Abstract
Objective:
A proinflammatory imbalance in the tumor necrosis factor (TNF) system may contribute to the pathogenesis of schizophrenia (SCZ) and bipolar disorders (BDs) and related comorbidities. We investigated the relative distribution of TNF-related molecules in blood and dorsolateral prefrontal cortex (DLPFC) in these disorders.
Method:
We measured plasma levels of TNF, soluble TNF receptor 1 (sTNFR1), soluble TNF receptor 2 (sTNFR2), and a disintegrin and metalloprotease-17 (ADAM17) using enzyme immunoassays and calculated the TNF/sTNFRs ratio (TNF/sTNFR1+sTNFR2) in a sample of 816 SCZ and BD spectrum patients and 624 healthy controls (HCs). TNF, TNFRSF1A (TNFR1), TNFRSF1B (TNFR2), and ADAM17 mRNA levels were determined in whole blood, and postmortem DLPFC obtained from an independent cohort (n = 80 SCZ, n = 44 BD, and n = 86 HC).
Results:
In peripheral blood, we show increased TNF-related measures in patients compared to HC, with an increased TNF/sTNFRs ratio (p = 6.00 × 10−5), but decreased TNF mRNA expression (p = 1 × 10−4), with no differences between SCZ and BD. Whole blood ADAM17 mRNA expression was markedly higher in BD vs SCZ patients (p = 1.40 × 10−14) and vs HC (p = 1.22 × 10−8). In postmortem DLPFC, we found no significant differences in mRNA expression of TNF pathway genes between any groups.
Conclusions:
SCZ and BD patients have increased plasma TNF pathway markers without corresponding increase in blood cell gene expression. ADAM17 expression in leukocytes is markedly different between the two disorders, while alterations in TNF-related gene expression in DLPFC are uncertain. Further studies are necessary to elucidate the aberrant regulation of the TNF pathway in severe mental disorders.
Keywords: DLPFC, cytokines, working memory, mRNA, postmortem
Introduction
A dysregulation of the immune system due to chronically activated macrophages and T cells has been proposed to contribute to the pathogenesis of schizophrenia (SCZ)1 and bipolar disorder (BD).2 Several lines of evidence support a proinflammatory profile in both diseases, where alterations in the tumor necrosis factor (TNF) pathway, consisting of soluble and membrane-bound TNF (formerly TNF-alpha) and its two receptors, have been reported in peripheral blood.2,3 However, the regulation and the site of production of TNF-related molecules in these disorders are far from clear.
TNF is a proinflammatory cytokine expressed by macrophages, other leukocyte subsets, and endothelial cells,4–6 as well as by neurons, astrocytes, and microglia that have a macrophage phenotype. It signals through two distinct membrane-bound receptors: TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2) of which TNFR1 is widely distributed in a range of cells and tissues whereas TNFR2 is more selectively expressed by immune cells, endothelial cells, specific neuronal subtypes, and glia cells.7 Under physiological conditions, TNF has been known to regulate synaptic transmission, neurotransmission, homeostatic synaptic scaling, neurogenesis, and long term potentiation.8–10 TNF and its receptors are produced as transmembrane proteins with an extracellular domain that can be proteolytically cleaved from the cell surface by metalloproteases like a disintegrin and metalloprotease-17 (ADAM17) resulting in soluble TNF, soluble (s)TNFR1 and sTNFR2. Increased circulating TNF, sTNFR1, and sTNFR2 levels have been reported by several studies in SCZ3 and BD,2,11 but to this end, no large studies have evaluated if this leads to a proinflammatory imbalance between TNF and its soluble receptors, ie, the TNF/sTNFRs ratio that reflects activity in the TNF system and correlates with TNF bioactivity.12
Previously, modestly sized studies have demonstrated increased TNF mRNA expression in monocytes and lymphocytes in SCZ and BD.13–15 To our knowledge, however, TNF, TNF receptor superfamily [TNFRSF] 1A, and TNFRSF1B (denoted by TNFR1 and TNFR2, respectively, in the present manuscript), and ADAM17 mRNA expression in whole blood cells have not been investigated in a well-powered sample of patients with severe mental disorders.
The dorsolateral prefrontal cortex (DLPFC) is associated with a range of complex behaviors frequently referred to as executive functions, including working memory,16 cognitive and behavioral flexibility, and abstract reasoning, all of which have been implicated in SCZ and to a lesser extent in BD.17–19 We have previously found that general cognitive abilities were negatively associated with plasma TNFR1 levels in adults with SCZ and BD.20 Moreover, a postmortem study found elevated levels of TNFR1 mRNA in the frontal cortex in SCZ and increased transmembrane TNF in BD suggesting alterations in the TNF pathway in the central nervous system (CNS) in both illnesses.21 However, large postmortem studies of TNF pathway gene expression in the DLPFC are lacking.
The aim of the present study was to further determine the role of TNF pathway–related molecules at the protein and mRNA levels in BD and SCZ patients. First we investigated these markers, including the balance between TNF and its receptors, in peripheral blood in a large cohort (n = 1440). We then investigated whether TNF proteins and their proportion are associated with working memory, a task affiliated with the DLPFC. Lastly, mRNA levels of these molecules were examined in the DLPFC in an independent cohort (n = 210). We hypothesized that patients with SCZ and BD would present with a distinct systemic and cortical pattern of TNF pathway markers reflecting the relative contribution of these different compartments to the sustained systemic TNF activation that is proposed to be operating in these patients.
