Abstract
It is widely acknowledged that cultured myoblasts can not differentiate at very low density. Here we analyzed the mechanism through which cell density influences myogenic differentiation in vitro. By comparing the behavior of C2C12 myoblasts at opposite cell densities, we found that, when cells are sparse, failure to undergo terminal differentiation is independent from cell cycle control and reflects the lack of p27Kip1 and MyoD in proliferating myoblasts. We show that inhibition of p27Kip1 expression impairs C2C12 cell differentiation at high density, while exogenous p27Kip1 allows low-density cultured C2C12 cells to enter the differentiative program by regulating MyoD levels in undifferentiated myoblasts. We also demonstrate that the early induction of p27Kip1 is a critical step of the N-cadherin-dependent signaling involved in myogenesis. Overall, our data support an active role of p27Kip1 in the decision of myoblasts to commit to terminal differentiation, distinct from the regulation of cell proliferation, and identify a pathway that, reasonably, operates in vivo during myogenesis and might be part of the phenomenon known as “community effect”.
INTRODUCTION
Skeletal muscle cell differentiation, in vivo and in vitro, involves irreversible withdrawal from cell cycle and expression of a number of muscle-specific genes coordinated by the activity of muscle-specific transcription factors belonging to the MyoD family, Myf-5, MyoD, Myogenin and MRF-4, also known as Muscle Regulatory Factors (MRFs) (reviewed in Molkentin and Olson, 1996; Perry and Rudnicki, 2000). The achievement of the terminally differentiated state implies a close functional cross-talk between MRF and cell cycle regulators. pRb and p21Cip1 were shown to play an important role in the growth arrest of differentiating myoblasts. Their level and/or activity are positively linked to MyoD, which cooperates in the induction of cell cycle arrest (reviewed in Lassar et al., 1994; Maione and Amati, 1997; Walsh and Pearlman, 1997; Kitzmann and Fernandez, 2001; Wei and Paterson, 2001). Moreover, in vivo studies demonstrated a transient expression of the cyclin-dependent kinase (Cdk) inhibitor (CKI) p27Kip1, which correlates with the timing and expansion of the MyoD-initiated myotomal subdomain indicating a short-term role of p27Kip1 as the cells are withdrawing from the cell cycle (Zabludoff et al., 1998).
Besides the complex network controlling the execution of myogenic program, progression of muscle-determined cells toward stable differentiated state requires the formation of a “community” by similar cells that allows competent cells to respond to inductive signals (Gurdon et al., 1993; Cossu et al., 1995; Buckingham, 2002). This phenomenon, known as “community effect”, has a fundamental role in embryo development as it determines proper cell differentiation within a tissue and demarcation between tissues (Gurdon, 1988). The community effect in in vitro myogenic differentiation is resembled by the need for an optimal culture density in order to achieve full muscle gene expression. The formation of a “community” involves the establishment of cell-cell contacts that allows intercellular signaling interactions between similar adjacent cells.
Cell-cell contact depends on the interaction of a variety of surface molecules, whose functions have a central place in tissue organization as well as in many responses to environment stimuli and insults. Members of the cadherin family are regarded as the major players involved in cell-cell recognition and adhesion. It was shown that N-cadherin-dependent cell interactions play a critical role in regulating the signaling pathways required for differentiation of muscle progenitor cells during Xenopus development (Holt et al., 1994). In vitro studies have demonstrated that the inhibition of N-cadherin-mediated contact perturbs muscle differentiation (Knudsen et al., 1990; Mege et al., 1992; George-Weinstein et al., 1997; Charrasse et al., 2002). Conversely, forced expression of N-cadherin, as well as N-cadherin engagement achieved with different approaches, induces muscle-specific gene expression and myogenesis in vitro (Redfield et al., 1997; Goichberg and Geiger, 1998; Seghatoleslami et al., 2000; Gavard et al., 2004). Although these studies reasonably indicate N-cadherin-mediated signals as an important component of the cell-density-dependent myogenesis, they fail to supply information on how different cell densities, which influence entering of determined muscle cells to terminally differentiate state, specifically affect cell cycle and myogenic regulators.
In the present study we have investigated the mechanisms through which cell density influences myogenic differentiation in vitro and the pathway(s) involved therein. We compared the behavior of C2C12 myblasts induced to differentiate when dispersed or confluent. We demonstrate that failure of C2C12 cells to terminally differentiate at low density is not associated by a defect in cell cycle exit, suggesting the existence of a negative control of skeletal muscle differentiation specifically associated with cell density and independent from cell cycle control. On these premises we have identified a novel and important role for the CKI p27Kip1 in muscle differentiation concerning the maintenance of appropriate MyoD protein levels in proliferating myoblasts at high density by preventing its degradation. Furthermore, the results obtained suggest a functional link between cadherin-cadherin interactions, p27Kip1 and terminal differentiation, and support an active role for p27Kip1 in an early stage of the decision to enter the terminally differentiated state, distinct from regulation of cell proliferation.
MATERIALS AND METHODS
Cells and Culture Conditions
Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% Fetal Bovine Serum (FBS) (referred to as growth medium, GM). Muscle differentiation was induced, after 24 h in GM, by incubating cells in DMEM supplemented with 0.5% FBS (referred to as differentiation medium, DM).
C2C12 cells conditionally expressing human p27Kip1 were generated as follow: 5 μg of p3'SS plasmid (Stratagene), containing the LacI repressor and the hygromycin resistance genes, and 5 μg of pOPI3p27Kip1 plasmid (provided by J. Wyke), containing human p27Kip1 cDNA and neomycin resistance gene, were cotransfected into C2C12 cells; clones were selected with hygromycin B and G418, and screened for the ability to express p27Kip1 protein following addition of 10 mM isopropyl-β-d-thiogalactoside (IPTG) to the culture medium.
The L6C5 cells, a subclone of rat myogenic cell line L6, were provided by S. Adamo (University of Rome “La Sapienza”). Expression of N-cadherin in L6C5 cells was achieved by cotransfection with pBATMNC (provided by M. Takeichi, RIKEN Center for Developmental Biology, Kobe, Japan), containing mouse N-cadherin cDNA, and pBabeHygro (provided by H. Land, University of Rochester Medical Center, Rochester, NY, USA) carrying the hygromycin resistance gene (L6C5-N). Control L6C5 cells were obtained by transfection with pBabeHygro.
C2C12A5LacI is a clone of C2C12 steadily expressing the LacI repressor of the IPTG-inducible system (Stratagene) generated by transfecting the LacI-repressor containing plasmid p3'SS.
Transfections were performed by DNA-calcium-phosphate coprecipitation method.
3T3 fibroblasts from p27 knock out mice (referred to as MEF p27Kip1-/-) were kindly provided by S. Coats (Amgen, Thousand Oaks, CA, USA).
Low and high density conditions were achieved by seeding isolated myoblasts at 5 × 102/cm2 and confluent myoblasts at 2 × 104/cm2, respectively.
Inhibition of p27Kip1 expression was achieved by lipofecting 1,5 × 105 C2C12 cells in 35 mm collagen-coated dishes with C-5 propyne-modified phosphorothioate oligonucleotides, as described (Coats et al., 1996). p27Kip1-specific antisense and mismatch oligonucleotides, as well as GS2888 cytofectin were kindly provided by J. Wolf (Gilead Sciences).
Proteasome inhibitor MG132 (Sigma-Aldrich) was used at 50 μM; Roscovitine (Calbiochem) was used at 5 μM.
Viruses and Infection
pBabePurop27Kip1 and pBabePurop27Kip1VPKK retroviral vectors were kindly provided by B. Amati (European Institute of Oncology, Milan, Italy).
pBabeHygroMyoD and pBabeHygroMyoDSP3 were generated by subcloning the EcoRI/HindIII fragments of murine MyoD and MyoDSP3 cDNAs from pCB6-MyoD and pCB6-MyoDSP3 (Song et al., 1998, made available by G. Falcone, CNR, Rome) into pBluescript IIKS plasmid (Stratagene) in antisense orientation. The SalI/EcoRI fragments from pBluescript IIKS were EcoRI/SalI subcloned into the retroviral vector pBabeHygro (provided by H. Land).
High titer viral stocks were obtained from the ecotropic Phoenix packaging cell line (kindly provided by G. Nolan, Stanford University, CA, USA) transiently transfected with the retroviral vectors described above.
Infection was performed in 60 mm dishes. 2×105 C2C12 cells were seeded and treated for 2 h with 3 μg/ml polybrene in GM. 1 ml of viral stock was added and infection was allowed to proceed overnight. On the next day, cells were fed GM. Selective agents, puromycin or hygromycin B, were added on the second day postinfection.
