Abstract
AIM
To find new biomarkers for uveal melanoma (UM) by analyzing the serum peptidome profile.
METHODS
Proteomic spectra in patients with UM before and after operation were analyzed and compared with those of healthy controls. Magnetic affinity beads were used to capture serum peptides and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometer were used to compile serum peptide profiles.
RESULTS
A panel of 49 peptides were differentially expressed between UM patients and controls, of which 33 peptides were of higher intensities in patient group and 16 peptides were of higher intensities in control group. Based on combined use of these potential markers, peptides with mean molecular masses of 1467 and 9289.0 Da provide high sensitivity (83.3%), specificity (100%) and accuracy rate (93.0%) together to differentiate melanoma patients from healthy controls. At the time point of 6mo postoperatively, the levels of many peptides differentially expressed before surgery showed no more statistical difference between the patients and the control group. Fibrinogen α-chain precursors were identified as potential UM markers.
CONCLUSION
We have shown that a convenient and fast proteomic technique, affinity bead separation and MALDI-TOF analysis combined with bioinformatic software, facilitates the identification of novel biomarkers for UM.
Keywords: uveal melanoma, protein biomarker, peptidome profile, magnetic bead fractionation, mass spectrometry
INTRODUCTION
Uveal melanoma (UM) is the most common malignant intraocular tumor in adult humans, with an annual incidence of 0.31 (Black), 0.38 (Asian), 1.67 (Hispanic) and 6.02 (non-Hispanic white) per million population[1]. Despite the high accuracy of clinical diagnosis and advances in local treatment, more than 50% of UM patients develop metastasis within 10-year of initial diagnosis[2]. The prognosis for these metastatic patients is very poor; thus, it is clinically important to find clinical and molecular biomarkers for early disease detection and evaluation of metastatic potential of UM.
With the advancement of profiling methodologies in the past decades, gene expression and protein levels in tissues and body fluids can be monitored closely and globally during the course of human diseases. Recently, proteometric technologies identified many UM-related proteins and peptides[3]. Early in 2001, on the basis of two proteins with molecular weights (MW) of 4543.43 and 6853.30 Da, Missotten et al[4] could distinguish aqueous humor of melanoma eyes from control eyes in 89% of cases. Pardo et al's[5] research team conducted the first proteomic analysis of UM cells by using two-dimensional electrophoresis (2-DE) and mass spectrometry (MS), representing the first step towards the establishment of a UM protein database as a valuable resource for the study of this malignancy. Later research into the proteomics of primary UM cell cultures and cell lines had suggested the involvement of cell adhesion protein MUC18 and HMG-1 in the invasion potential of UM cells[6]. Overexpression of the oncogene DJ-1 was also noted to be an indicator of this malignancy. However, the in vitro environment created by standard cell-culture procedures does not properly replicate in vivo conditions[7].
In proteomics, it is well accepted that plasma or serum is the ultimate diagnostic fluid. A blood sample represents the summation of metabolic events in a wide variety of fluids and tissues and thus offers the opportunity to assess the status of an individual's health. Cancer cells release protein biomarkers into the extracellular environment and some of these products can end up in the bloodstream and serve as potential serum biomarkers. Therefore, we conduct the current study to analyze the proteomics of serum samples of UM patients before and after tumor removal surgery and compare to healthy controls.
SUBJECTS AND METHODS
The institutional review board of the Second Hospital Affiliated to Anhui Medical University and the Beijing Tongren Hospital approved the study, and the protocol adheres to the tenets of the Declaration of Helsinki. Written informed consent was obtained for each patient prior to enrollment into the study.
Patients and Blood Sample Preparation
A total of 18 patients (10 men and 8 women) with a clinical diagnosis of UM (17 of choroidal melanoma and 1 of ciliary melanoma) at the Tongren Eye Center of Beijing Tongren Hospital (Beijing, China) were recruited for this study. The mean age was 39.4y (range 21-67y). All of the patients underwent transscleral or transretinal local resection (11 cases) or enucleation (7 cases) of the affected eyes. Systemic evaluation to screen out contraindications for operation and metastatic lesions were also performed. Tissues or eyeballs acquired from the surgery were sent for immunohistochemical examinations and all confirmed UM of which 17 cases were of spindle cell type and 1 case of epithelioid cell type.
Venous blood samples were drawn after patients' fasting for at least 6h in pre-surgery mornings and obtained in a 5 mL BD vacutainer®, glass red-top tubes. After sample collection, the tubes are then allowed to clot at room temperature for no more than 4h (2-3h mostly) and centrifuged at 3000 rpm for 20min at room temperature. Sera (the upper phase) were transferred to five 0.5 mL Eppendorf tubes with approximately 200 µL serum in each and frozen at -80°C for future use.
Blood samples from 25 healthy individuals (13 men; mean age 33.8y) with no known malignancies were also collected, prepared and stored at Beijing Tongren Hospital following the same collection procedures.
After surgery, the patients were followed for ocular and systemic checkup at regular intervals. Fasting blood samples were collected at one month (15 cases; mean interval after surgery, 39.5d) and six months (10 cases; mean interval after surgery, 182.5d) post-operatively.
Magnetic Bead Fractionation
For proteome fractionation, serum samples were thawed at room temperature for 15min and processed with ClinProt purification reagent sets from Bruker Daltonics immediately. Three types of functionalized magnetic beads (MB) including MB-hydrophobic interaction chromatography (MB-HIC C8), MB-weak-cation-exchange chromatography (MB-WCX) and MB-immobilized metal ion affinity chromatography containing copper ions (MB-IMAC Cu) beads were chosen initially to test their affinity capabilities on two randomly selected serum samples of a UM patient. MB-IMAC Cu beads managed to capture the largest number of peptide peaks compared with the other two functionalized beads and hence were utilized for the proteome fractionation in this study.