Methods
Plasma and Leukocyte Cohort
Study Design and Ethics.
The TOP Study at the NORMENT Centre, Oslo University Hospital, and collaborating Norwegian hospitals22 was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. The biobank was approved by the Norwegian Directorate of Health. All participants provided written informed consent after receiving a description of the study.
Participants.
The main inclusion criteria were DSM-IV diagnoses of SCZ spectrum disorders or bipolar spectrum disorders, IQ > 70 and age between 18 and 65 years (for details see22). Healthy volunteers without any history of severe psychiatric disorders (or in any of their first-degree relatives), or substance/alcohol abuse/dependency from the same catchment area were randomly selected from the National Population Registry (www.ssb.no). (For details see22) For the present analyses, patients and controls were not included if they had coexisting autoimmune or inflammatory disease, cancer, ongoing infections, used anti-inflammatory drugs, or had C-reactive protein levels above 20 mg/L.
Clinical Assessments.
Diagnosis was obtained using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). Clinical symptoms were evaluated using the Young Mania Rating Scale (YMRS), Inventory of Depressive Symptoms (IDS), Calgary Depression Scale for Schizophrenia (CDSS), Positive and Negative Syndrome Scale (PANSS), while functioning was measured using the Global Assessment of Functioning split version function (GAF-F) and symptom scale (GAF-S). The clinical assessment team consisted of clinical psychologists and psychiatrists, who were all trained until satisfactory inter-rater reliability was obtained.23,24 Psychotic symptoms were examined in the SCZ group using the PANSS 5 factorial model,25 while depressive and hypomanic/manic symptoms were investigated in the BD group using YMRS, IDS, and CDSS.
Neurocognitive Assessment.
Psychologists trained in standardized neuropsychological testing performed neurocognitive assessment. Working memory was assessed with the Digit Span Test—backward (Wechsler Adult Intelligence Scale [WAIS]-III), letter number sequencing (WAIS-III), and the Working Memory—Mental Arithmetic (WM-MA) Test—commissions.26 For details see27.
Cytokine Assessment.
We used enzyme-linked immunosorbent assay to quantify protein levels due to its high specificity and sensitivity28 and measured plasma levels of TNF using a high sensitivity enzyme immunoassay from Cloud Corp (Housten, TX) while sTNFR1, sTNFR2, and sADAM17 were analyzed using EIAs from R&D systems (Minneapolis, MN). Intra- and inter-assay coefficients of variance for proteins were less than 10%. The ratio between TNF and sTNFRs may provide an estimate of the molar balance in serum between TNF molecules and sTNFRs. In molecular terms, this ratio was defined as TNF (pmol/L)/(sTNFR1 + sTNFR2)(pmol/L) × 100, assuming a molecular mass of (17 × 3) kD and 30 kD for TNF (trimer) and both types of sTNFRs, respectively.
RNA Isolation and RT-PCR.
Total RNA was isolated from whole blood using the Tempus 12-Port Isolation kit (Applied Biosystems; Ambion, Austin, TX) and quantified using the ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The tubes were stored at −80°C. Reverse transcription was performed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) with ~0.5 μg total RNA in 96-well PCR plates (Applied Biosystems). Each plate contained 80 samples that were randomly distributed to atone for the differences in reverse transcription or subsequent real-time PCR efficiency.
Quantification of mRNA is described in detail in supplementary material. We used Primer Express software version 3.0 (Applied Biosystems) to design sequence-specific mRNA (primer spanning exon-exon junction) oligonucleotide primers for the full-length TNF, TNFR1, TNFR2, and ADAM17 mRNA. Melt curves were evaluated for all primers. Data were normalized to β-actin.
Brain Cohort
Postmortem Brain Sample Collection.
Details of tissue acquisition, handling, processing, dissection, clinical characterization, diagnoses, neuropathological examinations, RNA extraction, and quality control measures were described previously.29 Toxicological analysis was performed on every case. For control cases, subjects with evidence of macro- or microscopic neuropathology, drug use, alcohol abuse, or psychiatric illness were excluded.
Postmortem Brain RNA Extraction and Sequencing.
Details of postmortem DLPFC RNA extraction and sequencing, and RNA Seq data processing were previously described.30 Total RNA was extracted from ~100 mg of postmortem tissue homogenates of DLPFC gray matter approximating BA9/46 in postnatal samples using the RNeasy kit (Qiagen) according to the manufacturer’s protocol. The poly(A)-containing RNA molecules were purified from 1 μg DNase-treated total RNA and, following purification, fragmented into small pieces using divalent cations under elevated temperature. Reverse transcriptase and random primers were used to copy the cleaved RNA fragments into first-strand cDNA, and the second-strand cDNA was synthesized using DNA polymerase I and RNase H. We performed the sequencing library construction using the TruSeq RNA Sample Preparation v2 kit by Illumina (see supplementary material for details).
Postmortem Analyses: RNA Sequencing Data Processing.