Immunofluorescence
Skeletal muscle Myosin Heavy Chain (MHC), p27Kip1 and Lac I expression was revealed in cells fixed for 10 min in 3.3% para-formaldehyde and permeabilized with 0.25% TritonX-100 by using the following antibodies: monoclonal antibody (mAb) to MHC (MF20) (provided by D. Fishman, Cornell University, New York), mAb to p27Kip1 (BD, Transduction Lab) and rabbit polyclonal antibody to LacI (Stratagene).
Bromodeoxyuridine-labeled cells were processed as described (Grossi et al., 1998).
FITC- and TRITC conjugated goat anti-mouse and anti-rabbit antibodies were from Cappel (West Chester, PA).
Nuclei were stained with 0.2 μg/ml DAPI.
Stained cells were examined using the Leica DMRE microscope equipped with 20 ×, 40 × and 100 × lenses. Single images were recorded on Leica DC250 camera and processed using the Qwin software (Leica).
Protein Extraction and Western Blot
Cells were lysed in boiling Sample Buffer 1 × (50 mM Tris-HCl pH 6.8, 2%SDS, 10% glycerol, 100 mM dithiothreitol (DTT)). 30 μg of protein were resolved on 8% or 12% SDS-PAGE (according to the different molecular weights) and then transferred onto nitrocellulose. After saturation in 5% milk in tris-buffered saline (TBS)-T (TBS plus 0.02% Tween20), filters were incubated with the following antibodies: monoclonal anti-p27Kip1 (BD, Transduction Lab); anti-Rb (PharMingen); rabbit polyclonal anti-human p27Kip1 (UBI), anti-cycD1 (sc-718), anti-p57Kip2 (sc-8298), and anti-cycE (sc-481) from Santa Cruz Biotechnology; rabbit polyclonal anti-MyoD antibody R3B2 (raised, in collaboration with S. Alemà, ICB, CNR, Rome and M. Crescenzi, ISS, Rome, against murine MyoD expressed in bacteria); monoclonal anti-MHC (MF20, a gift of D. Fischmann); monoclonal anti-Myogenin (IF5D, Wright et al., 1989, made available by G. Cossu, University of Rome “La Sapienza”); rabbit polyclonal anti-p21Cip1 (provided by C. Schneider, Laboratorio Nazionale CIB, Trieste, Italy); rabbit polyclonal anti-LacI antibody (Stratagene); mouse monoclonal anti β-tubulin (ICN). After washing, membranes were incubated with horseradish-peroxidase-conjugated species-specific secondary antibodies (BIO-RAD) followed by enhanced chemiluminescence system (Amersham).
Immunoprecipitation and Kinase Activity Assays
To evaluate Cdk2 and Cdk4-associated kinase activities, cells were lysed for 30 min at 4°C in a lysis buffer containing 50 mM Tris-HCl pH 8, 150 mM NaCl, 1% Nonidet-P40, 0.5% sodium deoxycholate, 0.5 mM phenylmethylsulfonylfluoride, leupeptin at 5 μg/ml, aprotinin at 5 μg/ml and pepstatin at 5 μg/ml. Protein extracts (2 mg per sample) were incubated over night at 4°C with 5 μg of specific antibodies [anti-Cdk2 goat antiserum (M2) or anti-Cdk4 goat antiserum (C-22) from Santa Cruz Biotechnology]. The following day, 50 μl of a protein G-agarose bead suspension (Pierce) was added to each sample at 4°C for 1 h. After extensive washes, immunoprecipitates were resuspended in 50 μl of kinase buffer (50 mM HEPES pH 7.5, 10 mM MgCl2, 10 mM β-glycero phosphate, 2.5 mM EGTA, 1 mM DTT, 50 mM NaF, 50 mM sodium orthovanadate) supplemented with 50 μM unlabeled ATP, 1 μg Histone H1 or 2.5 μg of GST-Rb fusion protein (Santa Cruz) as substrate, and 10 μCi [γ-32P]ATP per sample and incubated for 30 min at 30°C. The reactions were stopped by adding 2 × SDS loading buffer and boiling for 10 min. Labeled proteins were resolved on an SDS-12% polyacrilammide gel and either exposed to film or immunoblotted.
RNA Isolation and Northern Blot Analysis
Total RNA was prepared and analyzed by Northern blot as described (Russo et al., 1997). To detect p27Kip1, MyoD and constitutive transcript glyceraldehyde-3-phosphate dehydrogenase (GAPDH), inserts were excised with the appropriate restriction enzymes from the following plasmids and used as probes: pETmp27, containing a 500 base pairs mouse p27Kip1 cDNA (provided by G. Del Sal, Laboratorio Nazionale CIB, Trieste, Italy); pEMC11S, containing a 1,5Kb mouse MyoD cDNA (obtained from H. Weintraub); a plasmid containing a 1.2 Kb cDNA of the avian GAPDH (obtained from C. Schneider, Laboratorio Nazionale CIB, Trieste, Italy).
RESULTS
Myogenic Differentiation in Vitro Is Density-dependent
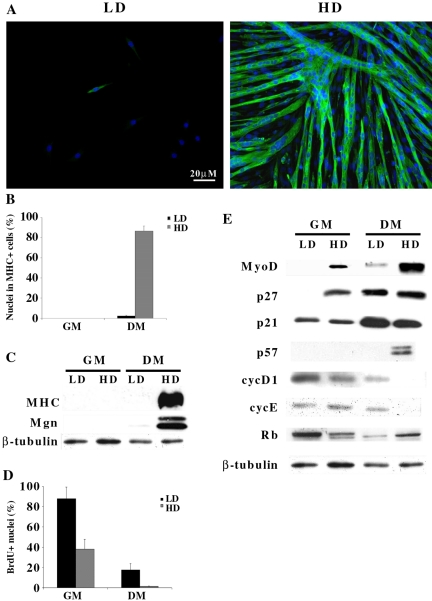
To assess the role of cell density in skeletal muscle differentiation, we studied the differentiative response of the C2C12 murine myogenic cell line to different culture conditions. Cells were plated in growth medium (GM) at two extremely different densities: 5 × 102/cm2 (referred to as low density, LD) and 2 × 104/cm2 (referred to as high density, HD). After 24 h, cells were shifted to differentiation medium (DM) and maintained in DM for 3 d. Analysis of skeletal muscle myosin heavy chain (MHC) (Figure 1A, B and C) and Myogenin (Figure 1C) expression revealed that only cells plated at HD were able to terminally differentiate, while cells plated at LD displayed a very poor differentiation potential, indicating that cell density can deeply influence the differentiative ability of C2C12 myoblasts.
Figure 1.
Myogenic differentiation in vitro is density-dependent. C2C12 myoblasts were seeded at low (5 × 102/cm2, LD) and high (2 × 104/cm2, HD) density in GM. Cells were shifted to DM the day after plating and analyzed after further 3 d. Proteins were extracted from C2C12 cells cultivated at the indicated densities after 24 h in GM or after shifting for 72 h in DM. (A) Immunofluorescence staining for MHC (green). Nuclei were counterstained in blue (Dapi) and individual pictures of the same field, taken with a DC camera, were merged using a LEICA Microsystems Imaging Equipment. (B) Percentage of nuclei belonging to MHC positive cells. (C) Western blot analysis of differentiation associated markers. (D) C2C12 cells were labeled with 50 μM BrdU for 2 h before shifting in DM (GM) or after 3 d in DM (DM). Cells in S phase were evidenced by BrdU staining. (E) Western blot analysis of MyoD and cell cycle regulatory proteins. Anti-β-tubulin antibody was used to normalize the amount of protein loaded. Data are the means ± SD of three independent experiments.
Because cell cycle progression and myogenic differentiation are two very close and mutually exclusive events (Okazaki and Holtzer, 1966; Nadal-Ginard, 1978), we asked whether the failure of C2C12 cells to differentiate at LD could be ascribed to a high proliferative rate retained in the differentiation condition. The data shown in Figure 1D indicate that LD-cultured C2C12 cells were able to exit the cell cycle when serum deprived and that the residual percentage of cells in S phase (17.5%) did not account for the virtually complete absence of terminally differentiated myocites (2%, Figure 1B).
