Abstract
In addition to providing a regulated linkage between the membrane and the actin cytoskeleton, ezrin participates in signal transduction pathways. Here we describe that expression of the ezrin Y145F mutant delays epithelial cell spreading on fibronectin by inhibiting events leading to FAK activation. The defect in spreading was rescued by the overexpression of catalytically functional Src. We demonstrate that ezrin Y145 is phosphorylated in A431 cells stimulated with epidermal growth factor (EGF) and in v-Src–transformed cells. Moreover in cells devoid of Src, SYF-/- fibroblasts, ezrin Y145 phosphorylation could only be detected upon the introduction of an active form of Src. The phosphorylation of ezrin at Y145 required prior binding of the Src SH2 domain to ezrin. Our results further show that Src activity influences its binding to ezrin and a positive feedback mechanism for Src-mediated Y145 phosphorylation is implied. Interestingly, cells expressing ezrin Y145F did not proliferate when cultured in a 3D collagen gel. Collectively, our results demonstrate a key signaling input of Src-dependent ezrin phosphorylation in adhesion-mediated events in epithelial cells.
INTRODUCTION
Signal transduction through ERM (Ezrin/Radixin/Moesin) proteins has emerged as an important means of coordinating localized and dynamic cellular processes that require membrane cytoskeletal reorganization (Bretscher et al., 2002; Gautreau et al., 2002). By linking the cytoplasmic face of the plasma membrane to the actin cytoskeleton, ERM proteins act as structural scaffolds and provide a platform for the transmission of signals in response to extracellular cues. Ezrin is well documented to participate in several cortical actin-based processes such as membrane projections (Berryman et al., 1995; Lamb et al., 1997; Mackay et al., 1997), cell adhesion (Takeuchi et al., 1994; Hiscox and Jiang, 1999; Pujuguet et al., 2003), cell motility (Crepaldi et al., 1997; Ng et al., 2001), and also in signaling to cell survival (Gautreau et al., 1999). Recent genetic studies in Drosophila described that the loss of the single Drosophila ERM protein disrupted epithelial integrity (Polesello et al., 2002; Speck et al., 2003), further highlighting the crucial function of ERM proteins in regulating cell-signaling events that affect actin organization and polarity of cells.
The active conformation of ezrin is adopted following the disengagement of the N-terminal and C-terminal domain interaction (Gary and Bretscher, 1995; Pearson et al., 2000) achieved by binding phosphatidylinositol 4,5 bisphosphate (PIP2) at the plasma membrane and its subsequent phosphorylation on the C-terminal threonine 567 (Fiévet et al., 2004). The freed C-terminal region binds F-actin (Algrain et al., 1993; Turunen et al., 1994) and the exposed FERM (band four point one/ERM) domain located at the N-terminal region associates either directly or indirectly with a number of transmembrane proteins, namely adhesion molecules and ion exchangers (for review see Bretscher et al., 2002).
Phosphorylation of ezrin is required for both conformational activation and for signaling to downstream events. The “activating” C-terminal threonine phosphorylation on T567 was first described to be downstream of the Rho pathway (Matsui et al., 1998). Additional studies have implicated protein kinase C (PKC) α in the phosphorylation of ezrin T567 (Ng et al., 2001) and PKCθ was demonstrated in vivo to phosphorylate the equivalent conserved threonine residue in moesin (Pietromonaco et al., 1998). Other residues of ezrin have recently been described as targets for serine/threonine kinases. For instance, in gastric parietal cells ezrin S66 phosphorylation is mediated by protein kinase A for acid secretion (Zhou et al., 2003), and phosphorylation of ezrin T235 by cyclin dependent kinase 5 (cdk5) was observed during pRb-induced cell senescence (Yang and Hinds, 2003). Ezrin was initially identified as a substrate for tyrosine phosphorylation by EGFR (Bretscher, 1989) and phosphorylation of residues Y145 and Y353 were detected to high stoichiometry after EGF treatment of human epithelial-derived A431 cells by in vivo 32P biosynthetic labeling, phospho-peptide mapping and site-directed mutagenesis (Krieg and Hunter, 1992). Phosphorylation of ezrin at Y353 has been delineated to signal survival during epithelial cell differentiation via the phosphatidylinositol 3-kinase (PI3K)/Akt pathway (Gautreau et al., 1999), but as of yet the role of ezrin phosphorylation at Y145 is unknown.
Src family kinases are implicated in a wide variety of cellular functions including extracellular matrix–dependent adhesion, spreading, migration, and growth (Parsons and Parsons, 1997). The activity of the Src kinases is regulated through simultaneous dephosphorylation/phosphorylation of specific tyrosine residues. Dephosphorylation of tyrosine 527/529 (avian/murine Src) disrupts the intramolecular association with the N-terminal Src homology 2 (SH2) domain allowing Src activation, which requires autophosphorylation at tyrosine 416/418 (avian/murine Src) in the catalytic domain. We have previously shown that Src and ezrin act cooperatively in enhancing scattering of mammary carcinoma cells (Elliott et al., 2004). Here we investigate the molecular connections between Src and ezrin and address whether Src controls ezrin phosphorylation at Y145. Specifically, we analyze the role of ezrin Y145 phosphorylation in cell adhesion-based signaling.
MATERIALS AND METHODS
Plasmid Constructs and Cell Culture
The plasmids pCB6 encoding VSVG-tagged ezrin Y145F, Y190F, and Y190F/Y204F were obtained by site-directed mutagenesis (Stratagene, La Jolla, CA). The pCB6 plasmids encoding wild-type ezrin and PIP2- ezrin were previously described (Algrain et al., 1993; Barret et al., 2000). The plasmids pGEX-2T coding for ezrin deleted of the last 52 amino acids (ezrin Δ52) and ezrin Δ52 Y145F were obtained by introducing a stop codon by PCR and site-directed mutagenesis, respectively. All plasmids were verified by sequencing. Wild-type Src, Src Y527F, and Src R295F/Y527F (Kmiecik and Shalloway, 1987) were described previously (Hung and Elliott, 2001). GST-Src-SH2 fusion protein was provided by Dr. A Bretscher. LLC PK1 pig kidney–derived epithelial cells (CCL 101; American Type Culture Collection, Manassas, VA) and Madin-Darby canine kidney (MDCK) ts v-Src cells (Behrens et al., 1993) were grown in DMEM (Invitrogen. Carlsbad, CA) containing 10% (vol/vol) fetal bovine serum and 2 mM l-glutamine. LLC-PK1 cells stably expressing VSVG-tagged ezrin mutants Y145F, Y190F, and Y190F/Y204F were derived as previously described (Gautreau et al., 2000) and maintained in selection media containing 0.7 mg/ml geneticin/G418 (Invitrogen). A fivefold expression of VSVG-ezrin Y145F in clones 2 and 3 over endogenous ezrin was observed and these two independent clones were used in all subsequent experiments.