The Illumina Real Time Analysis module performed image analysis, base calling, and ran the BCL converter (CASAVA v1.8.2), generating FASTQ files containing the sequencing reads. These reads were aligned to the human genome (UCSC hg19 build) using the spliced-read mapper TopHat (v2.0.4) using the reference transcriptome to initially guide alignment, on the basis of known transcripts of Ensemble Build GRCh37.67 (the “-G” argument in the software). A normalized reads per kilobase million (RPKM) metric were calculated for each gene by dividing the number of reads mapping to the gene divided by the length of the gene (in kilobases).
Statistical Analysis
Plasma and Leukocyte Cohort Analyses.
Statistical analyses were performed using the SPSS software package for Windows, version 22.0. Data normality was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Differences in demographic data between groups were investigated using the chi-square test for categorical variables, the Kruskal–Wallis test for continuous variables, and Tukey and the Mann–Whitney U test for post hoc analyses. We used t tests for normally distributed variables, and nonparametric tests (Mann–Whitney U test) for skewed distributions to investigate differences between groups. Correlations were examined by using Spearman’s rank correlation.
Potential confounders (age, sex, smoking status, body mass index, time for blood sampling, and race) were investigated using nonparametric tests (Spearman’s rank correlation and Mann–Whitney U test), and confounders with a P value lower than 0.2 were controlled for in linear regression models. Results are given as standardized beta or t test from the regression analyses.
We calculated an aggregate working memory score from the three working memory tasks and controlled for age, gender, diagnosis and investigated interaction effects of diagnosis in general linear models.
We corrected for multiple testing according to Bonferroni, and alpha was set at P < .007 for the main analyses in the circulation (correcting for seven tests: TNF, sTNFR2, sADAM17, TNF mRNA, TNFR1 mRNA, TNFR2 mRNA, and ADAM17 mRNA) and P < 0.01 for the aggregate working memory score.
Postmortem Analyses.
Differential expression analysis was performed using LIMMA R package.31 For SCZ vs HC samples, gene RPKM was regressed against a binary diagnosis variable, covarying for eight principal components and covariates of age, sex, and mitochondrial mapping rate, with the number of components determined for the expression matrix of a set of 562 immune genes (Birnbaum et al, under review) using the sva R package (surrogate variable analysis).32 The optimum number of components was determined based on an iterative algorithm to remove the impact of mRNA quality on differences in expression between patients and controls that is not accounted for by controlling only for RNA integrity number (RIN) and demographic variables. For BD vs HC samples, gene RPKM was regressed against a binary diagnosis variable, covarying in this case for 10 principal components and age, sex, and mitochondrial mapping rate. A multiple testing correction, false discovery rate (FDR), was employed to correct for all immune genes in the matrix of immune genes from which the principal components were derived.
Results
Demographics and Clinical Characteristics
The sociodemographic and clinical characteristics of the participants are presented in tables 1 and 2.
Table 1.
Demographic and Clinical Characteristics of Participants
| Parameters | Plasma (cytokine/protein) Cohort | Leukocyte (mRNA) Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| SCZ | BD | HC | Post Hoc Analysis | SCZ | BD | HC | Post Hoc Analysis | |
| (N = 569) | (N = 247) | (N = 624) | (N = 224) | (N = 143) | (N = 184) | |||
| Male sex, N (%) | 340 (59.8) | 103 (41.7) | 330 (52.9) | SCZ > HC > BD | 127 (56.7) | 51 (35.7) | 108 (58.7) | HC, SCZ > BD |
| Ethnicity (Caucasian) | 453 (79.6) | 222 (89.9) | 611 (97.9) | HC > BD > SCZ | 178 (79.5) | 132 (92.3) | 184 (100) | HC > BD > SCZ |
| Tobacco (users)a | 300 (54.8) | 131 (54.6) | 55 (19.9) | SCZ, BD > HC | 118 (57.3) | 66 (56.4) | 24 (20.5) | SCZ, BD > HC |
| Medication | ||||||||
| Antipsychotics | 488 (85.8) | 128 (51.8) | — | SCZ > BD | 189 (84.4) | 76 (53.1) | — | SCZ > BD |
| Lithium | 9 (1.6) | 50 (20.3) | — | BD > SCZ | 4 (1.8) | 20 (14.0) | — | BD > SCZ |
| Antidepressants | 179 (31.5) | 95 (38.8) | — | BD > SCZ | 36 (16.1) | 65 (46.1) | — | BD > SCZ |
| Mood stabilizers | 76 (13.4) | 98 (40.2) | — | BD > SCZ | 61 (27.2) | 54 (38.0) | — | BD > SCZ |
| Age (years) | 27 (15) | 32 (18) | 32 (13) | BD, HC > SCZ | 29 (15) | 31 (16) | 31.5 (13) | NS |
| Body mass indexb | 25.7 (7.1) | 25.4 (5.6) | 24.4 (4.4) | BD, SCZ > HC | 25.9 (7.4) | 25.4 (5) | 24.5 (4.29) | SCZ > HC |
| Duration of illness (years) | 4 (8) | 9 (13) | — | BD > SCZ | 4.5 (7.8) | 8 (10.5) | — | BD > SCZ |
| Time of blood samplingc | 09:45 (1:10) | 09:27 (0:55) | 12:42 (6:50) | HC > SCZ > BD | 09:50 (1:00) | 09:30 (1:00) | 11:10 (6:50) | HC > SCZ > BD |
| PANSS total score | 62 (22) | 45 (13) | — | SCZ > BD | 63 (22) | 44 (13) | — | SCZ > BD |
| YMRS total score | 4.0 (9) | 2 (6) | — | SCZ > BD | 5 (9) | 1 (4) | — | SCZ > BD |
| IDS total score | 17 (20) | 15 (18) | — | NS | 17 (21) | 14 (19) | — | NS |
| CDSS total score | 5 (7) | 3 (6) | — | SCZ > BD | 5 (8) | 2.5 (7) | — | SCZ > BD |
| GAF-S | 40 (13) | 55 (16) | — | BD > SCZ | 39 (13) | 57 (15) | — | BD > SCZ |
| GAF-F | 41 (15) | 50 (20) | — | BD > SCZ | 40 (13) | 51 (17) | — | BD > SCZ |
Note: HC, healthy control; SCZ, schizophrenia spectrum; BD, bipolar disorder; NS, nonsignificant; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; IDS, Inventory of Depressive Symptoms; CDSS, Calgary Depression Scale for Schizophrenia; GAF-F, Global Assessment of Functioning—Function Scale; GAF-S, Global Assessment of Functioning—Symptom Scale.
