Abstract
AIM
To observe the attenuation of ethanol extract of Herba Scutellaria barbata (SE) against diabetic retinopathy (DR) and its engaged mechanism.
METHODS
C57BL/6J mice were intraperitoneally injected with streptozotocin (STZ, 55 mg/kg) for 5 consecutive days to induce diabetes. The diabetic mice were orally given with SE (100, 200 mg/kg) for 1mo at 1mo after STZ injection. Blood-retinal barrier (BRB) breakdown was detected by using Evans blue permeation assay. Real-time polymerase chain reaction (RT-PCR), Western blot and immunofluorescence staining were used to detect mRNA and protein expression. Enzyme-linked immunosorbent assay (ELISA) was used to detect serum contents of tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β.
RESULTS
SE (100, 200 mg/kg) reversed the breakdown of BRB in STZ-induced diabetic mice. The decreased expression of retinal claudin-1 and claudin-19, which are both tight junction (TJ) proteins, was reversed by SE. SE decreased the increased serum contents and retinal mRNA expression of TNF-α and IL-1β. SE also decreased the increased retinal expression of intercellular cell adhesion molecule-1 (ICAM-1). SE reduced the increased phosphorylation of nuclear factor kappa B (NFκB) p65 and its subsequent nuclear translocation in retinas from STZ-induced diabetic mice. Results of Western blot and retinal immunofluorescence staining of ionized calcium-binding adapter molecule 1 (Iba1) demonstrated that SE abrogated the activation of microglia cells in STZ-induced diabetic mice.
CONCLUSION
SE attenuates the development of DR by inhibiting retinal inflammation and restoring the decreased expression of TJ proteins including claudin-1 and claudin-19.
Keywords: scutellaria barbata, diabetic retinopathy, tight junction, inflammation, nuclear factor kappa B, microglia
INTRODUCTION
With the elevating of living standards, the incidence of diabetes is increasing in both developed and developing countries. Diabetic retinopathy (DR) is one of the most common and serious microvascular complications of diabetes mellitus (DM), and vison loss due to DR has become a major cause of blindness in adult[1]–[2]. The pathogenesis of DR is generally divided into two stages including non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR) according to the international clinical DR disease severity scale[3]. In the early NPDR stage, an increase in vasopermeability due to blood-retinal barrier (BRB) breakdown is a key factor for diabetic macular edema (DEM), which will lead to considerable vision loss in diabetic patients[4]–[5]. Moreover, NPDR will further progress into PDR, and retinal angiogenesis occurs in this later stage, which will cause vitreous hemorrhage, traction retinal detachment, and finally vision loss[2].
It has been reported that inflammation plays an important role in regulating the development of DR, and DR is generally considered as an inflammatory disease[6]. A variety of reports demonstrated the increased adhesion of leukocytes to retinal vessels in diabetic animals, and the levels of various pro-inflammatory cytokines were increased in the retina and vitreous in diabetes attributed to the activation of pro-inflammatory transcriptional factors including nuclear factor kappa B (NFκB)[7]–[11]. Such increased leukostasis and the elevated pro-inflammatory cytokines and other growth factors will contribute to the breakdown of BRB, which is the early key event in the development of DR[7]–[9]. By using pharmacologic inhibitors or anti-inflammatory agents to inhibit the production of inflammatory mediators, some therapeutic approaches have been identified that obviously attenuated the development of DR, especially the early stage of DR[11]–[12]. Recently, some traditional Chinese medicines or formulas have been reported to attenuate DR by inhibiting inflammation, such as Dang-Gui-Bu-Xue-Tang (an aqueous extract of Radix astragali, Angelica sinensis and Panax notoginseng)[13], Rhodiola sachalinensis (Gaoshan Hongjingtian)[14], and Dendroboum chrysotoxum Lindl[15].