Reagents and Antibodies
Reagents were as follows: PP2 Src inhibitor (4-amino-5-(′-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) (Calbiochem, La Jolla, CA), human recombinant Src (Panvera, Madison, WI), [γ-32P]ATP (Amersham Pharmacia, Piscataway, NJ), fibronectin (FN) from bovine plasma (Sigma, St. Louis, MO), collagen type I from rat tail (Becton Dickinson, Lincoln Park, NJ), dia-filtered HGF obtained from human fibroblast MRC5-conditioned medium (Crepaldi et al., 1997). Primary antibodies were as follows: Affinity-purified rabbit polyclonal anti-VSVG and anti-ezrin were previously described (Gautreau et al., 2000); monoclonal anti-phospho-tyrosine produced from mouse hybridoma 4G10; monoclonal anti-α-tubulin (Amersham); affinity-purified polyclonal to Src-family PTKs, anti-Src Src2, and anti-(P)Y145 ezrin (Santa Cruz Biotechnology, Santa Cruz, CA); polyclonal anti-(P)T567 ezrin (Cell Signaling, Beverly, MA); monoclonal anti-c-Src and anti-avian Src clone EC10 (Upstate Biotechnology, Lake Placid, NY); rabbit polyclonal anti-FAK for western blotting (Upstate Biotechnology), and the monoclonal anti-FAK for immunofluorescence (BD Transduction Laboratories, Lexington, KY); phosphorylation site-specific rabbit polyclonal anti-Src (P)Y418 and anti-FAK (P)Y397 (BioSource, Camarillo, CA).
Immunoprecipitations and GST Pull-downs
Where indicated, cells were pretreated with 0.1 mM pervanadate from a 50 mM stock solution freshly prepared by mixing equal volumes of 0.1 M H2O2 and 0.1 M Na3VO4. For immunoprecipitations, cells were lysed in 1 ml of RIPA lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 10 mM EDTA, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and protease inhibitor cocktail containing 10 μg/ml leupeptin, 10 U/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride) and carried out as previously described. For GST pull-down experiments, cells were lysed in 1 ml of modified lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% SDS, 1 mM EGTA, 1.5 mM MgCl2, 10% glycerol, 1% Triton, 0.5% sodium deoxycholate, 1 mM sodium orthovanadate, and protease inhibitor cocktail) and carried out as previously described.
Src Kinase Assay
Bacterially expressed GST-tagged ezrin proteins were purified according to standard procedures. Equal amounts of GST proteins (10 μg) were subjected to a kinase reaction with 50 ng recombinant Src in a buffer consisting of 20 mM HEPES, pH 7.5, 10 mM MgCl2, 100 μM Na3VO4, and 20 μM ATP (2 μCi [γ-32P]). Reactions were carried out at 30°C for 15 min and stopped in 3× SDS sample buffer, boiled, and resolved by SDS-PAGE. Gels were Coomassie blue stained, dried, and exposed to x-ray film.
Cell Spreading
Subconfluent cells grown in serum-free, CaCl2-free DMEM medium were trypsinized and treated with soybean trypsin inhibitor (0.5 mg/ml). Cells were collected by centrifugation and resuspended in serum-free, CaCl2-free DMEM containing heat-inactivated bovine serum albumin (BSA; 2 μg/ml) and held in suspension for 30 min at 37°C. Cell culture dishes (10 cm2) were precoated with FN (10 μg/ml) in phosphate-buffered saline (PBS) at 37°C for 1 h or overnight at 4°C and rinsed with PBS before use. Suspended cells were distributed onto FN-coated dishes (∼5 × 106 cells/dish) and incubated at 37°C for the indicated times. Phase contrast images were taken with a Leica microscope (Deerfield, IL); cells were classified as spread when flat and contrasted and unspread when round and bright. For biochemical analysis, the attached cells were carefully rinsed in cold PBS, and whole cell extracts were collected in 3× SDS sample buffer, boiled for 5 min, and immediately analyzed by immunoblotting or snap-frozen in liquid nitrogen and stored at -80°C.
Immunofluorescence Staining of Cells
Glass coverslips were coated with FN (10 μg/ml) in PBS for 1 h at 37°C and washed with PBS. Cells were detached by limited trypsin/EDTA, treated with soybean trypsin inhibitor (0.5 mg/ml), and resuspended in serum-free DMEM containing heat-inactivated BSA (2 μg/ml). Cells were distributed on FN-coated coverslips for 1.5 h at 37°C and washed with PBS before fixation with 4% paraformaldehyde for 10 min. Immunofluroescence was carried out as previously described. Cells were viewed using a fluorescence microscope (Leica). Images were acquired with a CCD camera (Princeton Scientific, Monmouth Junction, NJ), analyzed using Metaview Software (Universal Imaging, West Chester, PA) and were processed using Adobe Photoshop Software (San Jose, CA).
3D Collagen Gel Cultures
Collagen type I gels were prepared for tubulogenesis assays as described previously (Crepaldi et al., 1997). Single cells were cultured in gels submerged in medium supplemented with 100 U/ml HGF for 7 d. Gels were then fixed in paraformaldehyde for 30 min and immunofluorescence was carried out as described above but with extended washing times of 5 min each. Gels were mounted onto glass slides in 50% glycerol. Total number of DAPI-stained nuclei were counted in 15 fields. Results are representative of several experiments using different clones.
For quantification of apopotosis, cells were isolated by centrifugation after collagenase treatment of gels (Sigma), 2 mg/ml for 30 min at 37°C. Apoptosis was detected by using the cell death ELISA kit (Roche, Indianapolis, IN) according to the manufacturer's instructions. The absorbance readings taken at 600 nm reveal the relative quantification of fragmented histone complexed DNA resulting from apoptosis.
