. 54.1, p=0.01), and were less likely to have an asymmetric sensory (41% vs. 55%, p=0.029) or motor (29% vs. 46%, p=0.006) deficit compared to the no-reherniation group (Table 2 and Supplemental Table 1). Herniation level and morphology, opioid use, smoking, BMI, self-assessed symptom trend, surgical complications, and preference for surgery were not associated with revision surgery for reherniation.
Table 2.
Patient baseline demographic characteristics, comorbidities, and health status measures according to reherniation.
| No Reherniation (n=730) | Reherniation (n=73)* | P | |
|---|---|---|---|
|
| |||
| Mean Age (SD) | 41.1 (10.9) | 37.2 (9.3) | 0.003 |
| Female - no. (%) | 307 (42%) | 39 (53%) | 0.081 |
| Ethnicity: Not Hispanic - no. (%)† | 695 (95%) | 71 (97%) | 0.61 |
| White - no. (%) | 639 (88%) | 68 (93%) | 0.22 |
| Education - At least some college - no. (%) | 534 (73%) | 49 (67%) | 0.34 |
| Income - Under $50,000 - no. (%) | 332 (45%) | 41 (56%) | 0.10 |
| Marital Status - Married - no. (%) | 508 (70%) | 54 (74%) | 0.52 |
| Work Status - no. (%) | 0.51 | ||
| Full or part time | 424 (58%) | 43 (59%) | |
| Disabled | 114 (16%) | 8 (11%) | |
| Other | 191 (26%) | 22 (30%) | |
| Work Lift Demand - no. (%) | 0.047 | ||
| Not Important | 292 (40%) | 20 (27%) | |
| Important | 437 (60%) | 53 (73%) | |
| Disability compensation - no. (%)‡ | 148 (20%) | 14 (19%) | 0.94 |
| Mean Body Mass Index (BMI), (SD)§ | 28.1 (5.7) | 28.6 (5.7) | 0.49 |
| Smoker - no. (%) | 182 (25%) | 19 (26%) | 0.95 |
| Comorbidities - no. (%) | |||
| Hypertension | 90 (12%) | 9 (12%) | 0.85 |
| Diabetes | 24 (3%) | 4 (5%) | 0.52 |
| Osteoporosis | 9 (1%) | 0 (0%) | 0.71 |
| Heart Problem | 35 (5%) | 2 (3%) | 0.61 |
| Stomach Problem | 81 (11%) | 8 (11%) | 0.87 |
| Bowel or Intestinal Problem | 45 (6%) | 5 (7%) | 0.98 |
| Depression | 84 (12%) | 10 (14%) | 0.72 |
| Joint Problem | 119 (16%) | 11 (15%) | 0.92 |
| Other¶ | 309 (42%) | 25 (34%) | 0.23 |
| Number of Comorbidities - no. (%) | 0.27 | ||
| None or one | 530 (73%) | 59 (81%) | |
| Two or three | 147 (20%) | 10 (14%) | |
| Four or more | 47 ( 6%) | 3 ( 4%) | |
| Time since recent episode < 6 months | 565 (77%) | 54 (74%) | 0.60 |
| SF-36 scores, mean(SD)|| | |||
| Bodily Pain (BP) | 24 (18.3) | 18.3 (13.6) | 0.01 |
| Physical Functioning (PF) | 33.3 (23.7) | 25.3 (20.3) | 0.006 |
| Mental Component Summary (MCS) | 44.6 (11.4) | 45.4 (11) | 0.55 |
| Oswestry (ODI)** | 54.1 (19.8) | 60.4 (17.3) | 0.01 |
| Sciatica Frequency Index (0–24)†† | 16.6 (5.1) | 17.4 (4.7) | 0.20 |
| Sciatica Bothersome Index (0–24)‡‡ | 16.3 (5) | 17 (4.7) | 0.31 |
| Back Pain Bothersomeness (0–6)§§ | 4 (1.9) | 4.6 (1.4) | 0.012 |
| Leg Pain Bothersomeness (0–6) ¶¶ | 5 (1.4) | 5.2 (1.1) | 0.32 |
| Patient very dissatisfied with symptoms - no. (%) | 640 (88%) | 65 (89%) | 0.88 |
| Patient’s self-assessed health trend - no. (%) | 0.16 | ||
| Getting better | 58 ( 8%) | 8 (11%) | |
| Staying about the same | 324 (44%) | 24 (33%) | |
| Getting worse | 343 (47%) | 40 (55%) | |
| Treatment preference - no. (%) | 0.16 | ||
| Preference for non-surg | 118 (16%) | 12 (16%) | |
| Not sure | 109 (15%) | 5 ( 7%) | |