MB facilitated proteome fractionation was carried out as per the manufacturer's instructions. We diluted 5 µL of sample with 10 µL of a binding solution added to the bead slurry (5 µL) in a 0.2 mL polypropylene tube, mixed thoroughly by pipetting up and down several times, and incubated the tube for 1min. To separate the unbound solution, the tube was placed in a MB separator and the supernatant was removed carefully with a pipette. MBs were then washed three times with 100 µL wash buffer. Following binding and washing, the bound proteins/peptides were eluted from the MB with 5 µL of an acetonitrile-water (1:1 by volume). A portion of the eluted sample was diluted 1:10 in matrix solution comprised of α-cyano-4-hydroxycinnamic acid (HCCA, 0.6 g/L in 2:1 ethanol:acetone). Then 0.5 µL of the resulting mixture was spotted on the AnchorChipTM target (Bruker Daltonics, Germany) and allowed to air dry for approximately 5min at room temperature.
Matrix-assisted Laser Desorption/Ionization Time-of-flight Mass Spectrometry
For the proteome analysis, a linear matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS (Autoflex, Bruker Daltonics) was used with the following settings: ion source 1, 20.00 kV; ion source 2, 18.80 kV; lens, 6.60 kV; pulsed ion extraction, 100ns. Ionization was achieved by irradiation with a nitrogen laser operating at 20 Hz. For matrix suppression, we used a high gating factor with signal suppression up to 600 Da. Mass spectra were detected in linear positive mode. Mass calibration was performed with the calibration mixture of peptides and proteins in a mass range of 800-20 000 Da. All signals with a signal-to-noise (S/N) ratio >3 in a mass range of 1000-10 000 Da were recorded. AutoXecute acquisition control, a software tool, was applied for automatic data acquisition. We used the ClinProtTools (CPT) bioinformatics software (Ver. 2.0; Bruker Daltonics) for proteome pattern recognition which allowed differentiation between the cancer and control samples. A ±5 Da mass accuracy for each spectrum was observed and was probably due to varied sample position on the sample plate.
To optimize the MALDI-TOF MS analysis, we tested three different beads of varying functionalities: MB-HIC C8, MB-WCX and MB-IMAC Cu beads. Two serum samples, pre-operative and one-month post-operative, of a randomly selected UM patient, underwent three proteome assays each, with three types of functionalized MB and subsequent MALDI-TOF MS (Microflex, Bruker Daltonics). Most of the protein peaks were <10 kDa. Comparison of proteomic mass spectra in this range is sufficient for analysis.
Evaluation of Assay Precision and Diagnostic Efficacy
To evaluate the precision of the assay, we determined within-run and between-run variations by use of multiple analyses of bead fractionation and MS for 3 serum samples. For within-run and between-run variations, we examined 5-7 peaks with various intensities. Within-run imprecision was determined by evaluating the coefficients of variance (CV) for two samples, each with 3 assays within a run; between-run imprecision was determined by evaluating the CVs of 5 different assays for a sample over a period of 9d.
To assess the diagnostic efficacy, we calculated the means and standard deviation (SD) of the peaks of interest in the UM and control groups. After selecting the smaller SD of the two groups, the cutoff value was determined either as the corresponding mean plus 2 SD if this mean value is lower than that of the other group or as the corresponding mean minus 2 SD if this mean value is higher than that of the other group. The sensitivity (ratio of the cancer samples correctly designated with the cutoff value to all samples in the cancer group) and specificity (ratio of control samples correctly designated with the cutoff value to all samples in the control group) were analyzed accordingly. The 2 sided t-tests were used to evaluate the statistical significance of a potential marker between two groups.
Comparison of the spectral profiles of UM patients' serum samples collected pre- and post-operatively with normal groups was also performed to screen peptide of interest.
Bioinformatics and Identification of Protein Markers
Selected peptides were further purified by use of MB-IMAC Cu bead and directly identified by MALDI TOF/TOF analysis to obtain the peptide sequence. Peptide mass fingerprinting was performed with the Mascot search engine (Matrix Science) and a search of the National Cancer for Biotechnology Information (NCBI) protein-protein BLAST database (http://www.ncbi.nlm.nih.gov/BLAST/).
RESULTS
Reproducibility of Serum Proteome Profiling Using Copper Beads and Mass Spectrometry
Reproducibility was determined by calculating the mean CV of the normalized peak amplitudes for each of the 5 or 7 peptides with the highest average amplitudes in the mass spectra. These peptides were widely distributed in the range of 1000-10 000 Da. Table 1 summarized the within- and between-run CVs of the selected peptides. Within-run CVs of two serum samples and between-run CVs of a serum sample are all below 20%.