RESULTS
Lack of Y145 Phosphorylation Results in Delayed Spreading of LLC-PK1 Cells Due to Impaired Stress Fiber and Focal Adhesion Assembly
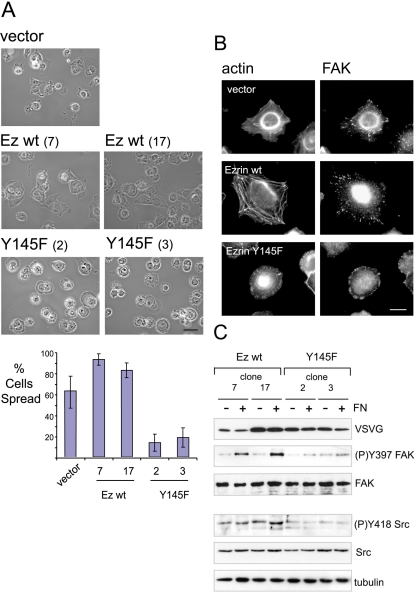
Recently, we reported a functional link between ezrin and the kinase c-Src (Elliott et al., 2004) and the phosphorylation of Y145 ezrin by p60lyk in T-cells was demonstrated (Autero et al., 2003). Because both ezrin and c-Src regulate cell adhesion, we hypothesized that c-Src may act through phosphorylation of ezrin at residue Y145 for the control of cell adhesion. To investigate the role of ezrin Y145 phosphorylation, we stably expressed ezrin Y145F in the LLC-PK1 epithelial cell-line derived from pig kidney. The exogenous protein was tagged at the COOH-terminus with the VSVG epitope and its expression was estimated to be fivefold higher relative to endogenous ezrin. To confirm that the introduced mutation did not affect the folding of ezrin Y145F, the resistance of the FERM domain to chymotrypsin treatment was tested (Gautreau et al., 2003). Ezrin Y145F yielded a similar pattern of digestion when compared with wild-type ezrin (unpublished data). Furthermore, the microvilli localization in polarized cells was unaltered, further indicating that the tyrosine-to-phenylalanine substitution had no major effect on ezrin membrane recruitment (unpublished data). Using two clonal cell lines for each of wild-type and mutant Y145F ezrin, we tested the effects of plating cells onto the extracellular matrix protein fibronectin, which triggers signaling through tyrosine kinases (Figure 1). Following a time course of plating cells onto fibronectin, we observed a significant delay in spreading by cells expressing Y145F ezrin compared with vector control or wild-type ezrin groups. By 1.5 h, an average of 63% of vector control cells and 88% of wild-type ezrin expressing cells were spread (Figure 1A). In contrast, only at most 25% of ezrin Y145F expressing cells were spread. The remaining cells were either partially rounded or completely unspread and notably lacked lamellipodia structures. By 3 h, a greater percentage of Y145F ezrin expressing cells were spread (unpublished data). By staining for focal adhesion kinase (FAK), we observed that focal adhesions in ezrin Y145F expressing cells were concentrated to the periphery of the cell in contrast to vector control cells or cells overexpressing wild-type ezrin, in which a dispersed pattern was observed (Figure 1B). Furthermore, the assembly of actin stress fibers was impaired in cells expressing ezrin Y145F. Because cell adhesion to the substrate was unaffected in the different cells (unpublished data), these data imply a role of ezrin Y145 phosphorylation in fibronectin-induced cell spreading.
Figure 1.
Ezrin Y145F mutant delays cell spreading onto fibronectin. (A) Phase contrast images of LLC-PK1 cells expressing vector alone (vector), ezrin wild-type expressing cells (two independent clones 7 and 17) and ezrin Y145F expressing cells (clones 2 and 3). Scale bar, 50 μm. Suspended cells were plated onto plastic dishes precoated with fibronectin (FN) and allowed to adhere for 1.5 h. Delayed cell spreading is observed with ezrin Y145F mutant expressing cells. For each cell clone, 10 random field views were analyzed for the mean percentage of cells spread shown in barchart; error bars, ±SD. (B) Fluorescence with anti-FAK antibody and phalloidin was performed in cells plated onto fibronectin-coated dishes for 1.5 h. Vector control cells and wild-type ezrin overexpressing cells show stress fibers and a disperse, punctate staining of FAK. In cells expressing ezrin Y145F, the assembly of F-actin is perturbed and FAK is concentrated to the periphery of the nonspread cells. Scale bar, 10 μm. (C) Cells extracts from two independent clones of each wild-type or Y145F ezrin expressing cells were prepared either from suspension cells or after plating onto fibronectin and analyzed by immunoblotting. FN-induced FAK Y397 phosphorylation is detected only in cells expressing wild-type ezrin. Lower phosphorylation level of Src Y418 in the ezrin Y145F expressing cells is detected compared with the wild-type ezrin expressing cells. Anti-tubulin antibody shows equal protein load. The results shown are representative of five independent experiments.
Ezrin Y145F Interferes with Fibronectin-induced FAK Activation in LLC-PK1 Cells
Cell spreading on fibronectin leads to an increase in FAK autophosphorylation concomitant with its activation. Having observed altered spreading by the ezrin Y145F expressing cells, we analyzed the induced FAK activation after 1.5 h of cell attachment onto fibronectin. In cells expressing ezrin Y145F, the induction of FAK Y397 phosphorylation was heavily reduced compared with cells expressing wild-type ezrin (Figure 1C) and vector control cells (unpublished data). Because the full extent of FAK activation is dependent on the catalytic activity of Src family kinases, we analyzed Src activity in the cells by monitoring Src autophosphorylation using an anti-phospho Y418 Src antibody (Cary et al., 2002). We observed lower levels of Src Y418 phosphorylation in cells expressing the Y145F mutant of ezrin compared with cells expressing wild-type ezrin. The level of Src Y418 phosphorylation remained low after 3 h of plating Y145F ezrin expressing cells. However at this time point, FAK Y397 phosphorylation has increased to a level comparable to that of control cells and this increase was concomitant to cell spreading (unpublished data).
Overexpression of Src Can Rescue the Spreading Defect of Ezrin Y145-expressing Cells
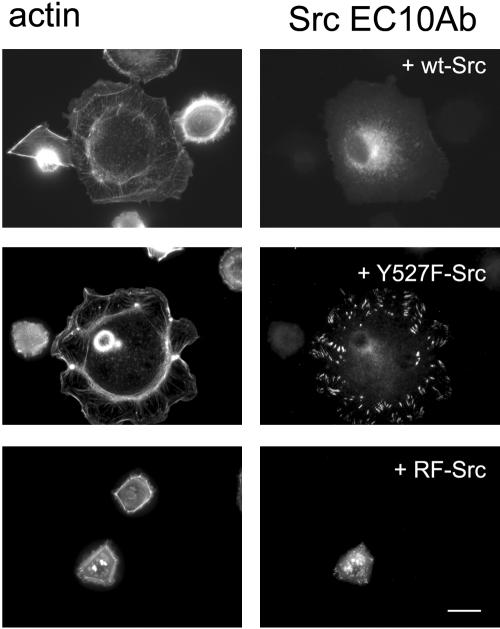
To determine if the observed defect in spreading was a consequence of reduced Src activity, we introduced exogenous Src (pp60src) in the ezrin Y145F expressing cells (Kmiecik and Shalloway, 1987). The overexpression of wild-type Src (wt Src) and activated Src (Y527F-Src), but not the kinase dead version of the Src Y527F mutant (RF-Src), were able to revert the defective spreading phenotype by the ezrin Y145F expressing cells after 1.5 h on fibronectin (Figure 2). Cells transfected with wild-type or activated Src showed larger cell surface extensions on fibronectin substrate. The differential localization, where wt-Src was predominantly perinuclear and Y527F-Src in focal contacts, is similar to previous studies in other cell types (Fincham and Frame, 1998; Schaller et al., 1999). The rescue of the spreading by overexpression of catalytically functional Src supports the notion that ezrin Y145F expression attenuates Src activity and thus Src-mediated cell spreading.