| Preference for surgery | 500 (68%) | 56 (77%) | |
| Pain Radiation - no. (%) | 715 (98%) | 72 (99%) | 0.97 |
| Straight Leg Raise Test - Ipsilateral - no. (%) | 479 (66%) | 41 (56%) | 0.14 |
| Straight Leg Raise Test - Contralateral/Both - no. (%) | 135 (18%) | 18 (25%) | 0.26 |
| Any Neurological Deficit - no. (%) | 574 (79%) | 51 (70%) | 0.12 |
| Reflexes - Asymmetric Depressed | 307 (42%) | 23 (32%) | 0.10 |
| Sensory - Asymmetric Decrease | 403 (55%) | 30 (41%) | 0.029 |
| Motor - Asymmetric Weakness | 338 (46%) | 21 (29%) | 0.006 |
| Herniation Level - no. (%) | 0.48 | ||
| L2-L3/L3-L4 | 40 ( 5%) | 2 ( 3%) | |
| L4-L5 | 282 (39%) | 32 (44%) | |
| L5-S1 | 407 (56%) | 39 (53%) | |
| Herniation Type - no. (%) | 0.17 | ||
| Protruding | 196 (27%) | 14 (19%) | |
| Extruded | 481 (66%) | 56 (77%) | |
| Sequestered | 52 ( 7%) | 3 ( 4%) | |
| Posterolateral herniation - no. (%) | 579 (79%) | 57 (78%) | 0.92 |
| Opioid Use - no. (%) | 369 (51%) | 42 (58%) | 0.31 |
A total of 820 patients had surgery. Surgical information was available for 810 patients. Of the 810 patients, 803 patients (730 patients who did not have recurrent disc herniation and 73 patients who had recurrent disc herniation) had at least 1 follow-up through 8 years and were included in the outcome analysis.
Race or ethnic group was self-assessed. Whites and blacks could be either Hispanic or non-Hispanic.
This category includes patients who were receiving or had applications pending for workers compensation, Social Security compensation, or other compensation.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Other = problems related to stroke, diabetes, osteoporosis, cancer, fibromyalgia, chronic fatigue syndrome (CFS), post-traumatic stress (PTSD), alcohol, drug dependence, heart, lung, liver, kidney, blood vessel, nervous system, hypertension, migraine, anxiety, stomach or bowel.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Stenosis Frequency Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Stenosis Bothersomeness Index ranges from 0 to 24, with lower scores indicating less severe symptoms.
The Low Back Pain Bothersomness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
The Leg Pain Bothersomeness Scale ranges from 0 to 6, with lower scores indicating less severe symptoms.
SD indicates standard deviation.
Multivariate analysis demonstrated that younger age, lack of a sensory or motor neurological deficit, and higher baseline ODI score were independent predictors of reherniation (Table 3). For every increase in age by one year, the rate of reherniation decreased by 4%. Patients with an asymmetric sensory deficit had a 39% lower rate of reherniation, while those with an asymmetric motor deficit had a 46% lower rate. For every one-point increase in baseline ODI score, the rate of reherniation increased by 2%. Lifting at work, baseline BP and baseline PF were significantly associated with reherniation in the univariate analysis, though they were no longer significant in the multivariate model. Gender was forced into the model but was not a significant predictor.
Table 3.
Results of Cox Proportional Hazards Model For Variables Predicting Time To Reherniation Surgery.