The expression of cell cycle regulators and of MyoD was then compared in C2C12 cells plated at LD and HD, cultured in growth or differentiation conditions. Western blot analysis (Figure 1E) shows that p21Cip1 was expressed equally at low and at high density, increasing in DM. p57Kip2 was only expressed in differentiated cells, confirming the tight association between p57Kip2 and myogenic terminal differentiation (Zhang et al., 1999). p27Kip1 was undetectable in proliferating C2C12 cells plated at LD (GM/LD), while it was expressed at high levels when they were plated at HD (GM/HD). The subsequent cultivation in DM allowed a significant and comparable accumulation of the protein in both culture conditions. The expression pattern of MyoD in C2C12 cells maintained in GM at LD and HD was similar to that of p27Kip1, while, unlike p27Kip1, MyoD levels remained very low in LD-cultured C2C12 cells in DM (compare DM/LD with DM/HD). Figure 1E also shows that pRb was present as hyperphosphorylated form when cells were in GM, whether at LD or HD, and that, after shifting to DM, protein levels increased in differentiated HD-cultured C2C12 cells and accumulated as hypophosphorylated form, while only the phosphorylation levels changed in LD-cultured C2C12 cells, denoting growth arrest without muscle differentiation. We also analyzed the levels of the cyc D1 and cyc E and found that they were still expressed in C2C12 cells at LD conditions after 3 d in DM (Figure 1E). This finding was very surprising, since it has been shown that the expression of these proteins, especially that of cyc D1, is linked to the presence of growth factors in the medium (Matsushime et al., 1994).
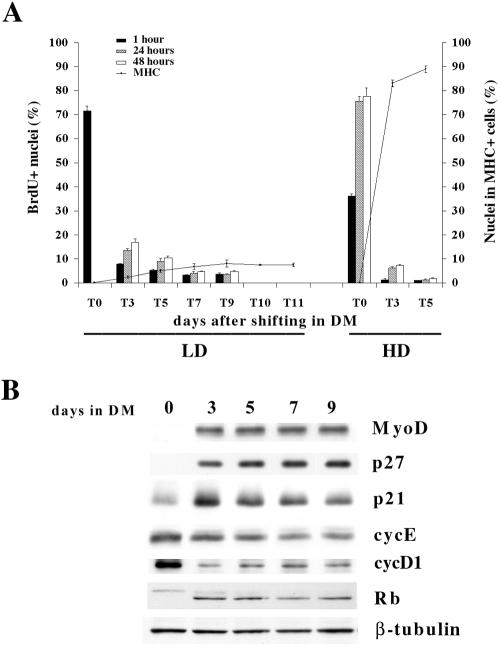
To avoid the possibility that lack of terminal differentiation in C2C12 cells cultured at LD was due to a delay in cell cycle exit, we extended the analysis to 9 d after shifting in DM and monitored DNA synthesis by BrdU labeling and staining. We also assessed the incidence of terminally differentiated cells at the different time points prolonging the analysis to 11 d. The results, shown in Figure 2A, indicate that LD-cultured C2C12 cells actually exited the cell cycle and that the majority of the cells were growth arrested at day 7 (T7) as assessed by 48 h BrdU labeling. The evaluation of differentiated cells evidenced that the incidence of MHC positive cells did not significantly increase neither at the same time point (T7) nor during the following times analyzed, (T9), (T10), (T11) (Figure 2A). Figure 2B shows that the expression of both p27Kip1 and MyoD proteins increased upon shift in DM and their levels did not vary throughout the analyzed period length. Both cyc D1 and E were found to be still expressed in growth arrested LD C2C12 cells, while pRb accumulated as hypophosphorylated form.
Figure 2.
LD-cultured C2C12 cells exit cell cycle upon serum depletion. (A) C2C12 cells, seeded at low or high densities, were labeled with 50 μM BrdU for 1 h or with 20 μM BrdU for 24 or 48 h at the indicated times. Cells in S phase were evidenced by BrdU staining. At the indicated times parallel cultures were processed for MHC expression by immunofluorescence. (B) Proteins were extracted from C2C12 cells cultivated at low density after 24 h in GM (0) or after shifting in DM at the indicated times. Anti-β-tubulin antibody was used to normalize the amount of protein loaded. Data are the means ± SD of three independent experiments.
Taken together these results suggest the existence of a negative regulatory pathway, controlling myogenic differentiation, which strictly depends on cell density itself and involves differential expression of key-regulators of both, cell cycle and myogenic differentiation.
p27Kip1 Specifically Regulates C2C12 Cell Differentiation
Because the relationship between p27Kip1 expression and high cell density has been documented (Hengst et al., 1994; Polyak et al., 1994; Dietrich et al., 1997; Nakatsuji et al., 2001), and we found that only myoblasts expressing high levels of p27Kip1 in GM execute the full differentiative program upon cultivation in DM, we asked whether p27Kip1 could be directly involved in the regulation of myogenic differentiation.
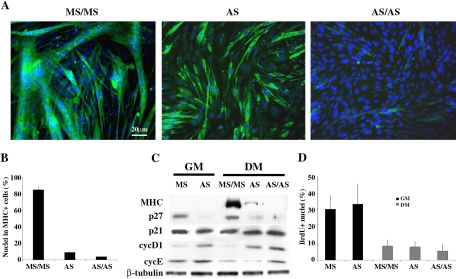
Two different approaches were used, aimed at providing direct evidence for a specific role of p27Kip1 in myogenic differentiation. In a first set of experiments we attempted to inhibit p27Kip1 expression in HD-cultured C2C12 myoblasts by an antisense strategy. For this purpose, cells, plated in GM at HD, were transfected with p27Kip1-specific antisense (AS) and mismatch (MS) oligonucleotides. 24 h later, cells were shifted to DM. After further 48 h a set of cultures were transfected again with the AS oligonucleotides in DM (AS/AS), to ensure a continuous inhibition of protein expression throughout the experiment. Figure 3 illustrates the extent of terminal differentiation of transfected HD-cultured C2C12 cells (Figure 3A), and shows that interference with p27Kip1expression resulted in a severe impairment of terminal differentiation, achieving 96% inhibition in AS/AS (Figure 3B). Western blot analysis indicates that p27Kip1 accumulation was prevented very efficiently by the AS oligonucleotides, both in GM and in DM (Figure 3C), suggesting a direct relationship between loss of p27Kip1 accumulation and lack of myogenic differentiation. The inhibition of p27Kip1 in growth conditions was accompanied by increased levels of both cyc D1 and cyc E, whose expression was unexpectedly retained in AS transfected C2C12 cells cultured in DM, despite growth factor deprivation (Figure 3C). Hence, we asked whether impairment of differentiation potential and increase of G1 cyclin expression would correspond to an altered growth behavior of C2C12 cells in DM. Figure 3D shows that p27Kip1 inhibition did not interfere with normal growth control, as MS and AS transfected cultures displayed similar percentage of cells in S phase, both in GM and in DM.
Figure 3.
p27Kip1 antisense oligonucleotides severely inhibit myogenic differentiation. C2C12 cells, plated at HD, were transfected in GM with either p27Kip1 antisense (AS) or mismatch (MS) oligonucleotides and, after 24 h, were shifted to DM for 3 d without further treatment (AS) or transfecting them again after 48 h in DM (MS/MS and AS/AS). (A) At the third day in DM, cells were fixed and MHC expression was revealed by immunofluorescence staining (green). Nuclei were counterstained in blue (Dapi) and individual pictures of the same field, taken with a DC camera, were merged using a LEICA Microsystems Imaging Equipment. (B) Percentage of nuclei belonging to MHC positive cells. (C) Proteins were extracted from parallel cultures after 24 h in GM or 72 h in DM. Extracts normalized for protein content were blotted and probed with antibodies specific for the indicated proteins. (D) C2C12 myoblasts, transfected as indicated, were labeled with 50 μM BrdU for 2 h after 24 h in GM or 72 h in DM. Cells in S phase were evidenced by BrdU staining. Data are the means ± SD of three independent experiments.
These data demonstrate that inhibiting p27Kip1 in HD cultures strongly influences the proper execution of the myogenic differentiation program without altering cell cycle response to growth factor deprivation, and indicate that p27Kip1 expression is required in order to reach a fully differentiated state. Accordingly, it would be predicted that forced expression of p27Kip1 in LD-cultured C2C12 cells could mimic HD conditions and promote terminal differentiation.