Herba Scutellaria barbata (SE), named Ban-Zhi-Lian in Chinese, is the dried whole plant of Scutellaria barbata D. Don. (Labiatae). It has been used as a drug for clearing away heat and toxic materials, promoting blood circulation and removing blood stasis, and reducing swelling and alleviating pain for thousands of years in China. Modern pharmacological studies demonstrated that S. barbata had a variety of activities including anti-cancer, anti-angiogenesis, anti-inflammation, anti-complement, and antioxidant[16]–[20]. In this study, we aimed to observe whether the ethanol extract of SE can attenuate NPDR in streptozotocin (STZ)-induced diabetic mice by inhibiting retinal inflammation and restoring the decreased expression of tight junction (TJ) proteins.
MATERIALS AND METHODS
Materials
Reagents
Antibodies for 536Ser phosphorylated NFκBp65, NFκBp65, Lamin B1 and β-actin were all purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody for Iba1 was bought from GeneTax Inc. (Alton Parkway Irvine, CA, USA). Antibodies for claudin-1 and claudin-19 were purchased from Santa Cruz (Santa Cruz, CA, USA). Antibody for intercellular cell adhesion molecule-1 (ICAM-1) was purchased from Biobasic Inc (Shanghai, China). Peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (H+L) and anti-mouse IgG (H+L) were purchased from Jackson ImmunoResearch (West Grove, PA, USA). Alexa Fluor 488 goat anti-Rabbit IgG were purchased from BD Biosciences (Franklin Lakes, NJ, USA). NE-PER nuclear and cytoplasmic extraction reagents, and Pierce BCA Protein Assay Kits were purchased from ThermoFisher Scientific (Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits were obtained from RapidBio (West Hills, CA, USA). Trizol reagent and 4′,6-Diamidino-2-phenylindole (DAPI) were purchased from Life Technology (Carlsbad, CA, USA). PrimeScriptRT Master Mix and SYBR Premix Ex TaqTM were purchased from Takara (Shiga, Japan). Other reagents unless noted were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of Herba Scutellaria barbata
The powder of SE was soaked in 80% ethanol for 2h at room temperature. The macerated plant material was extracted under reflux for 2h three times. The combined extraction was concentrated and dried under vacuum using a rotary evaporator under reduced pressure.
Experimental animals
Specific pathogen free male C57BL/6J mice (weight: 18-22 g) were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Science (Shanghai, China). The mice were fed with a standard laboratory diet and given free access to tap water, living in a controlled room temperature (22°C±1°C), humidity (65%±5%) with a 12:12-h light/dark cycle. All animals have received humane care in compliance with the institutional animal care guidelines approved by the Experimental Animal Ethical Committee of Shanghai University of Traditional Chinese Medicine.
Methods
Treatment of animals
Fifty mice were intraperitoneally injected (i.p.) with STZ (55 mg/kg) for 5 consecutive days, while the other sixteen mice were i.p. with physiological saline and served as control animals. The concentration of serum glucose was measured 7d after the last injection, and the mice with high glucose concentration (>16.5 mmol/L) were considered as diabetic mice. In this experiment, the glucose concentration in 48 mice was >16.5 mmol/L, and those mice were randomly divided into three groups: DM group (n=16), DM+SE (100 mg/kg; n=16), DM+SE (200 mg/kg; n=16), respectively. At 1mo after the injection of STZ, the mice were orally given with SE (100, 200 mg/kg, intragastric administration) for 1 consecutive month. At 2mo after the injection of STZ, the mice were anesthetized by sodium pentobarbital (30 mg/kg, i.p.), the blood samples were taken from the abdominal aorta, and the eyes were removed immediately. The body weight was monitored and the concentration of blood glucose was determined by Glucometer (Accu-Check Performa Nano, Roche Diagnostics, Germany) during the whole experimental process.