| Variable | Hazard Ratio (95% Confidence Interval) | P |
|---|---|---|
| Age | 0.96 (0.94–0.99) | 0.002 |
| Sex (Female vs. Male) | 1.48 (0.92–2.37) | 0.108 |
| Asymmetric sensory decrease (Any vs. None) | 0.61 (0.37–0.99) | 0.046 |
| Asymmetric motor weakness (Any vs. None) | 0.54 (0.32–0.91) | 0.021 |
| Oswestry Disability Index (ODI) | 1.02 (1.01–1.03) | 0.003 |
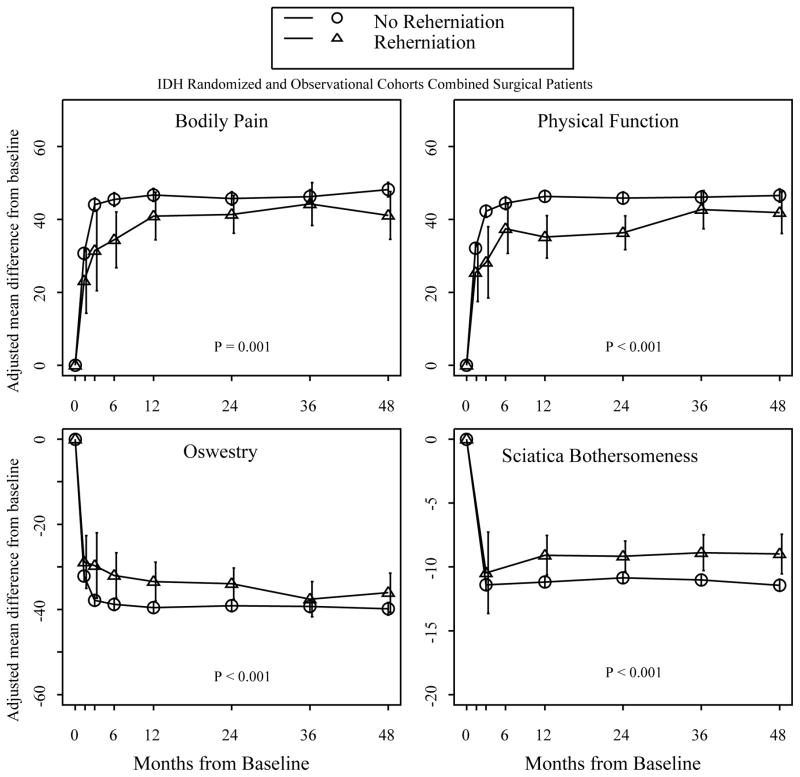
Both the reherniation and no-reherniation groups improved from baseline to 4-year follow up (Figure 1, Table 4). The time adjusted mean improvement from baseline to four-year follow-up was significantly less for the reherniation group on all outcome measures (BP 39.5 vs. 44.9, p=0.001; PF 37.1 vs. 44.5, p<0.001; ODI 33.9 vs. 38.3, p <0.001; SBI 8.7 vs. 10.5, p<0.001). The reherniation group was less likely to be very or somewhat satisfied with their symptoms four years after revision surgery (58.7% vs. 69.1%, p=0.008). Eleven percent of patients in each group remained on opioids at eight years. By four years, the only significant difference remaining between the two groups was less improvement on SBI for the reherniation group (−9 vs. −11.4, p=0.002).
Figure 1.
Outcomes of patients with reherniation and non-reherniation from zero to four years.
Table 4.
Mean change in scores over four years, satisfaction rate, and opioid use*.
| Mean Change in Score Compared with Baseline (Standard Error [SE]) or Percent
|
Difference (95% Confidence interval)† | P | ||
|---|---|---|---|---|
| No Reherniation (n = 730) | Reherniation (n = 73) | |||
|
| ||||
| SF-36 Bodily Pain (BP) (SE)†† | 44.9 (0.7) | 39.5 (1.7) | 5.4 (2.2, 8.6) | 0.001 |
| SF-36 Physical Function (PF) (SE)†† | 44.5 (0.6) | 37.1 (1.5) | 7.4 (4.5, 10.3) | <0.001 |
| SF-36 Mental Component Summary (MCS)†† | 6.6 (0.3) | 4.8 (0.7) | 1.8 (0.5, 3.1) | 0.008 |
| Oswestry Disability Index (ODI) (SE) ‡ | −38.3 (0.5) | −33.9 (1.2) | −4.4 (−6.7, −2.1) | <0.001 |
| Sciatica Bothersomeness Index (SE)§ | −10.5 (0.2) | −8.7 (0.5) | −1.8 (−2.7, −0.9) | <0.001 |
| Very/somewhat satisfied with symptoms (%) | 69.1 | 58.7 | 10.4 (2.8, 18) | 0.008 |
| Opioid use (%) | 11.9 | 11.3 | 0.6 (−3.6, 4.8) | 0.78 |
Scores are adjusted for age, sex, race, marital status, compensation, smoking status, asymmetric sensory decrease, asymmetric motor weakness, work lift demand, herniation location, working status, stomach comorbidity, depression, diabetes, other** comorbidity, self-rated health trend, duration of most recent episode, treatment preference, baseline score (for SF-36, ODI, and Sciatica Bothersomeness Index), and center.
Difference is the difference between no reherniation and reherniation.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Sciatica Bothersomeness index range from 0 to 24, with lower scores indicating less severe symptoms.