Table 1. Reproducibility of mass spectra profiled by copper beads and MALDI-TOF analysis.
| Sample No. | Mean mass (Da) | MI | CV (%) | MCV (%) |
| Within-run reproducibility | ||||
| PO 6-2 (n=3) | 5906 | 937.9 | 8.5 | 14.8 |
| 1467 | 263.5 | 32.6 | ||
| 4211 | 225.9 | 8.7 | ||
| 7767 | 222.3 | 7.8 | ||
| 9292 | 214.8 | 16.2 | ||
| C2 (n=3) | 5906 | 775.1 | 27.1 | 18.5 |
| 9294 | 290.2 | 21.8 | ||
| 4211 | 288.9 | 22.8 | ||
| 1467 | 257.9 | 16.2 | ||
| 2661 | 180.1 | 4.6 | ||
| Between-run reproducibility | ||||
| PO 1-10 | 5906 | 905.51 | 11.1 | 19.3 |
| 9290 | 634.33 | 23.4 | ||
| 4211 | 435.08 | 12.5 | ||
| 7766 | 419.97 | 26.6 | ||
| 2662 | 230.69 | 37.5 | ||
| 3264 | 198.70 | 13.1 | ||
| 5338 | 165.89 | 10.8 |
Reproducibility was determined by calculating the mean CV of the normalized peak amplitudes for each of the five or seven peptides with the highest average amplitudes. MI: Mean intensity; CV: Coefficient of variance; ICV: Individual coefficient of variance; MCV: Mean coefficient of variance of the runs.
Screen for Differentially Expressed Peptides/Proteins
No patients were found to have metastasis related to the intraocular tumor. Sixty-eight serum samples from 18 UM patients before and after surgery and 25 healthy controls were manually fractionated using the MB-IMAC Cu beads kit. Eluted samples are mixed with the matrix solution at a fixed proportion and later spotted on the AnchorChipTM targets as described above. Mass spectra were generated with MALDI-TOF MS (Autoflex, Bruker Daltonics).
A subset of 43 spectra from 18 pre-surgery UM patients and 25 controls were processed with CPT software to interrogate the dataset for the discovery of disease-specific biomarkers. This capability is contributable to visually inspect and distinguish peptide/protein peaks of significantly different intensities. Approximately 100 peaks were detected and calibrated by the CPT software and 49 peaks that differed significantly between the two groups were screened out.
To better characterize the pool of differentially expressed peptides/proteins, receiver operating characteristic curve (ROC curve) was used to assess the discriminatory efficacy of each peptide/protein. All of the 49 differentially expressed peptides demonstrated area under curve (AUC) between 0.70 and 0.90, which is suggestive of medium diagnostic accuracy for each peak. Among them, 14 peaks showed AUC higher than 0.85. Their mean MW were 1467, 1207.56, 1741.61, 2024.2, 4054.88, 4117.41, 4173.61, 4964.57, 1351.66, 1897.62, 3263.52, 1264.62, 1520.56 and 3192.64 Da. They were designated as the UM markers A to N respectively for subsequent characterization. All the P values of these markers were <0.001. For markers C, D, E, F, G, H and J, mean peak intensities in the UM patients group were stronger than those in the control samples. For markers A, B, I, K, L, M and N, mean peak intensities in the UM patients group were lower than those in the control samples.
The means and SD of the 14 peaks in the cancer and normal control groups were calculated. After selecting the smaller SD of the two groups, the cutoff value was determined either as the corresponding mean plus 2 SD if this mean value is lower than that of the other group or as the corresponding mean minus 2 SD if this mean value is higher than that of the other group. As shown in Table 2, the sensitivities of these UM markers A to N were 66.7%, 61.1%, 61.1%, 55.6%, 55.6%, 50.0%, 50.0%, 50.0%, 38.9%, 50.0%, 16.6%, 94.4%, 94.4% and 94.4%. The specificities of these UM markers were mostly over 90%, only with markers L, M and N around 30.0%. The accuracy rates ranged from 55.8% to 86.0%.
Table 2. Determination of the sensitivity and specificity for the 14 selected markers with ROC over 0.85.
| UM potential markers | A | B | C | D | E | F | G | ||
| MW (Da) | 1467 | 1207.56 | 1741.61 | 2024.2 | 4054.88 | 4117.41 | 4173.61 | ||
| Cancer mean intensity | 181.5 | 112.6 | 32.0 | 27.6 | 72.6 | 52.6 | 26.1 | ||
| Cancer SD | 168.2 | 82.1 | 20.3 | 13.1 | 33.8 | 16.8 | 7.4 | ||
| Cut-off value | 210.2 | 39.8 | 20.0 | 23.4 | 56.5 | 52.1 | 25.8 | ||
| Normal mean intensity | 455.2 | 232.6 | 12.2 | 12.7 | 34.2 | 33.2 | 17.2 | ||
| Normal SD | 122.5 | 64.7 | 3.9 | 5.4 | 11.1 | 9.5 | 4.3 | ||
| Sensitivity (%) | 66.7 | 61.1 | 61.1 | 55.6 | 55.6 | 50.0 | 50.0 | ||
| Specificity (%) | 100 | 96.0 | 92.0 | 96.0 | 96.0 | 100 | 100 | ||
| Accuracy rate (%) |
86.0 |
81.4 |
81.4 |
79.1 |
79.1 |
79.1 |
79.1 |
||
| UM potential markers |
H |
I |
J |
K |
L |
M |
N |
||
| MW (Da) | 4964.57 | 1351.66 | 1897.62 | 3263.5 | 1264.62 | 1520.6 | 3192.67 | ||
| Cancer mean intensity | 62.1 | 43.2 | 55.6 | 193.8 | 36.5 | 21.8 | 98.5 | ||
| Cancer SD | 34.6 | 28.2 | 48.9 | 95.4 | 18.7 | 6.5 | 42.4 | ||
| Cut-off value | 56.1 | 30.4 | 35.5 | 132.8 | 74.0 | 34.8 | 183.3 | ||
| Normal mean intensity | 25.8 | 81.6 | 17.7 | 296.9 | 66.3 | 32.0 | 160.7 | ||
| Normal SD | 15.2 | 25.6 | 8.9 | 82.0 | 19.3 | 7.2 | 45.7 | ||
| Sensitivity (%) | 50.0 | 38.9 | 50.0 | 16.6 | 94.4 | 94.4 | 94.4 | ||
| Specificity (%) | 96.0 | 100 | 92.0 | 100 | 32.0 | 32.0 | 28.0 | ||
| Accuracy rate (%) | 76.7 | 74.4 | 74.4 | 65.1 | 58.1 | 58.1 | 55.8 | ||
The means and SD of the peaks of interest in the cancer and normal control groups were calculated. After selecting the smaller SD of the two groups, the cutoff value was determined either as the corresponding mean plus 2 SD if this mean value is lower than that of the other group or as the corresponding mean minus 2 SD if this mean value is higher than that of the other group. The sensitivity (ratio of the cancer samples correctly designated with the cutoff value to all samples in the cancer group) and specificity (ratio of control samples correctly designated with the cutoff value to all samples in the control group) were analyzed accordingly. The accuracy rate (i.e. total consistent rate) was determined as the ratio of cancer and normal control samples correctly designated to the total number of samples tested. MW: Molecular weight; Cancer mean: Mean intensity in the UM patients group; Cancer SD: Standard deviation of all the peak intensities in the UM patients group; Normal mean: Mean intensity in the normal control group; Normal SD: Standard deviation of all the peak intensities in the normal control group.