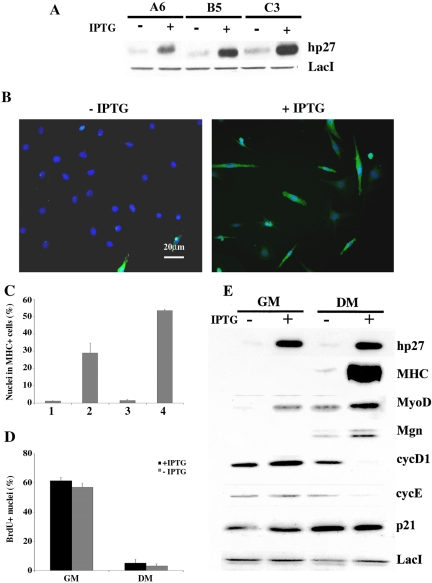
To achieve conditional expression of exogenous p27Kip1 in C2C12 myoblasts, we used an inducible mammalian expression system. Cells were cotransfected with p3'SS and pOPI3p27Kip1 expression vectors. Several clones were selected and three of them (A6, B5 and C3) were chosen for the strong induction of exogenous p27Kip1 expression upon addition of isopropyl-β-d-thiogalactoside (IPTG) (Figure 4A). Inducible and control cell lines were plated at LD, the inducer was added at different times and for different period lengths and the incidence of terminally differentiated cells was assessed after 5 d in DM (Figure 4B). Figure 4C illustrates the results obtained with clone A6 and shows that: i) the presence of IPTG throughout the experiment allowed more than 50% of the cells to undergo terminal differentiation (column 4); ii) the induction of exogenous p27Kip1 expression during only the first 24 h in GM, before shifting in DM, was sufficient to significantly enhance the proportion of differentiated C2C12 cells (column 2); iii) the addition of IPTG at the time of shifting in DM was ineffective (column 3). Similar results were obtained with the other two clones (not shown). We investigated whether the acquisition of competence for terminal differentiation was due to an additional antiproliferative effect exerted by exogenous p27Kip1. Data from bromodeoxyuridine (BrdU) incorporation showed that the proportion of C2C12 cells in S phase was comparable, both in GM and DM, in IPTG treated cells as well as in untreated cultures (Figure 4D).
Figure 4.
Forced expression of p27Kip1 recovers the ability of C2C12 myoblasts to differentiate at low density culture conditions. (A) Exogenous p27Kip1 expression in three engineered clones determined after 24 h in GM minus (-) or plus (+) IPTG. (B) A6 cells, plated at LD, were induced to differentiate in the absence or the presence of IPTG and MHC expression was analyzed after 5 d by immunofluorescence (green). Nuclei were counterstained in blue (Dapi) and individual pictures of the same field, taken with a DC camera, were merged using a LEICA Microsystems Imaging Equipment. (C) Analysis of MHC positive A6 cells following addition of IPTG at different times: for only 24 h in GM (column2),for 5 d in DM (column 3), or throughout the whole experiment, 24 h in GM + 5 d in DM, (column 4). Column 1 refers to untreated cells. (D) The percentage of cells in S phase was determined after 2 h BrdU labeling (50 μM) by immunofluorescence staining. (E) Protein extracts from A6 cells grown in GM for 24 h minus or plus IPTG or in DM for 5 d minus or plus IPTG were probed with the indicated antibodies. The expression of LacI repressor was used to normalize the amount of protein loaded. Data are the means ± SD of three independent experiments.
Western blot analysis (Figure 4E) revealed an interesting modulation of protein expression pattern dependent on exogenous p27Kip1. In fact, the early induction of p27Kip1 was associated with a strong accumulation of MyoD in GM that was probably responsible for the recovered ability of C2C12 cells to terminally differentiate at LD conditions. These findings indicate that p27Kip1 is acting upstream MyoD in the execution of the myogenic program. Another interesting aspect focuses on the levels of cyc D1 and cyc E expression. In fact, they were still expressed in LD-cultured C2C12 cells in DM conditions, while their expression was significantly down-regulated after exogenous p27Kip1 induction.
Overall these data lead to the conclusion that p27Kip1 is required to achieve terminal differentiation with a role parallel to, but distinct from, cell cycle control.
At Low Cell Density p27Kip1 and MyoD Are Subject of a Major Degradation Rate
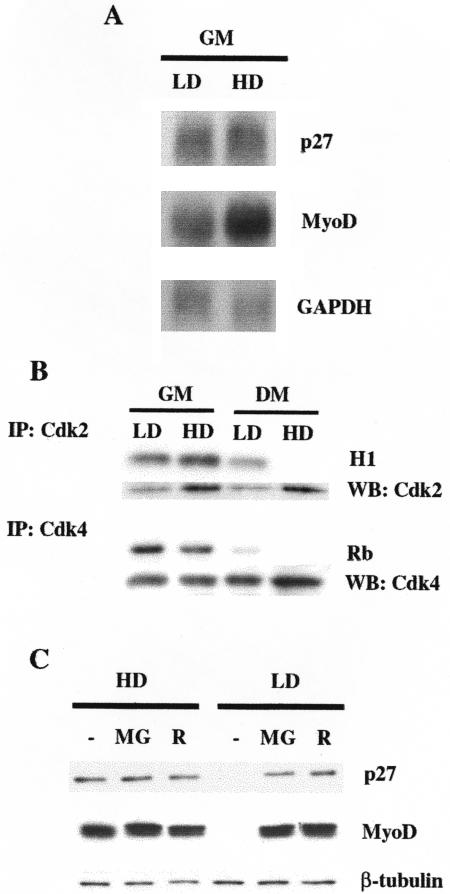
To understand how p27Kip1 and MyoD expression was regulated at the different cell densities, we first analyzed p27Kip1 and MyoD mRNAs by Northern Blot. Figure 5A shows that cell density did not dramatically influence MyoD mRNA accumulation and did not perturb p27Kip1 transcript levels. Thus, the discrepancy between mRNA and protein accumulation in C2C12 cells at LD suggests the existence of a posttranscriptional regulation of MyoD and p27Kip1 expression.
Figure 5.
At LD conditions MyoD and p27Kip1 show a major posttranslational control. (A) Northern Blot analysis of p27Kip1 and MyoD transcript accumulation. (B) Kinase activities precipitated by anti-Cdk2 or anti-Cdk4 antibodies from C2C12 at the indicated culture conditions after 24 h in GM or after shifting for 72 h in DM as assayed by phosphorylation of histone H1 (H1) and GST-Rb fusion protein (Rb) respectively. The amount of Cdk2 and Cdk4 in the immunoprecipitates was assessed by Western blot. (C) C2C12 cells, grown in GM at HD or LD were treated with either 50 μM MG132 (MG) or 5 μM Roscovitine (R) for 6 h. (-) denotes treatment with dimethyl sulfoxide. Extracts normalized for protein content (β-tubulin) were probed with antibodies specific for the indicated proteins.
Although the existence of a transcriptional control of p27Kip1 has been demonstrated (Hengst and Reed, 1996), different studies reported that the decrease of p27Kip1 levels during the G1/S transition involves ubiquitin-proteasome mediated proteolysis, which is mainly controlled by cyc E -Cdk2-dependent phosphorylation on Thr187 (Pagano et al., 1995; Vlach et al., 1997; Millard et al., 1997). Similarly it has been demonstrated that Cdk-dependent phosphorylation of MyoD can target it to a rapid degradation via proteasome (Song et al., 1998), and that direct phosphorylation of MyoD on Ser200 by cyc E-Cdk2 plays a crucial role in modulating MyoD half-life and myogenic activity (Kitzmann et al., 1999; Reynaud et al., 1999; Tintignac et al., 2000).
The analysis of Cdk activity in the various culture conditions indicate that, despite the elevated levels of the CKI p21Cip1 (see Figures 1 and 2), LD-cultured C2C12 cells in GM displayed a kinase activity associated to Cdk2 higher than HD-cultured C2C12 cells in the same culture conditions (compare histone H1 phosphorylation with the amount of Cdk2 in the respective immunoprecipitates), while Cdk4-associated activity was quite comparable in GM in the two density conditions. Moreover, Cdk2 retained a significant residual activity in C2C12 cells cultured at low density in DM conditions (Figures 5B). Thus, in order to verify whether the lack of p27Kip1 and MyoD expression in LD-cultured C2C12 cells could reflect a major degradation driven by cycE-Cdk2-dependent phosphorylation, we examined the effects of the proteasome inhibitor MG132, and the Cdk2-selective inhibitor roscovitine on the accumulation of endogenous p27Kip1 and MyoD in cultures at LD and HD in GM conditions. As shown in Figures 5C, addition of MG132 induced a strong accumulation of p27Kip1 and MyoD in LD-cultured C2C12 myoblasts, while the protein levels were unaffected in HD cultures, demonstrating that, in myoblasts cultivated at LD, both p27Kip1 and MyoD are under a tight posttranslational control based on degradation via proteasome. Moreover, roscovitine treatment was very effective in recovering both p27Kip1 and MyoD expression, suggesting a prominent role for Cdk2-dependent phosphorylation in directing them toward proteasome-dependent degradation. It has to be noted that the residual activity of Cdk2 might be also responsible for the lower MyoD protein in LD C2C12 cells in DM, as roscovitine exposure determines accumulation of MyoD comparable to that observed in HD C2C12 in DM (Supplemental Figure S1).