Categorical data are given as percent in brackets, while continuous data are given as median with interquartile range. Post hoc analysis is performed using Pearson chi-square for categorical data, and Kruskal–Wallis and Mann–Whitney tests for continuous data.
Missing: aN = 369, bN = 319, cN = 422 in the plasma cohort.
Table 2.
Postmortem Brain Cohort Samples Descriptives
| Parameters | Brain Cohort | ||
|---|---|---|---|
| SCZ | BD | HC | |
| (N = 80) | (N = 44) | (N = 88) | |
| Male sex, N (%) | 54 (73.8) | 23 (52.3) | 69 (78.4) |
| Ethnicity (Caucasian) | 80 (100) | 44 (100) | 88 (100) |
| Mean age (SE) | 46.5 (1.8) | 46.8 (2.1) | 44.7 (1.7) |
| Mean RIN (SE) | 8.2 (0.06) | 8.3 (0.08) | 8.4 (0.05) |
Note: SCZ, schizophrenia; BD, bipolar disorder; HC, healthy control; SE, standard error; RIN, RNA integrity number.
Plasma Levels of TNF-Related Molecules
The plasma levels of TNF-related molecules in absolute values are summarized in table 3. The patient group as a whole as well as the SCZ and the BD groups separately had significantly higher levels of TNF, sTNFR1, and sTNFR2 as well as the TNF/sTNFRs ratio as an estimate of TNF activity compared to HC, also in adjusted analysis (ie, confounders). In contrast, patients had lower levels of sADAM17 compared to HC in adjusted analysis. This pattern, however, was restricted to the SCZ group, with no significant difference between the BD and HC group (table 4).
Table 3.
Unadjusted Data: Protein Levels in Plasma, Relative mRNA Expression in Peripheral Blood, and mRNA Expression in the Dorsolateral Prefrontal Cortex
| Parameters | Schizophrenia | Bipolar Disorder | Healthy Controls | |||
|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |
| Plasma cytokine/protein | ||||||
| TNF (pg/mL) | 352 | 1.20 (1.28) | 168 | 1.16 (1.04) | 358 | 1.09 (1.03) |
| sTNFR1 (ng/mL) | 569 | 1.76 (0.74) | 247 | 1.67 (0.52) | 624 | 1.60 (0.77) |
| sTNFR2 (ng/mL) | 553 | 4.76 (1.43) | 239 | 4.60 (1.47) | 591 | 4.51 (1.44) |
| ADAM17 (pg/mL) | 548 | 189 (282) | 240 | 227 (358) | 594 | 214 (341) |
| Leukocyte mRNA | ||||||
| TNF mRNA | 224 | 0.89 (0.44) | 142 | 0.92 (0.41) | 184 | 1.00 (0.58) |
| TNFR1 mRNA | 224 | 1.02 (0.32) | 143 | 1.08 (0.46) | 184 | 1.00 (0.31) |
| TNFR2 mRNA | 224 | 0.98 (0.36) | 143 | 1.00 (0.46) | 184 | 1.00 (0.43) |
| ADAM17 mRNA | 224 | 1.02 (0.29) | 142 | 1.24 (0.46) | 184 | 1.00 (0.38) |
| DLPFC mRNA | ||||||
| TNF mRNA | 80 | 0.03 (0.05) | 44 | 0.03 (0.06) | 86 | 0.02 (0.05) |
| TNFR1 mRNA | 80 | 3.87 (3.10) | 44 | 3.19 (2.43) | 86 | 2.35 (0.77) |
| TNFR2 mRNA | 80 | 1.74 (1.20) | 44 | 1.37 (0.63) | 86 | 1.44 (0.64) |
| ADAM17 mRNA | 80 | 3.25 (0.95) | 44 | 2.83 (0.91) | 86 | 3.40 (0.94) |
Note: TNF, tumor necrosis factor; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2; ADAM17, a disintegrin and metalloprotease-17 protein; DLPFC, dorsolateral prefrontal cortex; IQR, interquartile range.
Data are given as median with interquartile range due to skewed distributions.
Table 4.