Evans blue permeation assay
BRB breakdown was evaluated as described in our previously published papers[15],[21]. In brief, mice were injected with 2% Evans blue (10 µL/g, i.p.) in phosphate buffered saline (PBS), and blood was extracted through the left ventricle at 2h after injection. The mice were further perfused with PBS to completely remove the Evans blue dye in vessels. Retinas were carefully dissected and the weight was determined after thoroughly drying. Next, the retinas were incubated in 120 µL formamide for 18h at 70°C to extract Evans blue dye. The extract was centrifuged twice at 10 000× g for 1h at 4°C, and the absorbance was determined at 620 nm. The concentration of Evans blue dye in the extracts was calculated using a standard curve of Evans blue dye in formamide and then normalized to the dried retinal weight.
Real-time polymerase chain reaction analysis
Total RNA in retinas was isolated by using Trizol reagent, and the RNA content was determined by measuring the optical density at 260 nm. cDNA was synthesized according to the instruction described in the kits. Real-time polymerase chain reaction (RT-PCR) was performed by using kits, and the relative expression of target genes was normalized to actin, analyzed by the 2−ΔΔCt method and given as ratio compared with the control. The primer sequences used in this study are shown in Table 1.
Table 1. The list of primers for RT-PCR.
| Target | Primer | Sequence |
| Tnf | FP | 5′-AGGCACTCCCCCAAAAGAT-3′ |
| RP | 5′-CAGTAGACAGAAGAGCGTGGTG-3′ | |
| Il1β | FP | 5′-AGTTGACGGACCCCAAAAG-3′ |
| RP | 5′-CTTCTCCACAGCCACAATGA-3′ | |
| Icam1 | FP | 5′-CCGCTGTGCTTTGAGAACT-3′ |
| RP | 5′-GGTCCTTGCCTACTTGCTG-3′ | |
| Actin | FP | 5′-TACAGCTTCACCACCACAGC-3′ |
| RP | 5′-TCTCCAGGGAGGAAGAGGAT-3′ | |
| Cldn1 | FP | 5′-CAGAAGATGTGGATGGCTGTC-3′ |
| RP | 5′-GGGGTCAAGGGGTCATAGAA-3′ | |
| Cldn5 | FP | 5′-TTGGAAGGGGCTGTGGAT-3′ |
| RP | 5′-CGGTCAAGGTAACAAAGAGTGC-3′ | |
| Cldn19 | FP | 5′-GCAAACTCTACGATTCACTCCTG-3′ |
| RP | 5′-CCACGACACTGAGCACCAT-3′ | |
| Ocln | FP | 5′-TTCCTCTGACCTTGAGTGTGG-3′ |
| RP | 5′-CTCTTGCCCTTTCCTGCTTT-3′ | |
| Tjp1 | FP | 5′-CTCCAGGTGCTTCTCTTGCT-3′ |
| RP | 5′-TATCTTCGGGTGGCTTCACT-3′ |
FP: Forward primer; RP: Reverse primer.
Western-blot analysis
Cytosolic and nuclear proteins in retinas were isolated as described in NE-PER nuclear and cytoplasmic extraction kits. After centrifugation, protein concentration of the resulting supernatants was determined. The protein concentration in each sample was normalized to the equal protein concentration. The protein samples were subjected to SDS-PAGE and then electrophoretically transferred onto an immobilon-P PVDF membrane (Millipore). The membranes were incubated with primary and secondary antibodies. Immunoblots were visualized using a chemiluminescent reagent. The grey densities of the protein bands were normalized by using β-actin or Lamin B1 density as an internal control, respectively.
Enzyme-linked immunosorbent assay analysis
The whole blood was centrifuged at 3000 rpm, 4°C for 15min, and serum was collected for ELISA analysis as described in kits.
Immunofluorescence staining
Paraffin-embedded sections of retinas (5 mm) were de-paraffinized in xylene, and re-hydrated in an ethanol gradient with distilled water. Retinas were incubated with 5% bovine serum albumin to minimize non-specific binding after endogenous peroxidase activity was quenched. After rinsing three times, retinas were incubated with Iba1 antibody at 4°C overnight, and further incubated with Alexa fluor 488 goat anti-rabbit IgG (H+L) antibody at room temperature for 1h. After rinsing three times again, retinas were incubated with DAPI for 10min, and images were captured under an inverted microscope (IX81, Olympus, Japan).