Figure 2.
Src activity is essential for the spreading of LLC-PK1 cells on fibronectin. Cells expressing ezrin Y145F mutant (clone 2 shown) transfected with chicken Src (wt-Src), activated mutant of Src (Y527F-Src), or kinase dead mutant of Src (RF-Src). Cells were stained for F-actin and exogenous Src using an antiavian specific Src antibody (EC10 antibody). Overexpression of wild-type and activated Src but not kinase dead Src in ezrin Y145F cells promotes cell spreading and induces F-actin assembly. Bar, 10 μm.
Direct Phosphorylation of Ezrin Y145 by Src
We next investigated the potential of Src to phosphorylate ezrin at Y145 in LLC PK1 cells. Tyrosine phosphorylation of ezrin was barely detectable after EGF stimulation of LLC-PK1 cells. However we readily detected ezrin tyrosine phosphorylation after treatment of cells with pervanadate for 5 min (Figure 3A). The tyrosine phosphorylation of ezrin was partially sensitive to pretreatment of cells with the Src family kinase inhibitor PP2 (shown) or SU6656 (unpublished data). This corresponded with an almost complete inhibition of phosphorylation at Y145, as detected with a phospho-Y145 specific antibody, indicating that Src kinases control ezrin phosphorylation at Y145. To confirm that phosphorylation of ezrin Y145 is Src dependent under physiological conditions, we observed that EGF stimulation of A431 cells indeed resulted in phosphorylation of endogenous ezrin at Y145 and that this induced phosphorylation was sensitive to the PP2 inhibitor (Figure 3B). In addition, we detected in MDCK epithelial cells expressing a temperature-sensitive (ts) v-Src (Behrens et al., 1993), a time-dependent induction of tyrosine 145 phosphorylation on endogenous ezrin when cells were switched to the permissive temperature activating v-Src (Figure 3C). To further validate that ezrin Y145 phosphorylation was dependent on Src, we exploited the use of SYF-/- (Src-/Fyn-/Yes-) cells. We did not detect Y145 phosphorylation on endogenous ezrin after pervanadate treatment of cells (Figure 3D); however, ezrin Y145 phosphorylation was detected when SYF-/- cells were transfected with an activated form of Src (Src Y527F).
Figure 3.
Ezrin Y145 phosphorylation by Src. (A) Tyrosine phosphorylation ((P)Y) and phosphorylated Y145 ezrin ((P)Y145) were detected on immunoprecipitated VSVG-ezrin from LLC-PK1 cells untreated or treated with 0.1 mM pervanadate for 5 min (-/+ pvd). Pretreatment with the Src kinase inhibitor, PP2 (20 min; 10 μM) inhibits ezrin Y145 phosphorylation. (B) Endogenous ezrin immunoprecipitated from A431 cells. EGF treatment (3 min; 100 nM) induces ezrin Y145 phosphorylation, which is sensitive to the PP2 inhibitor. (C) Immunoprecipitated ezrin from MDCK ts v-Src cells at different times after v-Src activation (40.5°C switched to 35°C). Immunoblots detect time-dependent induced ezrin tyrosine phosphorylation and ezrin (P)Y145 upon v-Src activation. (D) Immunoprecipitated ezrin from SYF-/- cells. Only in cells transfected with Src Y527F is ezrin phosphorylated at Y145. (E) Src directly phosphorylates ezrin at Y145 in vitro. Top: kinase assays show phosphorylation of GST-truncated ezrin (Ez wt Δ52), but not of GST-wild-type ezrin (Ez wt). Reduced phosphorylation was detected on GST-truncated ezrin with the Y145F mutation. Bottom: Coomassie gel stains GST-purified ezrin variants used in the assay. Results are representative of three independent experiments. (F) VSVG-tag immunoprecipitations from cells expressing wild-type ezrin or the PIP2- mutant of ezrin. Pervanadate treatment does not induce Y145 phosphorylation of the ezrin PIP2- mutant.
Next we assessed whether Src directly phosphorylates ezrin Y145 by performing in vitro Src kinase assays using purified GST-tagged versions of ezrin (Figure 3E). Phosphorylation of full-length GST-ezrin (Ez wt) could not be detected in the assay. The full-length protein, however, is known to be in a closed conformation due to the N-/C-terminal intramolecular interaction. In contrast, GST-ezrin deleted of its extreme 52 C-terminal amino acids (Ez wt Δ52), which relieves the N-/C-terminal interaction, was readily phosphorylated by Src, suggesting that the phosphorylatable region is masked when ezrin is in a closed conformation. To confirm that Y145 is a target for Src, a tyrosine-to-phenylalanine point mutation of the truncated ezrin was generated. The incorporation of phosphate on the mutant protein (Y145F Δ52) was strongly reduced, implying that Y145 of ezrin is a major target for Src phosphorylation. In cells, a mutant form of ezrin that is maintained in a closed conformation, the PIP2 binding mutant of ezrin (Fiévet et al., 2004), was not phosphorylated at Y145 (Figure 3F). In conclusion, these results demonstrate that Src is able to directly phosphorylate ezrin at residue Y145, exclusively when ezrin is in an open conformation, i.e., when ezrin is targeted to the membrane where Src is present.
Ezrin Interacts with Src SH2 Domain
Because of the ability of Src kinases to associate with tyrosine-phosphorylated proteins via their SH2 domains, we subsequently addressed whether ezrin itself is a binding partner of Src. By taking the SH2 domain of Src fused with glutathione S-transferase (GST-SH2 Src) as bait, pull-down experiments were performed with extracts from LLC-PK1 cells expressing wild-type ezrin. In pervanadate-treated conditions, a specific interaction of the SH2 domain of Src with ezrin was observed (Figure 4A). To determine whether the Src SH2 binding site on ezrin could be masked by the N-/C-terminal interaction, we performed a pull down with extracts from cells expressing the PIP2 binding mutant of ezrin that is in a closed conformation. The ezrin PIP2- mutant could not interact with the SH2 domain of Src in the same conditions (Figure 4A). These results suggest that Src can bind to tyrosine phosphorylated ezrin specifically in its open conformation.
Figure 4.