Since not all of these markers manifested satisfactory sensitivity or specificity rate, we selected marker A (with a MW of 1467 Da) to combine with other markers and other differentially expressed peptides to discriminate between UM and normal control groups. The accuracy rate was calculated as the ratio of cancer and normal control samples correctly designated to the total number of samples tested. As summarized in Table 3, the sensitivities of each combination were between 65.0% and 80.0%. The specificities were all over 90.0%. The accuracy rates ranged from 83.7% to 90.7%. In which, AG combined markers (1467 Da and 4173.61 Da) manifested the highest accuracy rate (90.7%) with sensitivity (77.8%) and specificity (100%) better than any individual marker.
Table 3. Diagnostic efficacy of combined markers in detection of UM.
| Combination | AG | AD | AF | AI | AJ | AK | AE | AB | AC | AH |
| Cancer discriminated | 14 | 14 | 13 | 12 | 14 | 12 | 13 | 12 | 13 | 12 |
| Normal discriminated | 25 | 24 | 25 | 25 | 23 | 25 | 24 | 24 | 23 | 24 |
| Sensitivity (%) | 77.8 | 77.8 | 72.2 | 66.7 | 77.8 | 66.7 | 72.2 | 66.7 | 72.2 | 66.7 |
| Specificity (%) | 100.0 | 96.0 | 100.0 | 100.0 | 92.0 | 100.0 | 96.0 | 96.0 | 92 | 96.0 |
| Accuracy (%) | 90.7 | 88.4 | 88.4 | 86.0 | 86.0 | 86.0 | 86.0 | 83.7 | 83.7 | 83.7 |
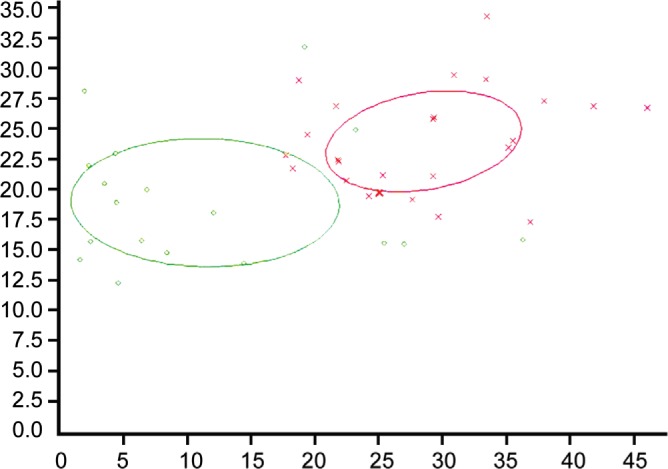
Among the 49 differentially expressed peptides between the UM and normal groups, peptides with an AUC lower than 0.85 were also included as discriminators to combine with marker A. On the basis of two peptides (1467 and 9289.0 Da; Figure 1), the serum samples of UM patients and normal groups could be distinguished in 93.0% of cases with high sensitivity (83.3%) and specificity (100%).
Figure 1. Dot graph depicting combined use of two peptides (1467 and 9289.0 Da) to discriminate samples between UM and normal groups.
In addition, CPT bioinformatics software (Version 2.0; Bruker Daltonics) provides many algorithms for the generation of diagnostic panels. With genetic algorithm and K-nearest neighbor algorithm (K=3), a panel of four peaks 2024 (marker D), 3194 (marker N), 4396 and 4645 Da managed to achieve an accuracy rate of 95.0%.
Comparison of Spectral Profiles of Uveal Melanoma Patients Before and After Surgery
At the time point of post-operative 1mo (average, 39.5d after surgery) and post-operative 6mo (average, 182.5d after surgery), serum samples of UM patients were collected and analyzed.