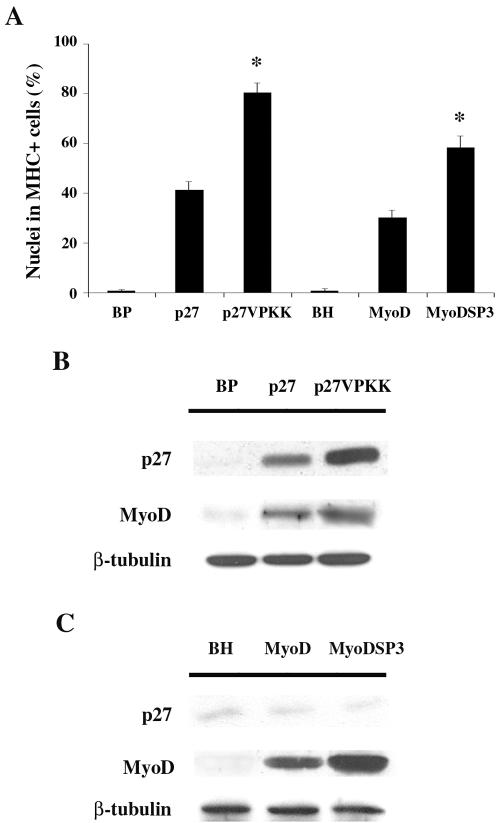
On the basis of these findings, we infected C2C12 cells with the retroviral vectors pBPp27VPKK, carrying mutated p27Kip1 (Val in place of Thr187), and pBHMyoDSP3 carrying mutated MyoD (Ala in place of Ser200). Infected cells were plated at LD, and the differentiation phenotype was analyzed after 3 d in DM and compared with that displayed by C2C12 cells expressing exogenous wild-type alleles or infected with the empty vectors (BP or BH). Figure 6A shows that overexpression of both p27VPKK and MyoDSP3 significantly improved myogenic differentiation when compared with overexpression of wild-type proteins. Expression of exogenous and endogenous p27Kip1 and MyoD was also analyzed in infected C2C12 cells kept at LD in GM. The results pointed up two important aspects. On the one hand, they confirm that forced expression of p27Kip at LD induces myoblasts to terminally differentiate as a consequence of MyoD accumulation (Figure 6B). On the other hand, as reasonably expected, overexpression of exogenous MyoD, either wild-type or mutated, allowed LD-cultured myoblasts to undergo differentiation, albeit to a different extent, without altering the p27Kip1 expression pattern (Figure 6C).
Figure 6.
Stabilized mutants of p27Kip1 and of MyoD improve terminal differentiation at LD. (A) C2C12 cells, infected with pBabePuro (BP) and pBabeHygro (BH) retroviral vectors, wild-type p27Kip1 and MyoD containing retroviral vectors or p27VPKK and MyoDSP3 expressing retroviral vectors, were plated at LD and, after 24 h in GM, shifted to DM. 3 d later the amount of MHC positive cells was assessed. (*) Values from p27VPKK and MyoDSP3 samples are statistically significant compared with those from the respective wild-type (p <0.001). Data are the means ± SD of three independent experiments. (B) Expression levels of p27Kip1, either wild-type or mutated, and MyoD in LD cultured C2C12 cells, infected as indicated, were assessed by Western Blot after 24 h in GM. (C) Expression levels of MyoD, either wild-type or mutated, and p27Kip1 in LD cultured C2C12 cells, infected as indicated, were assessed by Western Blot after 24 h in GM. Anti-β-tubulin antibody was used to normalize the amount of protein loaded.
Thus both, inhibitor treatment and overexpression of stabilized mutants, point out a major posttranslational regulation of p27Kip1 and MyoD at LD, and the role of Cdk2-dependent phosphorylation in controlling protein half-life. Moreover, data from p27Kip1 and MyoD overexpression experiments support a direct relationship between p27Kip1 and MyoD, with p27Kip1 acting upstream MyoD in coordinating muscle terminal differentiation.
N-Cadherin-dependent Cell-Cell Contact Induces p27Kip1 Expression and Leads Myoblasts to Terminally Differentiate at LD
To define whether the requirement of a high cell density for a full differentiation could reflect the production of specific factors, we cultivated C2C12 cells at LD with conditioned medium from HD cultures. We found that it was not able to promote terminal differentiation (data not shown), suggesting that direct cell-cell contact was required.
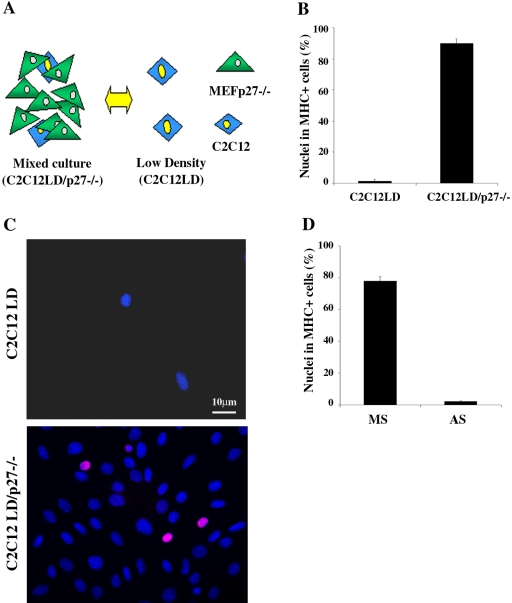
p27Kip1 is up-regulated during growth arrest induced by cell contact as the result of signal transduction triggered by interactions between molecules expressed on the surface of adjacent cells (Polyak et al., 1994). On this basis, we asked whether such a mechanism could be responsible for p27Kip1 accumulation and terminal differentiation in HD-cultured C2C12 cells. To address this issue we set up mixed cultures where 5 × 102/cm2 (LD) myoblasts were plated together with a large excess (2 × 104/cm2) of mouse fibroblasts, which provided HD culture conditions. A schematic representation of the experimental setting is illustrated in Figure 7A. To distinguish between murine myoblasts and fibroblasts in in situ analysis, we used a C2C12 cell line stably expressing the LacI repressor from the Lac-Switch inducible expression system (C2C12A5LacI, simply named C2C12), while to detect p27Kip1 specifically expressed by myogenic cells we used embryo fibroblasts from p27Kip1 knockout mice (MEFp27-/-) as coculture partner. C2C12 cells were plated at LD alone or in cocultivation with an excess of MEFp27-/- and, after 24 h in GM, were shifted to DM. As expected, terminal differentiation was largely prevented in single-culture myoblasts, while a large proportion (more than 90%) of C2C12 cells in mixed culture underwent myogenic differentiation (Figure 7B). Moreover, the commitment of LD-cultured C2C12 cells to differentiate in mixed culture was associated to an early expression of p27Kip1 when cells were still in GM (Figure 7C). To demonstrate a cause-effect relationship between early expression of p27Kip1 in GM and the subsequent differentiation of C2C12 cells in mixed culture, we inhibited p27Kip1 accumulation by transfecting cocultures with p27Kip1 AS oligonucleotides. Figure 7D shows that the interference with p27Kip1 expression in mixed culture strongly impaired C2C12 cell differentiation. These data demonstrate that the establishment of cell-cell contact with surrounding fibroblasts is sufficient to induce p27Kip1 expression and allows terminal differentiation in LD-cultured myoblasts, thus resembling HD culture conditions.
Figure 7.
Cell-cell contact with fibroblasts is sufficient to induce terminal differentiation of C2C12 cells cultured at LD. (A) Schematic representation of the experimental setting: C2C12A5LacI (simply named C2C12) cells cultured alone at LD (C2C12LD) or in mixed culture with an excess of mouse embryo fibroblasts from p27Kip1 knock out mice (C2C12LD/p27-/-). (B) C2C12 cells seeded as shown in (A) were shifted to DM 24 h after plating. 3 d later the incidence of differentiated C2C12 cells were evaluated by MHC staining. Data are the means ± SD of three independent experiments. (C) p27Kip1 expression in C2C12 myoblasts cultured at LD alone or in mixed culture with MEFp27-/- after 24 h in GM. Nuclei were counterstained in blue (Dapi); the pink colored nuclei result from the overlapping of the red (anti-p27Kip1) and blue (DAPI) colors and represent the double-stained cells. (D) C2C12LD/p27-/- mixed cultures were transfected in GM with p27Kip1 antisense (AS) or mismatch (MS) oligonucleotides and shifted to DM after 24 h. 3 d later the incidence of differentiated C2C12 cells was evaluated by MHC staining. Data are the means ± SD of three independent experiments.