Src SH2 domain binding to ezrin precedes Y145 phosphorylation. (A) GST or GST-Src SH2 domain were incubated with extracts of pervanadate-treated cells expressing ezrin wild-type and PIP2- ezrin. The proteins bound to the beads were subjected to SDS-PAGE and immunoblotted. Total cell lysate (VSVG input) and GST proteins (GST) were detected. Wild-type ezrin can interact with the SH2 domain of Src, whereas the PIP2- mutant of ezrin cannot. (B) The mutations Y190F and Y190F/Y240F abolish the interaction of ezrin with the SH2 domain of Src. (C) Reduced tyrosine phosphorylation, (P)Y, was observed on immunoprecipitated ezrin mutants Y145F and Y190F from pervanadate-treated cells compared with wild-type ezrin. Both ezrin Y145F and Y190F mutants showed a complete lack of immunoreactivity with the anti-(P)Y145 ezrin antibody. Results are representations of at least three experiments. (D) Cell extracts from wild-type or Y190F ezrin expressing cells were prepared either from suspension cells or after plating onto fibronectin (FN) and analyzed by immunoblotting. FN induced FAK Y397 phosphorylation is detected only in cells expressing wild-type ezrin. A lower phosphorylation level of Src Y418 is detected in cells expressing ezrin Y190F than in cells expressing wild-type ezrin.
Src SH2 Domain Binding to Ezrin Is a Prerequisite for Y145 Phosphorylation
In pursuit of the Src SH2 docking site on ezrin, we investigated potential interactions with residues Y190 and Y204. These tyrosine residues are located on the ITAM (immunereceptor tyrosine-based activation motif)-like motif present in the ERM proteins (Rozsnyay et al., 1996), which in ezrin comprises the amino acid sequence EYLKIAQDLEMYGINYFEI (189–207). In moesin the equivalent tyrosines were shown to interact with Syk, a nonreceptor tyrosine kinase (Urzainqui et al., 2002). GST-Src SH2 domain pull-down experiments revealed that the single mutation Y190F in itself was sufficient to completely abrogate interaction with the SH2 domain of Src (Figure 4B). Because analysis by chymotrypsin digestion and the localization in cells of the Y190F mutant of ezrin validated that the mutation did not affect the conformation of the protein (unpublished data), these results implied that phosphorylation at Y190 can act as a docking site for Src SH2 domain. Furthermore, immunoprecipitated ezrin Y190F was not phosphorylated at Y145 in contrast to wild-type ezrin (Figure 4C). We tested the effects of plating Y190F ezrin expressing cells onto fibronectin and observed similar defects in cell spreading and the delayed activation of FAK as was demonstrated by cells expressing the ezrin Y145F mutant (Figure 4D). This suggests that the binding of the SH2 domain of Src to phosphorylated Y190 is a prerequisite for ezrin Y145 phosphorylation.
Src Activity Influences Its Interaction with Ezrin
To determine whether the attenuation of Src activity in the ezrin Y145F mutant cells (see Figure 1C) affected Src binding to ezrin Y145F, we performed GST-SH2 Src pulldown experiments from extracts of cells expressing wild-type ezrin or ezrin Y145F. Interestingly, we observed that the SH2 domain of Src could interact with the ezrin Y145F mutant but to a lesser degree when compared with wild-type ezrin (Figure 5A). We could exclude the possibility of phosphorylated Y145 also serving as a Src SH2 docking site, because no interaction was detected with either nonphosphorylated or phosphorylated peptides comprising Y145 (unpublished data). Therefore, the reduced Src SH2 domain interaction with ezrin Y145F was likely due to reduced Y190 phosphorylation. We questioned whether the Src SH2 domain binding to ezrin was dependent on Src-induced phosphorylation. GST-Src SH2 domain pull-down experiments were performed with MDCK cells expressing temperature-sensitive v-Src. The Src SH2 domain could only bind with endogenous ezrin when v-Src was activated in cells (Figure 5B). The results indicate that the ezrin/Src interaction is influenced by Src activity.
Figure 5.
Y145 phosphorylation sustains Src binding to ezrin. (A) GST or GST-Src SH2 domain were incubated with extracts of pervanadate-treated cells expressing ezrin wild-type and Y145F ezrin. Ezrin Y145F shows reduced binding to the SH2 domain of Src compared with ezrin wild-type. (B) GST-Src SH2 domain pull-down experiments were performed with MDCK ts v-Src cells at either the nonpermissive temperature (40.5°C; time 0) or at different times of induced v-Src expression upon switch to the permissive temperature (35°C). An interaction with the SH2 domain of Src and endogenous ezrin is only detected in cells induced to express v-Src.
Ezrin Y145F Expression in LLC-PK1 Cells Inhibits Their Proliferation in 3D Collagen Gel
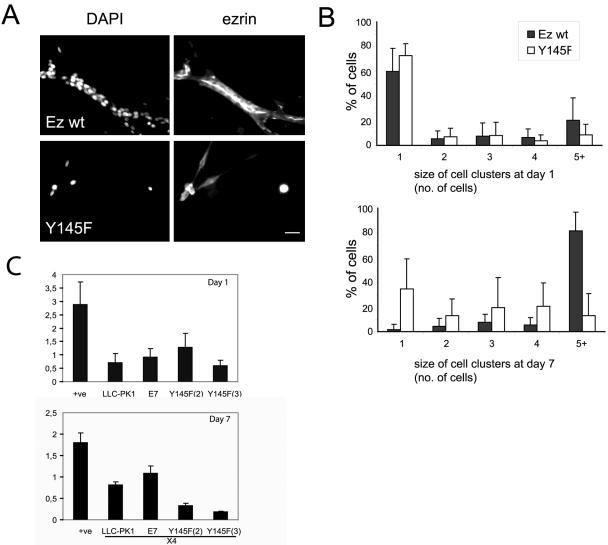
Ezrin has been described as a downstream effector of HGF receptor in the development of tubules (Crepaldi et al., 1997). We analyzed the consequence of the Y145F mutation of ezrin in a tubulogenesis assay. Single LLC-PK1 cells suspended within a 3D collagen matrix undergo clonal growth, form cysts, and in the presence of HGF, develop into tubules. After 7 d of culture in a 3D collagen gel, the cells expressing Y145F ezrin remained as single cells or in small clusters and did not form tubules, a dramatic contrast to cells expressing wild-type ezrin (Figure 6A). In contrast to our previous studies on cells expressing the ezrin Y353F mutant that underwent apoptosis when cultured in 3D collagen gel (Gautreau et al., 1999), ezrin Y145F expressing cells display a proliferation defect. This was assessed by quantification of cell number, which had not increased to the same extent as cells expressing wild-type ezrin (Figure 6B) or by using a cell proliferation assay (unpublished data). We confirmed that the defect was that of proliferation and not increased in cell death by DAPI staining that revealed intact nuclei in ezrin Y145F expressing cells (Figure 6A) and by using a cell death assay that indicated no increase in ezrin Y145F expressing cell death after 7 d of culture in collagen gels (Figure 6C). Interestingly, the defect of cell growth in 3D collagen was not as pronounced when cells were grown on plastic, on which Y145F expressing cell clones appeared only slightly reduced in their proliferation rate (unpublished data). These observations imply a role of ezrin Y145 phosphorylation in the integration of signals from the extracellular matrix and growth factors leading to cell proliferation.
Figure 6.