Altogether 47 peptides were differentially expressed between the post-operative 1mo UM patients group (15 cases) and the normal group. All the P values of these peptides were <0.05. With a close comparison of these 47 peptides with those 49 peptides differentially expressed between pre-operative UM patients and the normal group, we found that 41 peptides were overlapped, including all the previously denoted markers A-N. Four peptides (MW: 2093, 6691, 2864 and 8204 Da), all of very low mean intensities, were not detected in pre-operative UM patients and another 2 peptides (MW: 3884 and 2990 Da) differed between post-operative 1mo UM patients and the normal group but did not differ between pre-operative UM patients and the normal group.
Nine peaks differed significantly (P<0.05) between post-operative 6mo UM patients (10 cases) and the normal group. Of which, three peaks (MW: 2724, 1867 and 4645 Da) were also found in the dataset of 49 peptides differentially expressed between pre-operative UM patients and the normal group. The other 6 peaks (MW: 5966, 3303, 6029, 7635, 7564 and 3883 Da) differed between post-operative 6mo UM patients and the normal group but did not differ between pre-operative UM patients and the normal group. It is noted that there were no statistically significant differences in peak intensities of previously denoted markers A-N observed between post-operative 6mo UM patients and the normal group.
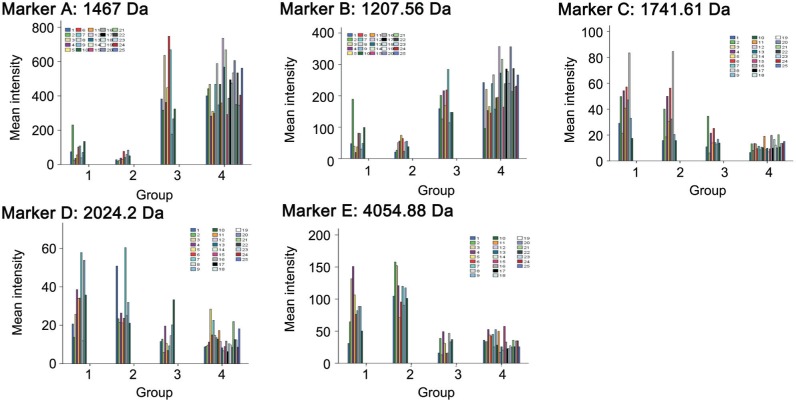
Univariate analysis of variance was employed to specifically analyze the differences in peak intensities of previously denoted markers A-N in 10 UM patients with complete pre-operative, post-operative 1mo and post-operative 6mo sera tested. Multiple comparisons were performed between each two of the time points. The mean intensities of each marker at the three time points, F and P values were summarized in Table 4. As shown in Figure 2, the dynamic variances in peak intensities of some markers in the 10 UM patients along the time before and after surgery and the corresponding peak intensities in normal group were visually depicted.
Table 4. Multiple comparisons between each of two subgroups of UM patients.
| Markers1 | A | B | C | D | E | F | ||||||
| MW (Da) | 1467 | 1207.56 | 1741.61 | 2024.2 | 4054.88 | 4117.41 | ||||||
| Normal mean | 455.61 | 232.38 | 12.00 | 12.77 | 34.13 | 33.17 | ||||||
| Mean intensity of UM I | 87.8 | 67.3 | 43.4 | 32.6 | 87.1 | 61 | ||||||
| Mean intensity of UM II | 45.4 | 46.4 | 36.5 | 30.5 | 113.1 | 72.6 | ||||||
| Mean intensity of UM III | 433 | 178.4 | 17 | 14.4 | 29.7 | 30.1 | ||||||
| F value | 33.775 | 27.529 | 13.919 | 6.052 | 24.934 | 27.615 | ||||||
| P value | <0.001 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | ||||||
| P value of I vs II | 0.419 | 0.284 | 0.199 | 0.721 | 0.04 | 0.059 | ||||||
| P value of II vs III | <0.001 | <0.001 | 0.001 | 0.009 | <0.001 | <0.001 | ||||||
|
P value of I vs III |
<0.001 |
<0.001 |
<0.001 |
0.004 |
<0.001 |
<0.001 |
||||||
| Marker1 |
H |
I |
J |
K |
L |
M |
N |
|||||
| MW (Da) | 4964.57 | 1351.66 | 1897.62 | 3263.52 | 1264.62 | 1520.56 | 3192.67 | |||||
| Normal mean | 25.8 | 81.6 | 17.6 | 296.9 | 66.2 | 30.8 | 160.9 | |||||
| Mean intensity of UM I | 75.3 | 29.6 | 77 | 161.4 | 28 | 18.3 | 82.6 | |||||
| Mean intensity of UM II | 70 | 17.5 | 62.6 | 161 | 23.5 | 16.2 | 92.2 | |||||
| Mean intensity of UM III | 20.1 | 94.9 | 24.1 | 243 | 69.1 | 31.1 | 126.6 | |||||
| F value | 10.994 | 14.718 | 7.001 | 7.901 | 20.113 | 14.897 | 5.821 | |||||
| P value | <0.001 | <0.001 | 0.006 | 0.002 | <0.001 | <0.001 | 0.008 | |||||
| P value of I vs II | 0.682 | 0.436 | 0.337 | 0.988 | 0.573 | 0.484 | 0.488 | |||||
| P value of II vs III | 0.001 | <0.001 | 0.017 | 0.002 | <0.001 | <0.001 | 0.017 | |||||
| P value of I vs III | <0.001 | <0.001 | 0.002 | 0.002 | <0.001 | <0.001 | 0.003 | |||||
UM I, II and III mean refer to the three subgroups of UM patients at three different time points respectively. I: Pre-operative; II: Post-operative 1mo; III: Post-operative 6mo. F value corresponds to the fixed factor of the grouping in univariate analysis of variance. P value below 0.05 was regarded as significant. The following three P values were generated by multiple comparisons between each of two subgroups of UM patients and were regarded as significant if <0.05. 1Peptide peaks of marker G (MW 4173.61 Da) was not detected in sera of UM II patients and multiple camparisons were not performed.