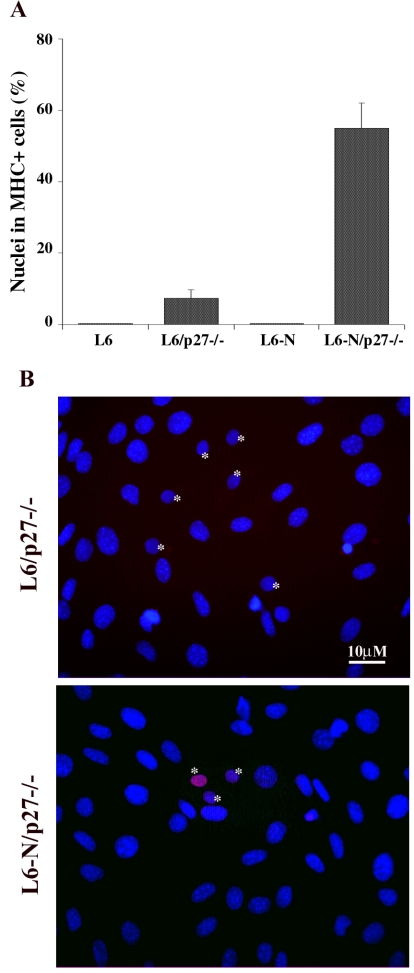
To investigate whether cadherin-mediated cell adhesion, particularly N-cadherin, was involved in density-dependent regulation of myogenic differentiation in vitro, we compared the differentiative response in mixed culture of the rat myogenic cell line L6C5, which is defective for N-cadherin expression (Pouliot et al., 1994), to that of L6C5-N cell line, a L6C5 derivative stably expressing mouse N-cadherin. The data reported in Figure 8A show that: i) both cell lines failed to undergo terminal differentiation when plated at LD (L6 and L6-N); ii) L6C5 cells were still unable to differentiate when plated at LD together with an excess of mouse fibroblasts, as very few L6C5 cells expressed MHC (L6/p27-/-); iii) only L6C5-N cells were responsive to HD culture conditions provided by adjacent heterologous cells and more than 50% of the cells underwent terminal differentiation (L6-N/p27-/-). The analysis of p27Kip1 expression in GM (Figure 8B) revealed that only myoblasts expressing exogenous N-cadherin were able to accumulate the CKI in mixed culture, suggesting a tight relationship between N-cadherin mediated adhesion, p27Kip1 expression and skeletal muscle terminal differentiation. Also in this experimental setting the inhibition of p27Kip1 by AS oligonucleotides in GM, strongly interfered with the differentiative potential of myogenic cells (data not shown), emphasizing that the essential prerequisite for efficient muscle differentiation is the early expression of p27Kip1.
Figure 8.
N-cadherin-mediated cell-cell contact allows p27Kip1 accumulation and terminal differentiation in LD myoblasts. (A) L6C5 and L6C5-N rat myogenic cells were plated at LD alone (L6 and L6-N) or in mixed culture with an excess of MEFp27-/- (L6/p27-/- and L6-N/p27-/-) and, after 24 h in GM, were placed in DM for 4 d. The incidence of differentiated cells was evaluated by MHC staining. (B) The expression of p27Kip1 was detected after 24 h in GM by immunofluorescence with specific antibodies. Nuclei were counterstained in blue (Dapi). The pink colored nuclei result from the overlapping of the red (anti-p27Kip1) and blue (DAPI) colors. Rat nuclei (*) can be distinguished from mouse nuclei characterized by intensely fluorescent chromocenters.
DISCUSSION
The results presented in this paper show that the activity of p27Kip1 is an important part of the mechanism regulating muscle differentiation at different cell densities and that it concerns the maintenance of proper MyoD levels in undifferentiated myoblasts. We demonstrate that p27Kip1 plays a central role in the decision of determined myoblasts to enter a stable differentiated state and that its function is independent from cell cycle regulation. We also propose that p27Kip1 is a crucial effector of the N-cadherin-dependent signaling involved in muscle-specific gene expression.
Here we show that in the context of LD conditions, determined myogenic C2C12 cells, as well as other myogenic cell lines and primary quail myoblasts (G. Messina, unpublished observations), although cell cycle arrested, are not able to achieve complete differentiation. The analysis of myogenic and cell cycle regulators revealed that a substantial difference between LD and HD cultures resides, under growth conditions, in the expression levels of MyoD and p27Kip1. The absence of p27Kip1 in GM at LD is expected, as its expression has been described to be positively influenced by cell-cell contact, in addition to serum deprivation (Hengst et al., 1994; Polyak et al., 1994). On the contrary, it was surprising to find undetectable levels of MyoD in growth conditions, as it is usually expressed both in proliferating and differentiated C2C12 cells. On the whole, the comparison of the expression pattern displayed by C2C12 cells at the two different cell densities highlights a possible functional link between lack of p27Kip1 and MyoD expression in GM and the lack of full myogenic commitment in sparse cultures.
While the role of p27Kip1 in cell cycle control is extensively documented, its possible specific involvement in myogenic commitment has never been invoked. In this study we demonstrated, by loss- and gain-of function approaches, that p27Kip1 is directly involved in the circuit that regulates the progression into a fully differentiated state, and that such a function is independent from its ability to control cell cycle exit. On the basis of the data presented it is apparent that p27Kip1 function is needed in a temporally defined window before exposure to differentiation stimuli, since induction of the protein limited to the 24 h before shifting cells to DM is sufficient to achieve differentiation in LD-cultured C2C12 cells. On the contrary, induction of exogenous p27Kip1 expression in DM is ineffective, suggesting that p27Kip1 plays an important role in coordinating the decision to undergo full myogenic commitment and in driving cells toward a differentiative fate in response to appropriate stimuli.
Prompted by the results we have presented an essential question might be asked: what is, beyond cell cycle control, the relationship between p27Kip1 and the execution of the myogenic differentiation program? An important player that appears to be in between is the muscle regulatory factor MyoD, whose levels in growth conditions, and partially in differentiation conditions, are influenced by cell density to the same extent of p27Kip1, and whose expression is modulated by exogenous p27Kip1. MyoD expression is mostly regulated at the transcriptional level, and in our experimental setting, the lower mRNA levels detected in C2C12 cells is certainly responsible for the decreased accumulation of MyoD protein at LD. However, we find that, at LD, post-translational regulation significantly contributes to modulate MyoD accumulation, as proteasome inhibitor treatment allows recovery of protein levels comparable to those observed in C2C12 cells at HD. It has been previously reported that MyoD half-life and myogenic activity are influenced by its phosphorylation state. Particularly, it has been described that Ser 200 is a target of cyc E-Cdk2 (Tintignac et al., 2000) and that the action of p57Kip2 in differentiating C2C12 cells contrasts this activity (Reynaud et al., 1999). The data we report suggest that a similar regulation occurs in C2C12 cells cultured in GM at LD, as treatment with roscovitine determines a significant accumulation of MyoD protein when cells are cultured at LD, while MyoD levels are not affected in HD cultures in GM, i.e., in the presence of p27Kip1, by neither inhibitors. It is reasonable that the role exerted by p27Kip1 on the execution of muscle differentiation concerns with the improvement of MyoD stability, by likely inhibiting cycE-Cdk2 activity, and that its absence in LD-cultured C2C12 cells determines a severely reduced accumulation of the MRF and, as a consequence, failure to terminally differentiate when transferred in DM. Cdk2 activity is also instrumental for controlling MyoD growth suppressive effects in growing myoblasts (Tintignac et al., 2000). Our data suggest that, although MyoD amount must be controlled in proliferating myoblasts, the decrease under threshold levels hinders myogenic cells to undergo terminal differentiation. This seems to be directly related to the activity of p27Kip1 rather than p57Kip2. Indeed, in our culture conditions p57Kip2 expression is undetectable in growth conditions, while it increases upon differentiation, as also previously reported (Reynaud et al., 1999). Results from the over expression of wild-type and degradation-resistant forms of p27Kip1 and MyoD confirm a specific role of p27Kip1 in promoting terminal differentiation as a direct consequence of induced MyoD accumulation in growth conditions. Therefore, it is conceivable that p27Kip1 and p57Kip2, although both involved in MyoD stabilization, act at different stages: at the decision to enter differentiation program and during terminal differentiation, respectively.