Proliferation of ezrin Y145F expressing cells is blocked when cultured in 3D collagen gel. Single cells expressing wild-type ezrin (clone 7) or ezrin Y145F mutant (clone 3) were embedded in a 3D collagen gel and cultured for 7 d in the presence of HGF. (A) Fluorescence images at day 7 with DAPI and anti-ezrin antibody. Wild-type ezrin expressing cells form branching tubules with ezrin localized to the inner/apical pole of the tubules. The ezrin Y145F expressing cells are seen as either single cells or in small clusters, with intact nuclei as observed by DAPI staining. Bar, 10 μm. (B) Barcharts show the mean percentage of cells ± SD, from 15 field views, in different sizes of cell clusters at either day 1 or day 7. Ezrin wild-type expressing cells show a shift to increased cell cluster size after day 7, in contrast to Y145F ezrin expressing cells. (C) Apoptosis of untransfected cells (LLC-PK1) and cells expressing either wild-type ezrin (E7) or ezrin Y145F (clones 2 and 3) was measured at day 1 (top graph) and 7 (bottom graph) of culture into collagen gel. At day 7 the values were taken of the cell sample diluted 4 times (4×). (+ve) is a positive control for detection of apoptosis. Error bars, ±SD. Culture of ezrin Y145F expressing cells in collagen does not result in increased cell apoptosis.
DISCUSSION
In this study we have demonstrated that ezrin Y145 is a direct target for phosphorylation by the tyrosine kinase Src, 1) when ezrin is in its open conformation, and 2) subsequent to the binding of Src SH2 domain to the putative Y190 phosphorylation of ezrin. Analysis of the ezrin Y145F mutant in cells indicate that Src-dependent phosphorylation of ezrin plays a significant role in signaling events leading to epithelial cell spreading and proliferation.
Our findings emphasize the importance of the membrane-actin associated form of ezrin for its signaling functions via phosphorylated Y145 in epithelial cells. We determined that exclusively the open conformation of ezrin can be phosphorylated on Y145 by Src, indicating that Y145 is not accessible when the N- and C-terminal domains interact. This finding is corroborated by structural studies of the ezrin FERM domain that speculate the region containing residue Y145 to be highly mobile in the activated FERM domain but fully buried in the dormant state (Smith et al., 2003). We report that binding of Src to ezrin is essential and precedes Y145 phosphorylation. The Src interaction was identified to occur via binding of its SH2 domain to a phosphorylated tyrosine distinct from Y145, at residue Y190. Because of the lack of antibody that specifically recognizes the phosphorylated residue Y190, we could not formally establish that this residue is phosphorylated in our cells. However, the tyrosine to phenylalanine substitution that abolishes a putative phosphorylation at Y190, the first tyrosine in the ITAM-like motif in ezrin (Rozsnyay et al., 1996), was sufficient to abrogate the Src/ezrin interaction and Y145 phosphorylation. Indeed, cells expressing Y190F displayed similar defects in cell spreading on fibronectin as was observed with ezrin Y145F cells (unpublished data), supporting the hypothesis that Y190 phosphorylation is upstream of Y145 phosphorylation. Furthermore we observed that binding of Src SH2 domain to ezrin only occurred with the open, membrane-actin associated form of ezrin, which is in contrast to the ITAM-dependent interaction of Syk with full-length moesin and ezrin (Urzainqui et al., 2002).
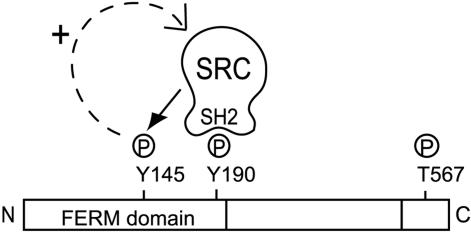
Evidence from this study suggests that a positive feedback loop exists whereby Src-mediated ezrin Y145 phosphorylation sustains Src activity. First, we have seen that Src binding to ezrin is necessary for ezrin Y145 phosphorylation. Second, ezrin Y145 phosphorylation positively influences Src activity because Src Y418 phosphorylation is reduced in Y145F ezrin expressing cells. Third, a reduced Src SH2 domain interaction with the mutant ezrin Y145F was detected. Fourth, Src activity can influence Src SH2 domain binding to ezrin because binding to ezrin from MDCK ts v-Src cells was only detected when v-Src was activated. Together a positive feedback model can be proposed, where Src-mediated ezrin Y145 phosphorylation sustains Src activity that maintains Src binding to ezrin (Figure 7). How ezrin Y145 phosphorylation sustains Src activity is unclear. The activity of Src family kinases is tightly regulated by both positive and negative phosphorylation. Certain protein tyrosine phosphatases (PTPases) serve to activate Src family kinases by dephosphorylation of the C-terminal regulatory tyrosine. Studies of cells lacking PTPases, such as PTPα (Harder et al., 1998) and PTP-PEST (Sastry et al., 2002), have suggested their primary role as Src activators. Tyrosine phosphatases can also indirectly control Src activity through regulating the C-terminal Src kinase, Csk, a natural inhibitor of Src activation. For instance, the Shp2 tyrosine phosphatase has recently been reported in a mechanism of regulating the access of Csk to Src tyrosine kinases (Zhang et al., 2004). A possible function of ezrin Y145 phosphorylation could be to act as a docking site for a Src regulator, giving precedence to a role of ezrin as a scaffold protein.
Figure 7.
Feedback loop model of Src-mediated ezrin Y145 phosphorylation. Binding of Src SH2 domain to the putative phosphorylated Y190 is required and precedes Y145 phosphorylation. Ezrin Y145 phosphorylation affects the activation status of Src. The Src/ezrin interaction, by being dependent on Src activity, is positively influenced by ezrin Y145 phosphorylation.
Y145 is conserved in all three ERM proteins and it can be speculated that this phosphorylation occurs in a cell-type/function–specific manner involving other members of Src family tyrosine kinases. For instance, Y145 of ezrin is a substrate for Lck in T-cells (Autero et al., 2003). We demonstrate that Src phosphorylated Y145 ezrin participates in cell-matrix adhesion signaling in epithelial cells. The defective spreading observed by cells expressing the ezrin Y145F mutant on fibronectin was associated with the impairment of FAK activation. It is known that the activation of FAK upon the engagement of integrins to the extracellular matrix has an absolute requirement for the activity of Src family kinases (Fincham and Frame, 1998; Klinghoffer et al., 1999). The expression of ezrin Y145F in cells resulted in reduced Src Y418 autophosphorylation levels, suggesting that the defect in FAK activation was a consequence of attenuated Src activity. Furthermore, the rescued spreading of ezrin Y145F expressing cells by the introduction of catalytically functional Src, supports the notion that ezrin influences the activity of Src in signaling events required for cell spreading. Because in nonconfluent cells ezrin is found in both the dorsal microvilli and membrane projections such as lamellipodia and filopodia (unpublished data), it is plausible that ezrin can be found in a multiprotein complex with FAK and Src, as we have previously described that ezrin can interact with FAK (Poullet et al., 2001).