Figure 2. The variances in peak intensities of markers A-E in the 10 UM patients along the time before and after surgery and comparison with corresponding peaks in the normal group.
Groups 1, 2 and 3 refer to the three subgroups of UM patients at three different time points respectively. 1: Pre-operative; 2: Post-operative 1mo; 3: Post-operative 6mo. The mean intensities at different time points of a UM patient were demonstrated with bars of the same color. Group 4 refers to the normal group, each shown in a different color bar.
Identification of Uveal Melanoma Markers
With this bead-based proteomic technology, we found several potential UM markers. Markers A-N and another three peptides with molecular mass 9288.95, 4396 and 4645 Da were selected for further identification based on the highest peak intensities of these peptides. After fractionation with the same MB-IMAC copper beads kit, samples were subjected to MALDI TOF/TOF MS analysis and analyzed by FlexAnalysis software. The MS fingerprint was subjected to Mascot searching for protein identification.
In the same sample, markers A and B (Figures 3, 4) were identified to be fibrinogen alpha chain (fibrinopeptide A) precursors with a Mascot score of 187. The Mascot scores were 130 and 76 for markers A and B respectively. The mass accuracy was approximately 10 ppm. The sequence of marker A was determined to be G.EGDFLAEGGGVR.G and the sequence of marker B was A.DSGEGDFLAEGGGVR.G.
Figure 3. Serum protein profile of a sample from the normal group with highest intensity of marker A (MW: 1467 Da).
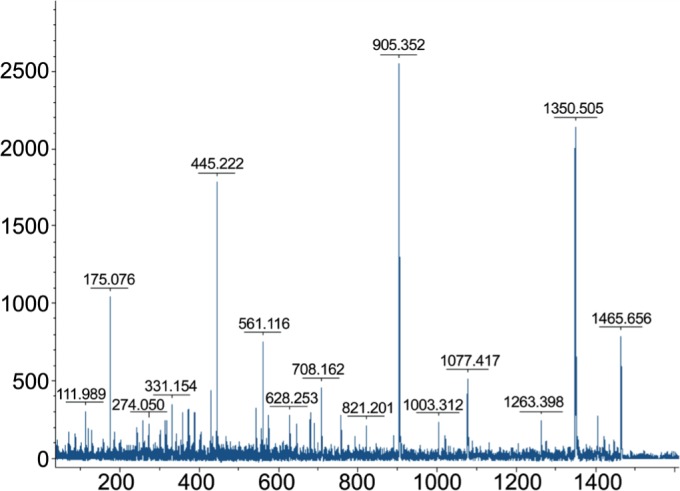
Figure 4. Serum protein profile of a sample from the normal group with highest intensity of marker B (MW: 1207.56 Da).
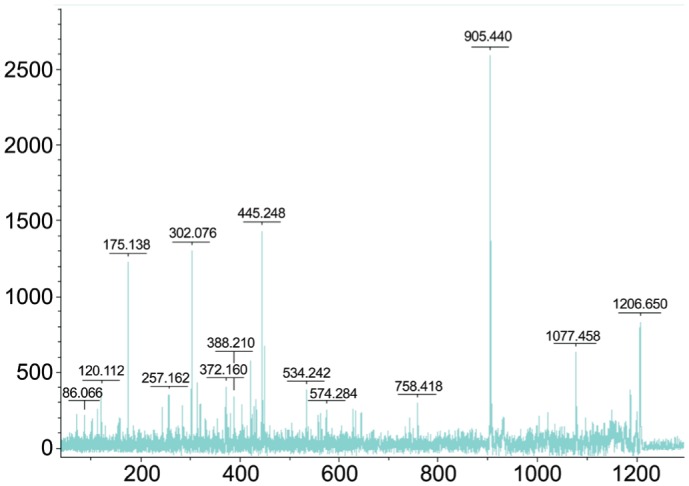
The sample labeled C20 was used for peptide identification of both markers (MW: 1467 D and 1207.56 Da). After fractionation with Cu-bead, this sample was subjected to MALDI-TOF/TOF MS and analyzed by FlexAnalysis softwares. The mass spectrum is shown with MW calculation (m/z values) along the x-axis and relative intensity along the y-axis on the top.
DISCUSSION
In current research, we utilized the affinity MB (ClinProt purification reagent sets from Bruker Daltonics) to fractionate the serum proteome of blood samples from UM patients and healthy controls. Chemically coated MB (particle size <1 µm; mean pore size 40 nm; specific surface area 100 cm2/g) are with various defined surface functionalities. The vast area provided by these MB facilitates selective affinity distillation of low MW protein/peptides (mainly from 800 Da to 20 000 Da). This platform of proteomics has been used to explore the proteome of oral cancers[8], nasopharyngeal cancer[9], head and neck carcinoma[10], cerebral glial carcinoma[11], prostate, bladder and breast cancer[12] as well as pneumonia and leukemia in recent years. We directly profiled protein/peptide patterns from affinity bead-purified serum samples with MALDI-TOF MS and determined a set of differentially expressed protein/peptides. To better characterize this dataset, we further selected several potential markers that discriminated UM patients' sera from healthy control samples.