Concerning the role of the CKIs in in vivo myogenesis, gene disruption studies showed that they are mostly redundant (Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996; Deng et al., 1995; Serrano et al., 1996; Zhang et al., 1997), and that only the loss of p57Kip2 significantly affects skeletal muscle differentiation (Zhang et al., 1997). The use of double mutant mice lacking p21Cip1 and p57Kip2, has provided evidence of cooperation between these two CKIs in the control of tissue growth and development (Zhang et al., 1999). In particular, the authors showed that mice lacking both p21Cip1 and p57Kip2 displayed defects in skeletal muscle in secondary myogenesis after Myogenin expression. On the contrary, the double p27Kip1 and p57Kip2 knockout mice apparently do not display defects in myogenesis more severe than those seen in p57Kip2-null embryos (Zhang et al., 1998).
In the light of these studies, our hypothesis envisages p27Kip1 as a necessary factor in the initial phases of myogenic differentiation, supported, and then substituted, in its function by the other members of the Cip1/Kip1 family and, probably, by the INK4 proteins. This view seems to be consistent with in vivo observations (Zabludoff et al., 1998) demonstrating that during myogenesis the initial CKI activity is represented by p27Kip1 and that subsequent signals transfer Cdk inhibition to other single or multiple CKIs. If this simple model is correct, it implies that any muscle phenotype in p27Kip1 knockout mice would be subtle, involving changes in the timing of cell cycle exit at the onset of differentiation, or in the level of MRF needed to initiate differentiation.
In the present study, by setting up culture conditions allowing protein expression patterns to be transiently modulated, we could point up a novel level of muscle differentiation control regulating the decision of determined myoblasts to enter terminal differentiation and depending on p27Kip1 expression and activity. On the basis of the data presented, this activity appears to be linked to the inhibition of Cdk2. However, roles for p27Kip1, other than Cdk inhibitor, have been proposed (Hauser et al., 1997; Zhang et al., 2003; reviewed in Coqueret, 2003), and we do not exclude additional functions for p27Kip1 in myogenesis regulation. Indeed, very preliminary observations indicate that a p27Kip1 mutant deficient in its interaction with both cyc E and Cdk2 was still partially able to promote differentiation of LD C2C12 cells, suggesting that p27Kip1 is probably acting also through noncanonical pathway(s) in addition to the well known Cdk inhibition (G. Messina, unpublished observations).
The important question now is: which are the molecules/pathways involved in the control of p27Kip1 levels that are missed in C2C12 cells cultured at LD? Among the possible mechanisms, we observed that the establishment of direct cell-cell contact plays an important role in determining p27Kip1 accumulation and muscle terminal differentiation. By using different cell combinations in mixed culture approaches we also found that N-cadherin-dependent cell-cell adhesion is part of the mechanism responsible for p27Kip1 expression in myoblasts cultured at HD, a function lost in LD cultures where cell-cell contact is prevented. It has been already described that p27Kip1 is an effector of the cell contact-induced cell cycle inhibition (Hengst et al., 1994; Polyak et al., 1994) and that it is part of N-cadherin-mediated contact inhibition of cell growth (Levenberg et al., 1999). Furthermore, many different studies suggest that N-cadherin-mediated adhesion is directly involved in muscle differentiation in vitro (Goichberg and Geiger, 1999, Charrasse et al., 2002; Gavard et al., 2004). In this regard, it has been described how N-cadherin-dependent cell-cell contact regulates muscle-specific gene expression through the Rho GTPase pathway (Charrasse et al., 2002). The authors hypothesize that, by modulating Rho GTPase activity, N-cadherin-mediated cell adhesion influences muscle-specific gene expression, including MyoD transcription, which, in turn, would control CKI-dependent cell-cycle arrest.
Although data from our mixed culture experiments confirm these observations, the results obtained from p27Kip1 and MyoD overexpression in LD-cultured C2C12 cells strongly suggest that p27Kip1 acts upstream MyoD, probably influencing its half-life. Hence, the data we have presented allow us to propose a model in which the establishment of N-cadherin-mediated cell-cell contact promotes p27Kip1 expression, which in turn supports MyoD accumulation and terminal differentiation. It must be underlined that the major activity of p27Kip1 occurs in a defined temporal window, when a growing myoblast has to decide, upon appropriate stimuli, whether to undertake terminal differentiation or not, and that the presence of p27Kip1 is required within that time window in order to maintain sufficient MyoD levels that will allow myoblasts to enter the terminal differentiative program.
In conclusion, our data highlight a specific role for the CKI p27Kip1 in skeletal muscle differentiation controlling progression from determined to fully differentiated state by regulating MyoD half-life, and show that an appropriate cell density is required in order to achieve full execution of the differentiative program, that reflects the expression of p27Kip1 primed by cadherin-mediated cell adhesion among many nearby similar cells. Such a pathway would constitute an integral part of the “community effect”, observed in vivo, which governs myogenesis and determines the precise temporal and spatial placement of body muscle formation.
Supplementary Material
Acknowledgments
We wish to thank S. Adamo, B. Amati, S. Coats, G. Cossu, M. Crescenzi, G. Del Sal, G. Falcone, D. Fischmann, H. Land, G. Micheli, G. Nolan, C. Schneider, M. Takeichi, J. Wolf, J. Wyke and all our colleagues who generously provided many reagents and helpful comments. We dedicate this paper to the late Franco Tatò, who transmitted us his passion for science and greatly contributed to this study.
This work was partially supported by grants from Associazione Italiana per la Ricerca sul Cancro and CNR/MURST (Legge 95/95).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0612) on January 12, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Buckingham, M. (2002). How the community effect orchestrates muscle differentiation. BioEssays. 25, 13-16. [DOI] [PubMed] [Google Scholar]
- Charrasse, S., Meriane, M., Comunale, F., Blangy, A., and Gauthier-Rouvière, C. (2002). N-cadherin-dependent cell-cell contact regulates Rho GTPases and β-catenin localization in mouse C2C12 myoblasts. J. Cell Biol. 158, 953-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats, S., Flanagan, W.M., Nourse, J., and Roberts, J.M. (1996). Requirement of p27Kip1 for restriction point control of the fibroblasts cell cycle. Science. 272, 877-880. [DOI] [PubMed] [Google Scholar]
- Coqueret, O. (2003). New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 13, 65-70. [DOI] [PubMed] [Google Scholar]
- Cossu, G., Kelly, R., Di Donna, S., Vivarelli, E., and Buckingham, M. (1995). Myoblast differentiation during mammalian somitogenesis is dependent upon a community effect. Proc. Nat. Acad. Sci. USA 92, 2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, C., Zhang, P., Harper, J. W., Elledge, S. J., and Leder, P. (1995). Mice lacking p21Cip1/Waf1 undergo normal development, but are defective in G1 checkpoint control. Cell. 82, 675-684. [DOI] [PubMed] [Google Scholar]
- Dietrich, C., Wallenfang, K., Oesch, F., and Wieser, R. (1997). Differences in the mechanisms of growth control in contact, inhibited and serum-deprived human fibroblasts. Oncogene. 15, 2743-2747. [DOI] [PubMed] [Google Scholar]
- Fero, M. L. et al. (1996). A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis and female sterility in p27(Kip1)-deficient mice. Cell. 85, 733-744. [DOI] [PubMed] [Google Scholar]
- Gavard, J., Marthiens, V., Monnet, C., Lambert, M., and Mege, R.M. (2004). N-cadherin activation substitutes for the cell contact control in the cell-cycle arrest and myogenic differentiation: Involvement of p120 and β-catenin. J. Biol. Chem. 279, 36795-36802. [DOI] [PubMed] [Google Scholar]
- George-Weinstein, M., Gerhart, J., Blitz, J., Simak, E., and Knudsen, K.A. (1997). N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev. Biol. 185, 14-24. [DOI] [PubMed] [Google Scholar]