Other comorbidities include: stroke, diabetes, osteoporosis, cancer, fibromyalgia, cfs, PTSD, alcohol, drug dependency, heart, lung, liver, kidney, blood vessel, nervous system, hypertension, migraine, anxiety, stomach, bowel.
Compared to primary surgery, repeat discectomy did not have a higher rate of perioperative complications including blood loss, length of hospital stay, nerve root injury, wound infection or dural tear (data not shown).
There were no differences in patient, herniation, or primary surgery peri-operative characteristics between the early and late reherniation groups (data not shown). There were no significant differences in four-year outcome following revision surgery between the early and late reherniation groups (Table 5).
Table 5.
Mean change in scores over four years and satisfaction rate for reherniation patients, according to whether or not reherniation patients had reherniation within 1 year of index surgery*.
| Mean Change in Score Compared with Baseline (Standard Error [SE]) or Percent
|
Difference (95% Confidence interval)† | P | ||
|---|---|---|---|---|
| Reherniation within 1 year of index surgery (n = 27) | Reherniation after 1 year of index surgery (n = 46) | |||
|
| ||||
| SF-36 Bodily Pain (BP) (SE)†† | 36.5 (4.1) | 40.1 (3.6) | −3.6 (−14.3, 7.1) | 0.51 |
| SF-36 Physical Function (PF) (SE)†† | 38.3 (4.1) | 38.7 (3.5) | −0.4 (−11, 10.2) | 0.94 |
| SF-36 Mental Component Summary (MCS)†† | 4.7 (1.7) | 4.1 (1.5) | 0.6 (−3.9, 5.1) | 0.81 |
| Oswestry Disability Index (ODI) (SE) ‡ | −32.4 (3.6) | −36 (3.1) | 3.6 (−5.7, 12.9) | 0.45 |
| Sciatica Bothersomeness Index (SE)§ | −9.1 (1.3) | −8.6 (1.1) | −0.5 (−3.9, 2.9) | 0.76 |
| Very/somewhat satisfied with symptoms (%) | 51.5 | 60.2 | −8.7 (−28.1, 10.7) | 0.38 |
Scores are adjusted for age, sex, and baseline score (for SF-36, ODI, and Sciatica Bothersomeness Index).
Difference is the difference between the reherniation within 1 year of index surgery and reherniation after 1 year of index surgery.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Sciatica Bothersomeness index range from 0 to 24, with lower scores indicating less severe symptoms.
Discussion
The current analysis demonstrated that 9.1% of patients undergoing a primary discectomy underwent revision surgery secondary to reherniation within the 8-year follow-up period, with 37.8% of all revision surgeries occurring in the first year after surgery. These findings are consistent with the current literature on reherniation and revision surgery. Davis et al. had a reherniation rate of 6% with a mean follow-up of 10-years, with one third of all reherniations occurring in the first postoperative year [28]. In another study of 25,366 IDH patients, 9.4% underwent repeat discectomy with mean follow-up of 4.1 years [29]. Moliterno et al. found a reherniation rate of 9.5%, with variable follow-up times [30]. Vik et al. found a reherniation rate of 8.6% at eight years, with nearly 50% of reoperations occurring in the first year [17]. Lastly, McGirt et al. had a 10.2% reherniation rate at 2 years with almost 50% occurring in the first year [31].
Younger patients with worse baseline symptoms and those without baseline neurological deficits were at increased risk of reherniation in the current study. These findings correlate with several studies identifying an association between younger age and reherniation [12, 18, 29]. In addition, Ahsan et al. reported that patients who reherniated had higher baseline ODI scores, but this was not statistically significant in their cohort of 18 reoperations [13]. Prior studies have reported that male sex, smoking, diabetes, disc protrusion, and elevated BMI were risk factors for reherniation [9–13, 15, 30]. These factors were not associated with reherniation in the current study. The literature on the absence of a neurological deficit as a risk factor for reherniation is small and contradictory, with Morgan-Hough et al. reporting an increased risk of reherniation among those with no neurological deficit and Cinotti et al. demonstrating a higher reherniation rate among those with a neurological deficit at baseline [18]. There is not a clear explanation for the association between the lack of a baseline neurological deficit and reherniation observed in the current study.
It is notable that smoking and increased BMI, known risk factors for primary disc herniation, were not found to be significant risk factors for reherniation in this analysis [32]. Occupational lifting, a third “high risk “ factor for primary disc herniation, was associated with reherniation in univariate analysis but did not maintain significance as an independent risk factor for reherniation [32]. Other studies have, however, found a link between smoking and reherniation as well as between occupational lifting and reherniation [10, 12].