Differences in Cytokine and mRNA Levels Between Patients and Controls After Controlling for Confounders
| Plasma Cohort (soluble cytokine) | Leukocyte Cohort (mRNA) | Brain Cohort (mRNA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β | t | n | β | t | t | Fold change | FDR | |
| Patients vs HC | |||||||||
| TNFR1 | 1003 | 0.14 | 4.47*** | 472 | 0.05 | 1.11 | |||
| TNFR2 | 1000 | 0.10 | 2.94** | 315 | 0.11 | 1.56 | |||
| TNF | 459 | 0.17 | 3.48** | 564 | −0.16 | −3.91*** | |||
| ADAM17 | 1415 | — | −2.08* | 428 | 0.16 | 3.15** | |||
| TNF/sTNFRs ratio | 455 | 0.20 | 4.07*** | ||||||
| SCZ vs HC | |||||||||
| TNFR1 | 748 | 0.15 | 3.82*** | 309 | 0.01 | 0.15 | 0.79 | 1.02 | 0.84 |
| TNFR2 | 828 | 0.09 | 2.56* | 239 | 0.14 | 1.71 | −0.40 | 0.99 | 0.92 |
| TNF | 319 | 0.16 | 2.38* | 323 | −0.18 | −3.02** | −0.04 | 1.00 | 0.99 |
| ADAM17 | 744 | — | −2.93** | 323 | 0.02 | 0.28 | −0.33 | 0.99 | 0.95 |
| TNF/sTNFRs ratio | 317 | 0.16 | 2.48* | ||||||
| BD vs HC | |||||||||
| TNFR1 | 479 | 0.17 | 3.50** | 264 | 0.05 | 0.81 | −0.61 | 0.99 | 0.77 |
| TNFR2 | 516 | 0.15 | 3.42** | 234 | 0.04 | 0.57 | −0.01 | 1.0 | 0.99 |
| TNF | 189 | 0.19 | 2.61** | 326 | — | −2.56* | 0.64 | 1.01 | 0.76 |
| ADAM17 | 315 | −0.03 | −0.48 | 222 | 0.40 | 5.92*** | 1.62 | 1.05 | 0.39 |
| TNF/sTNFRs ratio | 195 | 0.22 | 2.72** | ||||||
| BD vs SCZ | |||||||||
| TNFR1 | 478 | −0.07 | −1.46 | 367 | 0.06 | 1.12 | |||
| TNFR2 | 696 | −0.06 | −1.54 | 367 | −0.07 | −1.25 | |||
| TNF | 347 | −0.07 | −1.28 | 366 | 0.01 | 0.11 | |||
| ADAM17 | 510 | 0.06 | 1.42 | 366 | 0.39 | 8.03*** | |||
| TNF/sTNFRs ratio | 344 | −0.02 | −0.39 | ||||||
Note: SCZ, schizophrenia; BD, bipolar disorder; HC, healthy control; TNF, tumor necrosis factor; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2; ADAM17, a disintegrin and metalloprotease-17 protein; TNF/sTNFRs ratio, TNF/(sTNFR1 + sTNFR2).
The brain cohort: n(HC) = 86, n(SCZ) = 79, n(BD) = 44.
Results are given as t values from linear regression analyses after controlling for confounding factors.
*P < .05; **P < .01; ***P < .001.
TNF, TNFR1, TNFR2, and ADAM17 mRNA Expression in Whole Blood
The mRNA expression levels of TNF-related molecules in whole blood are summarized in table 3. The patient group as a whole as well as the SCZ and BD groups independently had lower levels of TNF mRNA compared to HC, with no difference between SCZ and BD (table 4). In contrast, there were no differences in TNFR1 mRNA and TNFR2 mRNA between patients and controls (table 4). However, patients had significantly higher levels of ADAM17 mRNA compared to HC after controlling for confounding factors. This effect was clearly driven by the BD group (table 4).
TNF Proteins and Working Memory
Performance on working memory tasks is presented in supplementary table S5. We found significant associations between working memory and TNF ratio and TNF that were significantly stronger in the SCZ group compared to HC and BD (data not shown) and remained significant after correcting for multiple testing and controlling for age, sex, PANSS score, and antipsychotics in the SCZ group (TNF ratio: F (5,120) = 2.90, adjusted R2 = 0.07, β = −0.23, P = .008) (TNF: F (5,122) = 3.35, R2 = 0.09, β = −0.26, p = .003) (supplementary table S6).
TNF, TNFR1, TNFR2, and ADAM17 mRNA Expression in Cortex
As shown in supplementary figure S1 and table S4, there were no differences in mRNA levels of TNF (FDR = 0.99), TNFR1 (FDR = 0.84), TNFR2 (FDR = 0.92), or ADAM17 (FDR = 0.95) between SCZ and HC. Similarly, no differences in mRNA levels of TNF (FDR = 0.76), TNFR1 (FDR = 0.77), TNFR2 (FDR = 0.99), or ADAM17 FDR = 0.39) were detected between BD and HC.