We describe a striking defect in cell growth by the ezrin Y145F expressing cells when cultured in a 3D collagen type I matrix, uncovering a novel function of ezrin in signaling cell proliferation in a three-dimensional environment. Epithelial cell tubulogenesis triggered by HGF involves a complex process of proliferation, adhesion, migration, and differentiation (reviewed in Rosario and Birchmeier, 2003). In line with our observation that the spreading of cells expressing ezrin Y145F is impaired when grown on fibronectin and that this defect can be reverted by overexpressing Src, we propose that signaling triggered by HGF is impaired when these cells are grown in the collagen matrix. Indeed, it has been reported that integrin signaling is up-regulated by HGF and this regulation is necessary for proliferation, motility, and branching morphogenesis (Saelman et al., 1995; Chiu et al., 2002). It has also been reported that HGF activity requires Src kinase. Src interaction with the HGF receptor, Met, is required for cellular proliferation, motility, and transformation mediated by Met (Ponzetto et al., 1994; Rahimi et al., 1998). Thus the counter effect of ezrin Y145 phosphorylation on Src may contribute to cell proliferation
We cannot completely exclude that the tubulogenesis defect observed with cells expressing ezrin Y145F is not dependent on Src activity. For example, the lack of tubules with cells expressing ezrin Y145F in a collagen matrix could be due to their inability to establish contacts. This seems unlikely because cells expressing an ezrin mutant (ezrin T567D) that impairs cell-cell adhesion do not form tubules but proliferate and form aggregates in a collagen matrix (Gautreau et al., 2000). Alternatively, the ERK pathway, which is essential for tubulogenesis (Khwaja et al., 1998; O'Brien et al., 2004) could be implicated downstream of ezrin Y145 phosphorylation. It remains to be investigated through which pathways ezrin signals epithelial cell proliferation in a 3D collagen gel.
The use of different forms of ezrin containing point mutations allowed us to uncover ezrin functions essential for tubulogenesis. Our data emphasize the need for a correct activation of the protein, which is itself necessary for its proper localization (Fiévet et al., 2004). This is also a prerequisite for the signaling functions of the protein because tyrosine phosphorylation is detected only when ezrin is in an active form, indicating that the structural and signaling functions of ezrin are linked. How the different functions performed by activated ezrin are coordinated during epithelial cell morphogenesis remains to be determined. Our data suggest that ezrin is involved at different steps of epithelial cell morphogenesis including the formation of cell-cell and cell-matrix adhesion and later during terminal differentiation in the formation of the apical surface. Proper cell-cell and cell-matrix adhesion are also required for the transmission of proliferation and survival signals. These results highlight the importance of spatial cellular environment and the integration of signals from the extracellular matrix and growth factors for the control of cell morphogenesis through ezrin.
A number of studies have implicated ezrin in cell transformation, invasion, and metastasis (for review see McClatchey, 2003). Because elevated Src activity is also associated with tumor progression, one possibility is that ezrin and Src cooperate in several functions such as migration, invasion, and adhesion during cancer development.
Acknowledgments
We are grateful to Drs Alexis Gautreau, Sandrine Etienne-Manneville, and Philippe Pujuguet for critical reading of the manuscript. We thank Dr. A. Bretscher for the kind gift of pGEX-SH2 Src domain and Drs. J. Behrens and W. Birchmeier for the MDCK ts v-Src cell line. This work was supported by grants from the Association pour la Recherche contre le cancer (ARC 4601), from the Ligue Nationale contre le Cancer (Equipe Labelisée to M.A.), and from the Canadian Breast Cancer Research Alliance to B.E.E. (grant 04315). J.S. is a recipient of a fellowship from Institut Curie and Centre National de la Recherche Scientifique (CNRS).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0721) on January 12, 2005.
References
- Algrain, M., Turunen, O., Vaheri, A., Louvard, D., and Arpin, M. (1993). Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 120, 129-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autero, M., Heiska, L., Ronnstrand, L., Vaheri, A., Gahmberg, C. G., and Carpen, O. (2003). Ezrin is a substrate for Lck in T cells. FEBS Lett. 535, 82-86. [DOI] [PubMed] [Google Scholar]
- Barret, C., Roy, C., Montcourrier, P., Mangeat, P., and Niggli, V. (2000). Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP(2)) binding site in the NH(2)-terminal domain of ezrin correlates with its altered cellular distribution. J. Cell Biol. 151, 1067-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens, J., Vakaet, L., Friis, R., Winterhager, E., Van Roy, F., Mareel, M. M., and Birchmeier, W. (1993). Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120, 757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman, M., Gary, R., and Bretscher, A. (1995). Ezrin oligomers are major cytoskeletal components of placental microvilli: a proposal for their involvement in cortical morphogenesis. J. Cell Biol. 131, 1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A. (1989). Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by EGF. J. Cell Biol. 108, 921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A., Edwards, K., and Fehon, R. G. (2002). ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell. Biol. 3, 586-599. [DOI] [PubMed] [Google Scholar]
- Cary, L. A., Klinghoffer, R. A., Sachsenmaier, C., and Cooper, J. A. (2002). SRC catalytic but not scaffolding function is needed for integrin-regulated tyrosine phosphorylation, cell migration, and cell spreading. Mol. Cell. Biol. 22, 2427-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, S. J., Jiang, S. T., Wang, Y. K., and Tang, M. J. (2002). Hepatocyte growth factor upregulates alpha2beta1 integrin in Madin-Darby canine kidney cells: implications in tubulogenesis. J. Biomed. Sci. 9, 261-272. [DOI] [PubMed] [Google Scholar]
- Crepaldi, T., Gautreau, A., Comoglio, P. M., Louvard, D., and Arpin, M. (1997). Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 138, 423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, B. E., Qiao, H., Louvard, D., and Arpin, M. (2004). Co-operative effect of c-Src and ezrin in deregulation of cell-cell contacts and scattering of mammary carcinoma cells. J. Cell. Biochem. 92, 16-28. [DOI] [PubMed] [Google Scholar]
- Fiévet, B. T., Gautreau, A., Roy, C., Del Maestro, L., Mangeat, P., Louvard, D., and Arpin, M. (2004). Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 164, 653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham, V. J., and Frame, M. C. (1998). The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 17, 81-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary, R., and Bretscher, A. (1995). Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell 6, 1061-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau, A., Fiévet, B. T., Brault, E., Antony, C., Houdusse, A., Louvard, D., and Arpin, M. (2003). Isolation and characterization of an aggresome determinant in the NF2 tumor suppressor. J. Biol. Chem. 278, 6235-6242.Epub 2002 Dec 6235. [DOI] [PubMed] [Google Scholar]