High performance liquid chromatography analysis
Analysis was performed on a prominence high performance liquid chromatography instrument (HPLC; Agilent) equipped with auto-sampler, quaternary pump, column heater compartment and DAD with an on-line degasser. The sample was separated on a Sepax HP-C18 column (4.6×250 mm, 5 µm). The mobile phase consisted of methanol, water and ethylic acid (v/v/v =35:61:4). The flow rate was 1.0 mL/min, and column temperature was set at 25°C. The DAD detector monitored signals between 190 nm and 400 nm, and the on-line UV spectra were recorded at 335 nm for scutellarin.
Statistical Analysis
Data were expressed as means±standard error of the mean (SEM). The significance of differences between groups was evaluated by one-way ANOVA with LSD post hoc test, and P<0.05 was considered as statistically significant differences.
RESULTS
Measurement of Body Weight and Blood Glucose Concentration
As shown in Figure 1A, the body weight of diabetic mice was lower than that of normal control mice (P<0.001). However, there was no obvious alteration in the body weight of mice after SE treatment. Next, Figure 1B showed that blood glucose concentration in diabetic mice was higher than that in normal control mice (P<0.001). Also, SE had no much effect on blood glucose concentration in diabetic mice.
Figure 1. Analysis of serum glucose level and body weight.
A: Body weight; B: Serum glucose level. n=16, eP<0.001 compared to control.
Herba Scutellaria Barbata Attenuated Blood-retinal Barrier Breakdown in Diabetic Mice
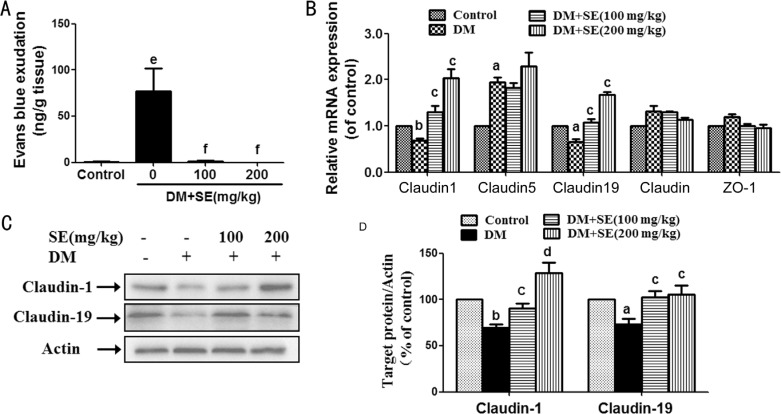
As shown in Figure 2A, the increased leakage of Evans blue dye was observed in retinas from STZ-induced diabetic mice (P<0.001), indicating the increased retinal vessel leakage. After diabetic mice were treated with SE (100, 200 mg/kg), such increased retinal vessel leakage was almost totally abrogated (P<0.001).
Figure 2. SE attenuated BRB breakdown and restored the decreased expression of claudin-1 and claudin-19 in retinas from STZ-induced diabetic mice.
A: BRB breakdown is detected by Evans blue dye leakage assay (n=6); B: Retinal mRNA expression of occludin, ZO-1, claudin-1, claudin-5 and claudin-19 (n=3-5); C: Retinal protein expression of claudin-1 and claudin-19, representative blots for claudin-1, claudin-19 and actin, and the results represent at least three independent experiments; D: The quantitative densitometric analysis of claudin-1 and claudin-19, and the results are presented as percentage of control (n=4-5). aP<0.05, bP<0.01, eP<0.001 compared to control; cP<0.05, dP<0.01, fP<0.001 compared to DM.