Correlation Between TNF-Related Molecules in Plasma (protein) and Whole Blood (mRNA)
The associations between protein and mRNA of the TNF-related molecules in plasma and whole blood, respectively, are presented in supplementary table S1. We found moderate but highly significant associations between TNFR1, TNFR2, and ADAM17 mRNA and significant weak associations between sTNFR1 and sTNFR2 in both the HC group and the patient group after controlling for confounders. Importantly, however, except for a weak correlation between mRNA and protein levels of TNFR1 in the patient group as a whole and those with SCZ, there was no significant correlation between protein and mRNA levels of the different TNF-related molecules (supplementary table S1).
Role of Medication
Patients using lithium (n = 19) had higher levels of ADAM17 mRNA and sTNFR1 levels compared to non-medicated (n = 85) patients in the BD group. Serum levels of lithium were associated with higher TNF levels and increased TNF/sTNFRs ratio (supplementary table S2), however, these results do not remain significant after correction for multiple testing. We found no other significant associations between medication groups (antipsychotics, mood stabilizers, and antidepressants), medication dosage, and cytokines/mRNA.
Clinical Characteristics and TNF Pathway Expression and Cytokines
We found weak correlations with small effect sizes between clinical symptoms (ie, PANSS, GAF, and CDSS) and TNF pathway–related gene expression and corresponding proteins. These results, however, do not survive correction for multiple testing (supplementary tables S3 and S4).
PANSS, GAF, and Duration of Illness in the SCZ Group.
We found weak negative associations after controlling for confounders between TNF mRNA and PANSS excited symptoms, TNFR1 mRNA and PANSS negative symptoms, TNF and GAF-symptom scale, sTNFR1 and GAF-symptoms scale, and TNF mRNA and duration of illness. Thus, it seems that decreased TNF and TNFR1 mRNA expression were associated with increased disease symptom severity, while increased circulating TNF and sTNFR1 were associated with increased symptom severity.
CDSS, IDS, YMRS, GAF and Duration of Illness in the BD Group.
We found a weak positive association after controlling for confounders between TNF mRNA and duration of illness in the BD group (the opposite of what we found in the SCZ group). Of the clinical symptoms only CDSS showed associations with cytokines and mRNA; increasing sTNFR2 levels and TNFR2 mRNA expression were associated with decreased depressive symptoms, while increased TNF mRNA levels were associated with increased depressive symptoms.
Discussion
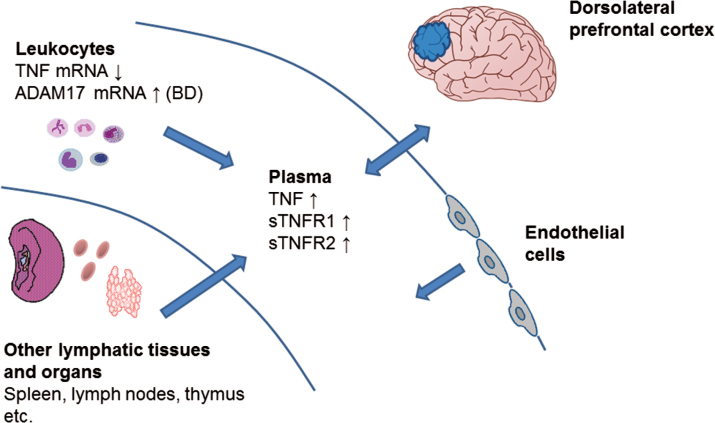
We found that patients with SCZ and BD had slightly increased plasma levels of TNF-related molecules with significantly increased TNF/sTNFRs ratio, which has been shown to be a surrogate marker of TNF bioactivity,12 compared to HC suggesting a subtle but potentially biologically relevant proinflammatory imbalance in the TNF system in these disorders. However, while TNF levels were elevated in plasma, TNF mRNA was decreased in whole blood suggesting other cellular sources of TNF than circulating leukocytes (figure 1). The increased ADAM17 mRNA expression detected in BD compared to SCZ in peripheral blood could potentially contribute to increased shedding of membrane-bound TNF in BD, suggesting a differential role for ADAM17 in BD compared to SCZ. We have previously reported that elevated plasma levels of sTNFR1 were associated with lower scores on several cognitive tests.20,33 Here, we found that a shift toward a proinflammatory imbalance in the TNF pathway was weakly but significantly associated with lower working memory scores potentially suggesting a link between the DLPFC and the TNF pathway, however, we observed no significant alterations in TNF system mRNA expression in the DLPFC of patients compared to HC.
Fig. 1.
Elevated soluble plasma cytokine levels in SCZ and BD may be a result of increased shedding from their membrane-bound form in BD, but blood leukocytes seem an unlikely source as TNF mRNA is downregulated in both disorders. There are no strong indications of alterations in TNF-related signaling molecules in prefrontal cortex, however, soluble plasma cytokines can pass through the blood brain barrier, and differential expression of these molecules may occur in other regions of the brain. The increase in plasma cytokines may also be a result of heightened immune activity in other lymphatic tissues and organs as well as endothelial cells.