The strongest predictor of reherniation in our analysis was younger age, with the rate of reherniation decreasing 4% with every additional year of age. Wilke et al. used an in-vitro model to show that discs of younger patients, with a well-hydrated nucleus pulposus, were more likely to reherniate under mechanical stress [33]. This study also showed that discs of patients older than age 55 were unlikely to reherniate due to fibrosis of the nucleus [33]. Increasing disc degeneration that accompanies aging likely protects against reherniation.
There are several limitations in this analysis. First, we did not analyze the size of the annular deficit during surgery, which has been associated with risk of reherniation in other investigations [14, 31]. Additionally, this analysis did not include patients with reherniations treated without revision surgery. Thus, we were unable to compare reherniation surgery directly with non-operative treatment for reherniation. Patients with reherniations treated non-operatively were included in the no-reherniation cohort, which may have resulted in this cohort having worse outcomes than if these patients had been excluded. Revision surgery also included 11 patients who received fusion in addition to revision discectomy; however, there were too few fusion patients to power an analysis of fusion versus no fusion for recurrent IDH.
Patients undergoing revision surgery improved significantly from baseline. However, the magnitude of improvement from baseline was somewhat less than in the primary discectomy patients. There were no outcome or baseline differences between patients who had reherniation surgery within 1-year from primary surgery compared with those who had surgery later. The findings of this study showing significant improvement with revision surgery are consistent with the majority of the literature on this topic [12, 13, 18, 34, 35]. When discussing the risk of reherniation with patients considering primary discectomy, they can be informed that there is less than a 10% chance of undergoing a second surgery for reherniation within 8 years, and that younger patients might be at somewhat higher risk for reherniation. Patients considering revision surgery can be informed that they will likely improve significantly following surgery but possibly not as much as with primary surgery. Further prospective investigation should assess the efficacy on non-operative treatment compared with surgery for reherniation.
Supplementary Material
Acknowledgments
National Institute of Arthritis and Musculoskeletal and Skin Diseases funds were received in support of this work.
Footnotes
Relevant financial activities outside the submitted work: board membership, grants.
Level of Evidence: 3
References
- 1.Lurie JD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: eight-year results for the spine patient outcomes research trial. Spine (Phila Pa 1976) 2014;39(1):3–16. doi: 10.1097/BRS.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlas SJ, et al. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 2005;30(8):927–35. doi: 10.1097/01.brs.0000158954.68522.2a. [DOI] [PubMed] [Google Scholar]
- 3.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine (Phila Pa 1976) 1983;8(2):131–40. [PubMed] [Google Scholar]
- 4.Shin BJ. Risk Factors for Recurrent Lumbar Disc Herniations. Asian Spine J. 2014;8(2):211–215. doi: 10.4184/asj.2014.8.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carragee EJ, et al. Activity restrictions after posterior lumbar discectomy. A prospective study of outcomes in 152 cases with no postoperative restrictions. Spine (Phila Pa 1976) 1999;24(22):2346–51. doi: 10.1097/00007632-199911150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bruske-Hohlfeld I, et al. Incidence of lumbar disc surgery. A population-based study in Olmsted County, Minnesota, 1950–1979. Spine (Phila Pa 1976) 1990;15(1):31–5. doi: 10.1097/00007632-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Leven D, et al. Risk Factors for Reoperation in Patients Treated Surgically for Intervertebral Disc Herniation: A Subanalysis of Eight-Year SPORT Data. J Bone Joint Surg Am. 2015;97(16):1316–25. doi: 10.2106/JBJS.N.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGirt MJ, et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64(2):338–44. doi: 10.1227/01.NEU.0000337574.58662.E2. discussion 344–5. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, et al. Risk Factors for Recurrent Lumbar Disc Herniation: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95(2):e2378. doi: 10.1097/MD.0000000000002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miwa S, et al. Risk Factors of Recurrent Lumbar Disc Herniation: A Single Center Study and Review of the Literature. J Spinal Disord Tech. 2013 doi: 10.1097/BSD.0b013e31828215b3. [DOI] [PubMed] [Google Scholar]