Statistically, these potential markers are of various degrees of sensitivity, specificity and accuracy rates and no single marker is suitable for effective screening alone. However, there are combined markers (for example, marker A and G) that yield better efficiency of discrimination than any individual marker. Another two peptides (1207 Da and 9289 Da) together can distinguish two groups with even higher accuracy rate. Given that oncogenesis is often heterogeneous and complicated, a single biomarker is hard to find to easily distinguish different groups. Combined use of multiple blood markers has been shown to be an advisable approach to improve diagnostic strength[9],[13]–[14].
The design of our study was also based on a well-accepted hypothesis that tumor cells synthesize, secrete and might eventually release a set of specific protein/peptides into the microenvironment around the tumor. Some of these protein/peptides (those of low MW especially) might end up in the blood circulation through tissue fluid or lymph. Detection of such protein/peptides might reveal tumor relevant information. By accomplishing surgical procedures to completely remove the tumor burden of these UM patients, we postulated that after a period of time, these tumor-related proteins/peptides could show possible patterns of approaching the normal levels. Our results that after 6mo the levels of most differentially expressed protein/peptides above mentioned returned to normal levels is in favor of this hypothesis. Besides, after comparison of differentially expressed proteins/peptides over a period of 6mo after the surgery, we discovered that some potential markers showed continual trend of increasing (e.g. markers A and B) or declining (e.g. markers C, D and E) and showed no statistical difference from that of the healthy controls.
Markers A and B were both identified to be fibrinogen alpha chain (fibrinopeptide A) precursors. Fibrinogen is a plasma glycoprotein synthesized in the liver and is composed of 3 structurally different subunits: 2 alpha chains, 2 beta chains and 2 gamma chains. The association of fibrinogen with regulation of tumor growth has been studied over decades. Local tumor cells may induce fibrinolysis, which may stimulate cell proliferation and self-regulated progression of the tumor[15]–[16]. A series of mechanisms regulating the level of fibrinogen in blood was reported to previously[15],[17]. Fibrinogen might play a role in tumor growth regulation. Abundant fibrinogen was discovered in the connective tissue of breast cancer while the adjacent normal tissue was not[18]. Zacharski et al[16] also reported increased amount of alpha and beta chains of fibrinogen around active tumor cells of small cell carcinoma of the lung. Increased levels of plasma fibrinogen were also reported in breast carcinoma and skin malignant melanoma patients[19]. A recent comparative proteomic research of oral cancer plasma found that the level of alpha chain of fibrinogen increased significantly compared to that of normal controls[8]. Our results showed decreased level of alpha chain precursor peptides of fibrinogen, indicating there might be different mechanism of fibrinolysis involved in the oncogenesis of UM. Further studies are needed to validate this finding.
Ideally, blood samples for biomarker measurement are collected centrally and processed immediately to avoid any unwanted changes in concentrations that could affect validity. In large-scale epidemiologic and clinical studies, however, this theoretical goal must give way to a more pragmatic approach. In our research, the reproducibility is evaluated with respect to CV, which were around 14%-20%. In a proteometric research on oral cancer plasma biomarkers, Cheng et al[20] reported CV being lower than 8%. We noticed that they used the ultraflex MALDI-TOF MS for proteomic profiling. In addition to the sensitivity of individual mass spectrometer (because true changes over time can be established only if measurement error is small), it should also be noted that serum sample storage time, thawing rounds, manual or automatic handling all could intervene with the reproducibility of proteomic profiling. Besides these, reliability and validity coefficients were influenced by variability in concentration, possibly because of the small magnitude of the individual protein/peptides.
The limitations should be mentioned here. One limitation is that we did not measure in duplicate or triplicate to adjust intra-assay variations. In addition, serum samples were not analyzed within one run due to our pre-set limitations on storage time to achieve sample quality control. In consequence, interassay variability cannot be avoided or ignored. Another limitation is that serum samples were stored in small volume (200 µL each). They were not randomized before analysis. Possible bias from order of draw, although unlikely, therefore cannot be ruled out. Among the many differentially expressed protein/peptides and selected potential markers, only two of them were identified. The nature of other differentially expressed proteins remains unknown, most likely because of extremely low amount of materials in the samples. Another limitation arised from the doubts in whether it is possible to eliminate tumor burden completely in our cases via surgical removal. There was hypothesis that UM patients might have developed micro-metastasis, even before they are diagnosed[21]. In the case of the existence of micro-metastasis, surgeries alone will not achieve its goal of removing tumor cells and hence their secretions or releases completely.
In conclusion, we have shown that a convenient, fast proteomic technique, affinity bead purification and MALDI-TOF analysis in combination with bioinformatic software, facilitates the detection and identification of novel biomarkers. This study using MALDI-TOF MS coupled with MB fractionation distinguished differentially expressed peptides but failed to identify most of these peptides probably due to extremely low amounts of them in the blood circulation. Proteomic pattern diagnosis is a promising tool for early disease detection and may help to reduce the number of invasive medical procedures in the future, such as biopsies and investigative surgeries.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81570891; No.81272981); the Beijing Natural Science Foundation (No.7151003); Advanced Health Care Professionals Development Project of Beijing Municipal Health Bureau (No.2014-2-003); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No.ZYLX201307).
Conflicts of Interest: Shi XY, None; Li Q, None; Wei WB, None; Tao LM, None.