The increased levels of TNF, sTNFR1, and sTNFR2 in SCZ and BD patients per se may not necessarily reflect a proinflammatory shift in the TNF pathway considering that soluble TNF receptors partake in regulating TNF activity by acting as decoy receptors and competing with membrane-bound receptors for TNF.34 However, our results suggest that while both plasma levels of TNF and its soluble receptors are elevated in SCZ and BD, there is also a proinflammatory imbalance as shown by increased TNF/sTNFRs ratio.12 In spite of this proinflammatory imbalance in plasma, and in contrast to previous more small-scaled studies,13,14TNF mRNA was downregulated in circulating leukocytes from both BD and SCZ patients implying other cellular sources for the elevated plasma levels in these disorders, such as endothelial cells and tissue macrophages (figure 1). Based on the distinct upregulation of ADAM17 mRNA in leukocytes in BD and the role of ADAM17 in the release of TNF and its receptor from their membrane to their soluble form, it is, however, possible that increased shedding of these molecules could contribute to their increased plasma levels in BD patients, potentially representing a distinct pattern of this disorder.
We predicted that these results in peripheral blood would be recapitulated in the DLPFC. Using a conservative method to control for mRNA quality in brain tissue, which is a major confounder of prior studies that control only for RIN and demographics, we find no evidence of the changes in peripheral blood in prefrontal cortical samples from either patients with SCZ or BD. It is important to recognize that our mRNA quality adjustment is rigorous and conservative and though potentially sensitive to type II error, is robust in controlling for Type I error. The lack of changes in the expression of TNF-related signaling molecules in the DLPFC per se cannot rule out altered TNF pathway activity in other regions of the brain or the possibility that inflammatory molecules in peripheral blood have CNS effects without changing intrinsic gene expression. Indeed, cytokines in peripheral blood are able to cross the blood brain barrier35 potentially influencing learning and memory,33 cognition,20 and neural activity and viability.36 Our findings raise questions, however, about whether TNF signaling in peripheral blood is a marker of a primary pathophysiological process or secondary to other disease mechanisms. Notably, enhanced TNF activity is associated with several comorbid metabolic conditions such as type 2 diabetes and cardiovascular disease.37,38 However, it is important to underscore that the cortex analyses were performed on whole cortex samples and not on isolated cells, and an up- or downregulation for example in microglia may be masked by expression in other cells. Correlations between clinical features, treatment and TNF measures in peripheral blood were largely uninformative showing a rather complex pattern. However, increasing depressive symptoms were associated with a moderate increase in TNF mRNA and lower levels of sTNFR2, TNFR1, and TNFR2 mRNA suggesting a proinflammatory imbalance in circulating immune cells of these patients, further supporting a potential link between TNF activity and depression as described by others.39 We found no significant difference in plasma/whole blood cytokine/mRNA levels between patients using antipsychotics in monotherapy and non-medicated patients and no significant correlation between defined daily dosage of antipsychotics and cytokine/mRNA levels. This is in contrast to previous smaller studies that have found that antipsychotics may influence the expression of TNF.40 However, plasma levels of TNF and TNF/sTNFRs ratio increased with higher lithium serum concentrations, and patients using lithium had higher sTNFR1 and ADAM17 mRNA levels compared to non-medicated bipolar patients, suggesting that lithium may promote activation of the TNF system.
There are some limitations to our study. Firstly, there is a difference in the time of blood sampling for our HC group compared to the patient group, but this was controlled for in the analysis of cytokine and mRNA levels. Secondly, current RNA sequencing quantification and mapping methods may lead to potential underestimation of mRNA levels. Further, although previous studies have measured TNF-related proteins in postmortem brain,41 we were unable to obtain reliable data on postmortem TNF pathway proteins. Finally, our data on the association of TNF molecules and clinical symptoms should be interpreted with caution as no consistent patterns were observed.
This is by far the largest study that investigates the TNF system in patients with severe mental disorders, including both plasma levels as well as mRNA expression in whole blood and in brain. Our data suggest a complex regulation of TNF-related molecules in peripheral blood of BD and SCZ patients with increased TNF/sTNFRs ratio in plasma, reflecting enhanced TNF activity as a major finding. The highly significant difference in ADAM17 mRNA expression between SCZ and BD may also implicate other ADAM17 substrates and respective systems in the pathology of BD and requires further elucidation. Our findings also suggest that circulating leukocytes are not a major source of soluble TNF levels in plasma. The lack of findings in the DLPFC leaves unresolved the issue of how peripheral blood alterations relate to the TNF system in the brain. Further studies are needed to investigate other cortical regions of the brain and the impact of our findings on the pathogenesis of severe mental disorders.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Supplementary Material
Acknowledgments
The authors thank the participants of the study and Thomas D. Bjella, Eivind Bakken, and Line Gundersen for their skillful research administrative assistance. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Smith RS. A comprehensive macrophage-T-lymphocyte theory of schizophrenia. Med Hypotheses. 1992;39:248–257. [DOI] [PubMed] [Google Scholar]