- 11.Shimia M, et al. Risk factors of recurrent lumbar disk herniation. Asian J Neurosurg. 2013;8(2):93–6. doi: 10.4103/1793-5482.116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suk KS, et al. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976) 2001;26(6):672–6. doi: 10.1097/00007632-200103150-00024. [DOI] [PubMed] [Google Scholar]
- 13.Ahsan K, et al. Discectomy for primary and recurrent prolapse of lumbar intervertebral discs. J Orthop Surg (Hong Kong) 2012;20(1):7–10. doi: 10.1177/230949901202000102. [DOI] [PubMed] [Google Scholar]
- 14.Carragee EJ, et al. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85-A(1):102–8. [PubMed] [Google Scholar]
- 15.Fritzell P, et al. Recurrent Versus Primary Lumbar Disc Herniation Surgery: Patient-reported Outcomes in the Swedish Spine Register Swespine. Clin Orthop Relat Res. 2015;473(6):1978–84. doi: 10.1007/s11999-014-3596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebow RL, et al. Asymptomatic same-site recurrent disc herniation after lumbar discectomy: results of a prospective longitudinal study with 2-year serial imaging. Spine (Phila Pa 1976) 2011;36(25):2147–51. doi: 10.1097/BRS.0b013e3182054595. [DOI] [PubMed] [Google Scholar]
- 17.Vik A, et al. Eight year outcome after surgery for lumbar disc herniation: a comparison of reoperated and not reoperated patients. Acta Neurochir (Wien) 2001;143(6):607–610. doi: 10.1007/s007010170066. discussion 610–11. [DOI] [PubMed] [Google Scholar]
- 18.Cinotti G, et al. Ipsilateral recurrent lumbar disc herniation. A prospective, controlled study. J Bone Joint Surg Br. 1998;80(5):825–32. doi: 10.1302/0301-620x.80b5.8540. [DOI] [PubMed] [Google Scholar]
- 19.Patel MS, et al. A comparative study of the outcomes of primary and revision lumbar discectomy surgery. Bone Joint J. 2013;95-B(1):90–4. doi: 10.1302/0301-620X.95B1.30413. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson B, Stromqvist B. Repeat decompression of lumbar nerve roots. A prospective two-year evaluation. J Bone Joint Surg Br. 1993;75(6):894–7. doi: 10.1302/0301-620X.75B6.8245078. [DOI] [PubMed] [Google Scholar]
- 21.Wera GD, et al. Failure within one year following subtotal lumbar discectomy. J Bone Joint Surg Am. 2008;90(1):10–5. doi: 10.2106/JBJS.F.01569. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer NJ, et al. Design of the Spine Patient outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2002;27(12):1361–72. doi: 10.1097/00007632-200206150-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 24.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–3. [PubMed] [Google Scholar]
- 26.Atlas SJ, et al. The Quebec Task Force classification for Spinal Disorders and the severity, treatment, and outcomes of sciatica and lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21(24):2885–92. doi: 10.1097/00007632-199612150-00020. [DOI] [PubMed] [Google Scholar]
- 27.Patrick DL, et al. Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976) 1995;20(17):1899–908. doi: 10.1097/00007632-199509000-00011. discussion 1909. [DOI] [PubMed] [Google Scholar]
- 28.Davis RA. A long-term outcome analysis of 984 surgically treated herniated lumbar discs. J Neurosurg. 1994;80(3):415–21. doi: 10.3171/jns.1994.80.3.0415. [DOI] [PubMed] [Google Scholar]
- 29.Keskimaki I, et al. Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine (Phila Pa 1976) 2000;25(12):1500–8. doi: 10.1097/00007632-200006150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Moliterno JA, et al. Results and risk factors for recurrence following single-level tubular lumbar microdiscectomy. J Neurosurg Spine. 2010;12(6):680–6. doi: 10.3171/2009.12.SPINE08843. [DOI] [PubMed] [Google Scholar]
- 31.McGirt MJ, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 2009;34(19):2044–51. doi: 10.1097/BRS.0b013e3181b34a9a. [DOI] [PubMed] [Google Scholar]
- 32.Saftic R, et al. Case-control study of risk factors for lumbar intervertebral disc herniation in Croatian island populations. Croat Med J. 2006;47(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- 33.Wilke HJ, et al. Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine (Phila Pa 1976) 2013;38(10):E587–93. doi: 10.1097/BRS.0b013e31828ca4bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haglund MM, et al. Outcome after repeat lumbar microdiscectomy. Br J Neurosurg. 1995;9(4):487–95. doi: 10.1080/02688699550041124. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulos EC, et al. Outcome of revision discectomies following recurrent lumbar disc herniation. Spine (Phila Pa 1976) 2006;31(13):1473–6. doi: 10.1097/01.brs.0000219872.43318.7a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.