REFERENCES
- 1.Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140(4):612–617. doi: 10.1016/j.ajo.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Vonk DT, Kim Y, Javid C, Gordon JD, Stea B. Prescribing to tumor apex in episcleral plaque iodine-125 brachytherapy for medium-sized choroidal melanoma: A single-institutional retrospective review. Brachytherapy. 2015;14(5):726–733. doi: 10.1016/j.brachy.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Abildgaard SK, Vorum H. Proteomics of uveal melanoma: a minireview. J Oncol. 2013;2013:820953. doi: 10.1155/2013/820953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Missotten GS, Beijnen JH, Keunen JE, Bonfrer JM. Proteomics in uveal melanoma. Melanoma Res. 2003;13(6):627–629. doi: 10.1097/00008390-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Pardo M, García A, Thomas B, Piñeiro A, Akoulitchev A, Dwek RA, Zitzmann N. Proteome analysis of a human uveal melanoma primary cell culture by 2-DE and MS. Proteomics. 2005;5(18):4980–4993. doi: 10.1002/pmic.200500030. [DOI] [PubMed] [Google Scholar]
- 6.Pardo M, García A, Thomas B, Piñeiro A, Akoulitchev A, Dwek RA, Zitzmann N. The characterization of the invasion phenotype of uveal melanoma tumour cells shows the presence of MUC18 and HMG-1 metastasis markers and leads to the identification of DJ-1 as a potential serum biomarker. Int J Cancer. 2006;119(5):1014–1022. doi: 10.1002/ijc.21942. [DOI] [PubMed] [Google Scholar]
- 7.Ramasamy P, Murphy CC, Clynes M, Horgan N, Moriarty P, Tiernan D, Beatty S, Kennedy S, Meleady P. Proteomics in uveal melanoma. Exp Eye Res. 2014;118:1–12. doi: 10.1016/j.exer.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Khurshid Z, Zohaib S, Najeeb S, Zafar MS, Slowey PD, Almas K. Human saliva collection devices for proteomics: an update. Int J Mol Sci. 2016;17(6):pii:E846. doi: 10.3390/ijms17060846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ZT, Liang ZG, Zhu XD. A review: proteomics in nasopharyngeal carcinoma. Int J Mol Sci. 2015;16(7):15497–15530. doi: 10.3390/ijms160715497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrique T, José Freitas da Silveira N, Henrique Cunha Volpato A, et al. HNdb: an integrated database of gene and protein information on head and neck squamous cell carcinoma. Database (Oxford) 2016;2016:pii:baw026. doi: 10.1093/database/baw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva J, Philip J, Entenberg D, Chaparro CA, Tanwar MK, Holland EC, Tempst P. Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal Chem. 2004;76(6):1560–1570. doi: 10.1021/ac0352171. [DOI] [PubMed] [Google Scholar]
- 12.Sacco F, Silvestri A, Posca D, Pirrò S, Gherardini PF, Castagnoli L, Mann M, Cesareni G. Deep proteomics of breast cancer cells reveals that metformin rewires signaling networks away from a pro-growth state. Cell Syst. 2016;2(3):159–171. doi: 10.1016/j.cels.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Lin YC, Wu Chou YH, Liao IC, Cheng AJ. The expression of mammaglobin mRNA in peripheral blood of metastatic breast cancer patients as an adjunct to serum tumor markers. Cancer Lett. 2003;191(1):93–99. doi: 10.1016/s0304-3835(02)00545-1. [DOI] [PubMed] [Google Scholar]
- 14.Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto VD, Annibale B, Buonadonna A, Pederzoli P, Delle Fave G. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27(1):6–11. doi: 10.1007/BF03350903. [DOI] [PubMed] [Google Scholar]
- 15.Weiskirchen R, Tacke F. Liver fibrosis: from pathogenesis to novel therapies. Dig Dis. 2016;34(4):410–422. doi: 10.1159/000444556. [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Memoli VA, Rousseau SM. Thrombin-specific sites of fibrinogen in small cell carcinoma of the lung. Cancer. 1988;62(2):299–302. doi: 10.1002/1097-0142(19880715)62:2<299::aid-cncr2820620212>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Palumbo JS, Talmage KE, Liu H, La Jeunesse CM, Witte DP, Degen JL. Plasminogen supports tumor growth through a fibrinogen-dependent mechanism linked to vascular patency. Blood. 2003;102(8):2819–2817. doi: 10.1182/blood-2003-03-0881. [DOI] [PubMed] [Google Scholar]
- 18.Rybarczyk BJ, Simpson-Haidaris PJ. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000;60(7):2033–2039. [PubMed] [Google Scholar]
- 19.Mannucci PM, Vaglini M, Maniezzo M, Magni E, Mari D, Cascinelli N. Hemostatic alterations are unrelated to the stage of tumor in untreated malignant melanoma and breast carcinoma. Eur J Cancer Clin Oncol. 1985;21(6):681–685. doi: 10.1016/0277-5379(85)90265-2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AJ, Chen LC, Chien KY, Chen YJ, Chang JT, Wang HM, Liao CT, Chen IH. Oral cancer plasma tumor marker identified with bead-based affinity-fractionated proteomic technology. Clin Chem. 2005;51(12):2236–2244. doi: 10.1373/clinchem.2005.052324. [DOI] [PubMed] [Google Scholar]
- 21.Pardo M, Dwek RA, Zitzmann N. Proteomics in uveal melanoma research: opportunities and challenges in biomarker discovery. Expert Rev Proteomics. 2007;4(2):273–286. doi: 10.1586/14789450.4.2.273. [DOI] [PubMed] [Google Scholar]