- 2. Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–1133. [DOI] [PubMed] [Google Scholar]
- 3. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–108. [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. [DOI] [PubMed] [Google Scholar]
- 5. Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imaizumi T, Itaya H, Fujita K, et al. Expression of tumor necrosis factor-alpha in cultured human endothelial cells stimulated with lipopolysaccharide or interleukin-1alpha. Arterioscler Thromb Vasc Biol. 2000;20:410–415. [DOI] [PubMed] [Google Scholar]
- 7. Naudé PJ, den Boer JA, Luiten PG, Eisel UL. Tumor necrosis factor receptor cross-talk. FEBS J. 2011;278:888–898. [DOI] [PubMed] [Google Scholar]
- 8. Clark IA, Vissel B. A neurologist’s guide to TNF biology and to the principles behind the therapeutic removal of excess TNF in disease. Neural Plast. 2015;2015:358263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. [DOI] [PubMed] [Google Scholar]
- 11. Doganavsargil-Baysal O, Cinemre B, Aksoy UM, et al. Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Hum Psychopharmacol. 2013;28:160–167. [DOI] [PubMed] [Google Scholar]
- 12. Aukrust P, Ueland T, Lien E, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–382. [DOI] [PubMed] [Google Scholar]
- 13. Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, et al. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol. 2010;13:1369–1381. [DOI] [PubMed] [Google Scholar]
- 14. Liu L, Jia F, Yuan G, et al. Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res. 2010;176:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Pandey GN, Ren X, Rizavi HS, Zhang H. Proinflammatory cytokines and their membrane-bound receptors are altered in the lymphocytes of schizophrenia patients. Schizophr Res. 2015;164:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnsten AF, Jin LE. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog Mol Biol Transl Sci. 2014;122:211–231. [DOI] [PubMed] [Google Scholar]
- 17. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39:889–905. [DOI] [PubMed] [Google Scholar]
- 18. Brandt CL, Eichele T, Melle I, et al. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br J Psychiatry. 2014;204:290–298. [DOI] [PubMed] [Google Scholar]
- 19. Porter RJ, Robinson LJ, Malhi GS, Gallagher P. The neurocognitive profile of mood disorders - a review of the evidence and methodological issues. Bipolar Disord. 2015;17(suppl 2):21–40. [DOI] [PubMed] [Google Scholar]
- 20. Hope S, Hoseth E, Dieset I, et al. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res. 2015;165:188–194. [DOI] [PubMed] [Google Scholar]
- 21. Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E. Different changes in cortical tumor necrosis factor-α-related pathways in schizophrenia and mood disorders. Mol Psychiatry. 2013;18:767–773. [DOI] [PubMed] [Google Scholar]
- 22. Dieset I, Djurovic S, Tesli M, et al. Up-regulation of NOTCH4 gene expression in bipolar disorder. Am J Psychiatry. 2012;169:1292–1300. [DOI] [PubMed] [Google Scholar]
- 23. Lagerberg TV, Kvitland LR, Aminoff SR, et al. Indications of a dose-response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry Res. 2014;215:101–104. [DOI] [PubMed] [Google Scholar]
- 24. Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P). Psychiatry Res. 1998;79:163–173. [DOI] [PubMed] [Google Scholar]
- 25. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simonsen C, Sundet K, Vaskinn A, et al. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr Bull. 2011;37:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demmo C, Lagerberg TV, Aminoff SR, et al. History of psychosis and previous episodes as potential explanatory factors for neurocognitive impairment in first-treatment bipolar I disorder. Bipolar Disord. 2016;18:136–147. [DOI] [PubMed] [Google Scholar]
- 28. Keustermans GC, Hoeks SB, Meerding JM, Prakken BJ, de Jager W. Cytokine assays: an assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–17. [DOI] [PubMed] [Google Scholar]
- 29. Lipska BK, Deep-Soboslay A, Weickert CS, et al. Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. [DOI] [PubMed] [Google Scholar]
- 30. Jaffe AE, Shin J, Collado-Torres L, et al. Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat Neurosci. 2015;18:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoseth EZ, Westlye LT, Hope S, et al. Association between cytokine levels, verbal memory and hippocampus volume in psychotic disorders and healthy controls. Acta Psychiatr Scand. 2016;133:53–62. [DOI] [PubMed] [Google Scholar]
- 34. Moelants EA, Mortier A, Van Damme J, Proost P. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol Cell Biol. 2013;91:393–401. [DOI] [PubMed] [Google Scholar]
- 35. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96:70–82. [DOI] [PubMed] [Google Scholar]
- 37. Carlsson AC, Östgren CJ, Nystrom FH, et al. Association of soluble tumor necrosis factor receptors 1 and 2 with nephropathy, cardiovascular events, and total mortality in type 2 diabetes. Cardiovasc Diabetol. 2016;15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moon YS, Kim DH, Song DK. Serum tumor necrosis factor-alpha levels and components of the metabolic syndrome in obese adolescents. Metabolism. 2004;53:863–867. [DOI] [PubMed] [Google Scholar]
- 39. Soczynska JK, Kennedy SH, Goldstein BI, Lachowski A, Woldeyohannes HO, McIntyre RS. The effect of tumor necrosis factor antagonists on mood and mental health-associated quality of life: novel hypothesis-driven treatments for bipolar depression? Neurotoxicology. 2009;30:497–521. [DOI] [PubMed] [Google Scholar]
- 40. Paterson GJ, Ohashi Y, Reynolds GP, Pratt JA, Morris BJ. Selective increases in the cytokine, TNFalpha, in the prefrontal cortex of PCP-treated rats and human schizophrenic subjects: influence of antipsychotic drugs. J Psychopharmacol. 2006;20:636–642. [DOI] [PubMed] [Google Scholar]
- 41. Marballi K, Cruz D, Thompson P, Walss-Bass C. Differential neuregulin 1 cleavage in the prefrontal cortex and hippocampus in schizophrenia and bipolar disorder: preliminary findings. PLoS One. 2012;7:e36431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.