- Gautreau, A., Louvard, D., and Arpin, M. (2000). Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J. Cell Biol. 150, 193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautreau, A., Louvard, D., and Arpin, M. (2002). ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr. Opin. Cell Biol. 14, 104-109. [DOI] [PubMed] [Google Scholar]
- Gautreau, A., Poullet, P., Louvard, D., and Arpin, M. (1999). Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 96, 7300-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, K. W., Moller, N. P., Peacock, J. W., and Jirik, F. R. (1998). Protein-tyrosine phosphatase alpha regulates Src family kinases and alters cell-substratum adhesion. J. Biol. Chem. 273, 31890-31900. [DOI] [PubMed] [Google Scholar]
- Hiscox, S., and Jiang, W. G. (1999). Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J. Cell Sci. 112, 3081-3090. [DOI] [PubMed] [Google Scholar]
- Hung, W., and Elliott, B. (2001). Co-operative effect of c-Src tyrosine kinase and Stat3 in activation of hepatocyte growth factor expression in mammary carcinoma cells. J. Biol. Chem. 276, 12395-12403. [DOI] [PubMed] [Google Scholar]
- Khwaja, A., Lehmann, K., Marte, B. M., and Downward, J. (1998). Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J. Biol. Chem. 273, 18793-18801. [DOI] [PubMed] [Google Scholar]
- Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A., and Soriano, P. (1999). Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmiecik, T. E., and Shalloway, D. (1987). Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49, 65-73. [DOI] [PubMed] [Google Scholar]
- Krieg, J., and Hunter, T. (1992). Identification of the two major EGF-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J. Biol. Chem. 267, 19258-19265. [PubMed] [Google Scholar]
- Lamb, R. F., Ozanne, B. W., Roy, C., McGarry, L., Stipp, C., Mangeat, P., and Jay, D. G. (1997). Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr. Biol. 7, 682-688. [DOI] [PubMed] [Google Scholar]
- Mackay, D. J., Esch, F., Furthmayr, H., and Hall, A. (1997). Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J. Cell Biol. 138, 927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, T., Maeda, M., Doi, Y., Yonemura, S., Amano, M., Kaibuchi, K., and Tsukita, S. (1998). Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 140, 647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey, A. I. (2003). Merlin and ERM proteins: unappreciated roles in cancer development? Nat. Rev. Cancer 3, 877-883. [DOI] [PubMed] [Google Scholar]
- Ng, T. et al. (2001). Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 20, 2723-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, L. E., Tang, K., Kats, E. S., Schutz-Geschwender, A., Lipschutz, J. H., and Mostov, K. E. (2004). ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7, 21-32. [DOI] [PubMed] [Google Scholar]
- Parsons, J. T., and Parsons, S. J. (1997). Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell Biol. 9, 187-192. [DOI] [PubMed] [Google Scholar]
- Pearson, M. A., Reczek, D., Bretscher, A., and Karplus, P. A. (2000). Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101, 259-270. [DOI] [PubMed] [Google Scholar]
- Pietromonaco, S. F., Simons, P. C., Altman, A., and Elias, L. (1998). PKC-theta phosphorylation of moesin in the actin-binding sequence. J. Biol. Chem. 273, 7594-7603. [DOI] [PubMed] [Google Scholar]
- Polesello, C., Delon, I., Valenti, P., Ferrer, P., and Payre, F. (2002). Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat. Cell Biol. 4, 782-789. [DOI] [PubMed] [Google Scholar]
- Ponzetto, C., Bardelli, A., Zhen, Z., Maina, F., dalla Zonca, P., Giordano, S., Graziani, A., Panayotou, G., and Comoglio, P. M. (1994). A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77, 261-271. [DOI] [PubMed] [Google Scholar]
- Poullet, P., Gautreau, A., Kadare, G., Girault, J. A., Louvard, D., and Arpin, M. (2001). Ezrin interacts with focal adhesion kinase and induces its activation independently of cell-matrix adhesion. J. Biol. Chem. 276, 37686-37691. [DOI] [PubMed] [Google Scholar]
- Pujuguet, P., Del Maestro, L., Gautreau, A., Louvard, D., and Arpin, M. (2003). Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol. Biol. Cell 14, 2181-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi, N., Hung, W., Tremblay, E., Saulnier, R., and Elliott, B. (1998). c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J. Biol. Chem. 273, 33714-33721. [DOI] [PubMed] [Google Scholar]
- Rosario, M., and Birchmeier, W. (2003). How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 13, 328-335. [DOI] [PubMed] [Google Scholar]
- Rozsnyay, Z., Sarmay, G., Zoller, M., and Gergely, J. (1996). Membrane-bound ezrin is involved in B-cell receptor-mediated signaling: potential role of an ITAM-like ezrin motif. Immunol. Lett. 54, 163-169. [DOI] [PubMed] [Google Scholar]
- Saelman, E. U., Keely, P. J., and Santoro, S. A. (1995). Loss of MDCK cell alpha 2 beta 1 integrin expression results in reduced cyst formation, failure of hepatocyte growth factor/scatter factor-induced branching morphogenesis, and increased apoptosis. J. Cell Sci. 108, 3531-3540. [DOI] [PubMed] [Google Scholar]
- Sastry, S. K., Lyons, P. D., Schaller, M. D., and Burridge, K. (2002). PTP-PEST controls motility through regulation of Rac1. J. Cell Sci. 115, 4305-4316. [DOI] [PubMed] [Google Scholar]
- Schaller, M. D., Hildebrand, J. D., and Parsons, J. T. (1999). Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol. Biol. Cell 10, 3489-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. J., Nassar, N., Bretscher, A., Cerione, R. A., and Karplus, P. A. (2003). Structure of the active N-terminal domain of Ezrin. Conformational and mobility changes identify keystone interactions. J. Biol. Chem. 278, 4949-4956. [DOI] [PubMed] [Google Scholar]
- Speck, O., Hughes, S. C., Noren, N. K., Kulikauskas, R. M., and Fehon, R. G. (2003). Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature 421, 83-87. [DOI] [PubMed] [Google Scholar]
- Takeuchi, K., Sato, N., Kasahara, H., Funayama, N., Nagafuchi, A., Yonemura, S., and Tsukita, S. (1994). Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J. Cell Biol. 125, 1371-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen, O., Wahlstrom, T., and Vaheri, A. (1994). Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J. Cell Biol. 126, 1445-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzainqui, A. et al. (2002). ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity 17, 401-412. [DOI] [PubMed] [Google Scholar]
- Yang, H. S., and Hinds, P. W. (2003). Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol. Cell 11, 1163-1176. [DOI] [PubMed] [Google Scholar]
- Zhang, S. Q. et al. (2004). Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol. Cell 13, 341-355. [DOI] [PubMed] [Google Scholar]
- Zhou, R., Cao, X., Watson, C., Miao, Y., Guo, Z., Forte, J. G., and Yao, X. (2003). Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J. Biol. Chem. 278, 35651-35659. [DOI] [PubMed] [Google Scholar]