- Goichberg, P., and Geiger, B. (1998). Direct involvement of N-cadherin-mediated signaling in muscle differentiation. Mol. Biol. Cell. 9, 3119-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi, M., La Rocca, S.A., Pierluigi, G., Vannucchi, S., Ruaro, E.M., Schneider, C., and Tatò, F. (1998). Role of Gas-1 down-regulation in mitogenic stimulation of quiescent NIH3T3 cells by v-src. Oncogene. 17, 1629-1638. [DOI] [PubMed] [Google Scholar]
- Gurdon, J.B. (1988). A community effect in animal development. Nature. 336, 772-774. [DOI] [PubMed] [Google Scholar]
- Gurdon, J.B., Tiller, E., Roberts, J., and Kato, K. (1993). A community effect in muscle development. Curr. Biol. 3, 1-11. [DOI] [PubMed] [Google Scholar]
- Hauser, P.J., Agrawal, D., Flanagan, M., and Pledger, J.W. (1997). The role of p27Kip1 in the in vitro differentiation of murine keratinocytes. Cell Growth Diff. 8, 203-211. [PubMed] [Google Scholar]
- Hengst, L., Dulic, V., Slingerland, J.M., Lees, E., and Reed, S.I. (1994). A cell cycle-regulated inhibitor of cyclin-dependent kinase. Proc. Natl. Acad. Sci. USA 91, 5291-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst, L., and. Reed, S. I. (1996). Translational control of p27Kip1 accumulation during the cell cycle. Science. 271, 1861-1864. [DOI] [PubMed] [Google Scholar]
- Holt, C.E., Lemaire, P., and Gurdon, J.B. (1994). Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopus mesoderm. Proc. Nat. Acad. Sci. USA 91, 10844-10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann, M., Vandromme, M., Schaeffer, V., Carnac, G., Labbè, J.C., Lamb, D., and Fernandez, A. (1999). Cdk1- and Cdk2-mediated phosphoylation of MyoD Ser200 in growing C2 myoblasts: role in modulating MyoD half-life and myogenic activity. Mol. Cell. Biol. 19, 3767-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann, M., and Fernandez, A. (2001). Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci. 58, 571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, K.A., Meyers, L., and McElwee, S.A. (1990). A role for the Ca(2+)-dependent adhesion molecule, N-cadherin, in myoblasts interaction during myogenesis. Exp. Cell Res. 188, 175-184. [DOI] [PubMed] [Google Scholar]
- Kiyokawa, H., Kineman, R.D., Manova-Todorova, K.O., Soares, V.C., Hoffman, E.S., Ono, M., Khanam, D., Hayday, A.C., Frohman, L.A., and Koff, A. (1996). Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 85, 721-732. [DOI] [PubMed] [Google Scholar]
- Lassar, A., Skapek, S., and Novitch, B. (1994). Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr. Opin. Cell Biol. 6, 788-794. [DOI] [PubMed] [Google Scholar]
- Levenberg, S., Yarden, A., Kam, Z., and Geiger, B. (1999). p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 18, 869-876. [DOI] [PubMed] [Google Scholar]
- Maione, R., and Amati, P. (1997). Interdependence between muscle differentiation and cell cycle control. Biochim. Biophys. Acta 1332, M19-30. [DOI] [PubMed] [Google Scholar]
- Matsushime, H., Quelle, D.E., Shurtleff, S.A., Shibuya, M., Sherr, C.J., and Kato, J.Y. (1994). D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14, 2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege, R.M., Goudou, D., Diaz, C., Nicolet, M., Garcia, L., Geraud, G., and Rieger, F. (1992). N-cadherin and N-CAM in myoblast fusion: compared localisation and effect of blockade by peptides and antibodies. J. Cell Sci. 103, 897-906. [DOI] [PubMed] [Google Scholar]
- Millard, S. S., Yan, J. S., Nguyen, H., Pagano, M.,. Kiyokawa, H., and Koff, A. (1997). Enhanced ribosomal association of 27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem. 272, 7093-7098. [DOI] [PubMed] [Google Scholar]
- Molketin, J.D., and Olson, E. (1996). Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6, 445-453. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard, B. (1978). Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 15, 855-864. [DOI] [PubMed] [Google Scholar]
- Nakatsuji, Y., and Miller, R.H. (2001). Density dependent modulation of cell cycle protein expression in astrocytes. J. Neurosci. Res. 66, 487-496. [DOI] [PubMed] [Google Scholar]
- Nakayama, K., Ishida, N., Shirane, M., Inomata, A., Inoue, T., Shishido, N., Horii, I., and Loh, D.Y. (1996). Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 85, 707-720. [DOI] [PubMed] [Google Scholar]
- Okazaki, K., and Holtzer, H. (1966). Myogenesis: fusion, myosin synthesis, and the mitotic cycle. Proc. Nat. Acad. Sci. USA 56, 1484-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, M., Tam, S.W., Theodoras, A.M., Beer, R.P., Del, S.G., Chau, V., Yew, P.R., Draetta, G.F., and Rolfe, M. (1995). Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27Kip1. Science. 269, 682-685. [DOI] [PubMed] [Google Scholar]
- Perry, R.L.S., and Rudnicki, M. (2000). Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5, D1750-1767. [DOI] [PubMed] [Google Scholar]
- Polyak, K., Lee, M.H., Erdjument-Bromage, H., Koff, A., Tempst, P., Robert, J.M., and Massaguè, J. (1994). Cloning of p27Kip1 a cyclin-Cdk inhibitor and potential mediator of extracellular antimitotic signal. Cell. 78, 59-66. [DOI] [PubMed] [Google Scholar]
- Pouliot, Y., Gravel, M., and Holland, P.C. (1994). Developmental regulation of M-cadherin in the terminal differentiation of skeletal myoblasts. Dev. Dyn. 200, 305-312. [DOI] [PubMed] [Google Scholar]
- Redfield, A., Nieman, M.T., and Knudsen, K.A. (1997). Cadherins promote skeletal muscle differentiation in three-dimensional cultures. J. Cell Biol. 138, 1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud, E.G., Pelpel, K., Guillier, M., Leibovitch, M.P., and Leibovitch, S.A. (1999). p57Kip2 stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts. Mol. Cell. Biol. 19, 7621-7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud, E.G., Leibovitch, M.P., Tintignac, L.A., Pelpel, K., Guillier, M., and Leibovitch, S.A. (2000). Stabilization of MyoD by direct binding to p57(Kip2). J. Biol. Chem. 275, 18767-18776. [DOI] [PubMed] [Google Scholar]
- Russo, S., Tatò, F., and Grossi, M. (1997). Transcriptional down-regulation of Myogenin expression is associated with v-ras-induced block of differentiation in unestablished quail muscle cells. Oncogene. 14, 63-73. [DOI] [PubMed] [Google Scholar]
- Seghatoleslami, M.R., Myers, L., and Knudsen, K.A. (2000). Upregulation of Myogenin by N-cadherin adhesion in three-dimensional cultures of skeletal myogenic BHK cells. J. Cell. Biochem. 77, 252-264. [DOI] [PubMed] [Google Scholar]
- Serrano, M., Lee, H., Chin, L., Cordon-Cardo, C., Beach, D., and DePinho, R.A. (1996). Role of the INK4a locus in tumor suppression and cell mortality. Cell. 85, 27-37. [DOI] [PubMed] [Google Scholar]
- Song, A., Wang, Q., Goebl, M.G., and Harrington, M.A. (1998). Phosphorylation of nuclear MyoD is required for its rapid degradation. Mol. Cell. Biol. 18, 4994-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintignac, L., Leibovitch, M.P., Kitzmann, M., Fernandez, A., Ducommun, B., Meijer, L., and Leibovitch, S.A. (2000). CycE-Cdk2 phosphorylation promotes late G1-phase degradation of MyoD in muscle cells. Exp. Cell Res. 259, 300-307. [DOI] [PubMed] [Google Scholar]
- Vlach, J., Hennecke, S., and Amati, B. (1997). Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. EMBO J. 17, 5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, J., and Perlman, H. (1997). Cell cycle exit upon myogenic differentiation. Curr. Opin, Genet. Dev. 7, 1377-1386. [DOI] [PubMed] [Google Scholar]
- Wei, Q., and Paterson, M. (2001). Regulation of MyoD function in the dividing myoblasts. FEBS Letters. 490, 171-178. [DOI] [PubMed] [Google Scholar]
- Wright, W.E., Sasoon, D.A., and Lin, W.K. (1989). Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 56, 607-617. [DOI] [PubMed] [Google Scholar]
- Zabludoff, S.D., Csete, M., Wagner, R., Yu, X., and Wold, B.J. (1998). p27Kip1 is expressed transiently in developing myotomes and enhances myogenesis. Cell Growth Diff. 9, 1-11. [PubMed] [Google Scholar]
- Zhang, P., Liegeois, N.J., Wong, C., Finegold, M., Hou, H., Thompson, J.C., Silverman, A., Harper, J.W., DePinho, R.A., and Elledge, S.J. (1997). Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 387, 151-158. [DOI] [PubMed] [Google Scholar]
- Zhang, P., Wong, C., DePinho, R.A., Harper, J.W., and Elledge, S.J. (1998). Cooperation between the Cdk inhibitors p27Kip1 and p57Kip2 in the control of tissue growth and development. (1998). Genes Dev. 12, 3162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P., Wong, C., LiuFinegold, D.M., Harper, J.W., and Elledge, S.J. (1999). p21(Cip1) and p57(Kip1) control muscle differentiation at the Myogenin step. Genes Dev. 13, 213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Ma, L., Enkemann, S.A., and Pledger, W.J. (2003). Role of Gadd45α in the density-dependent G1 arrest induced by p27Kip1. Oncogene. 22, 4166-4174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.