Herba Scutellaria Barbata Reversed the Decreased Expression of Tight Junction Proteins
As shown in Figure 2B, retinal mRNA expression of claudin-1 and claudin-19 was decreased in STZ-induced diabetic mice (P<0.05, P<0.01), whereas SE (100, 200 mg/kg) reversed such decrease (P<0.05). Retinal mRNA expression of claudin-5 was weakly increased in STZ-induced diabetic mice (P<0.05), but there was no alteration after SE (100, 200 mg/kg) treatment. Retinal mRNA expression of zonula occludens-1 (ZO-1) and occludin was not changed in STZ-induced diabetic mice with or without SE treatment.
Next, the results of Western-blot showed that the protein expression of claudin-1 and claudin-19 was decreased in retinas from STZ-induced diabetic mice (P<0.05, P<0.01), whereas SE (100, 200 mg/kg) reversed the decreased retinal protein expression of claudin-1 and claudin-19 (P<0.05, P<0.01) (Figure 2C, 2D).
Herba Scutellaria Barbata Reduced the Increased Expression of Intercellular Cell Adhesion Molecule-1, Tumor Necrosis Factor-α and Interleukin-1β
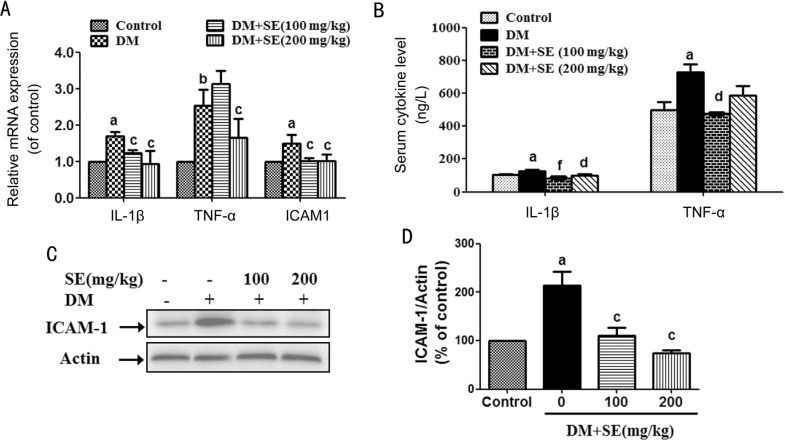
As shown in Figure 3A, retinal mRNA expression of ICAM-1, tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β was increased in STZ-induced diabetic mice (P<0.05, P<0.01). However, SE (100, 200 mg/kg) reduced the increased retinal mRNA expression of IL-1β and ICAM-1, and SE (200 mg/kg) reduced the increased retinal mRNA expression of TNF-α in diabetic mice (P<0.05) (Figure 3A). In addition, SE (100, 200 mg/kg) reduced the elevated serum content of IL-1β, and SE (100 mg/kg) reduced the elevated serum content of TNF-α in STZ-induced diabetic mice (P<0.01, P<0.001) (Figure 3B). SE (100, 200 mg/kg) also reduced the increased retinal ICAM-1 protein expression in STZ-induced diabetic mice (P<0.05) (Figure 3C, 3D).
Figure 3. SE reduced the increased ICAM-1, TNF-α and IL-1β expression in STZ-induced diabetic mice.
A: Retinal mRNA expression of TNF-α, IL-1β, and ICAM-1 (n=3-4); B: Serum contents of TNF-α and IL-1β (n=6); C: Retinal protein expression of ICAM-1, representative blots for ICAM-1 and actin, and the results represent four independent experiments; D: The quantitative densitometric analysis of ICAM-1, and the results are presented as percentage of control (n=4). aP<0.05, bP<0.01 compared to control; cP<0.05, dP<0.01, fP<0.001 compared to DM.
Herba Scutellaria Barbata Abrogated Retinal Nuclear Factor Kappa B Activation in Diabetic Mice
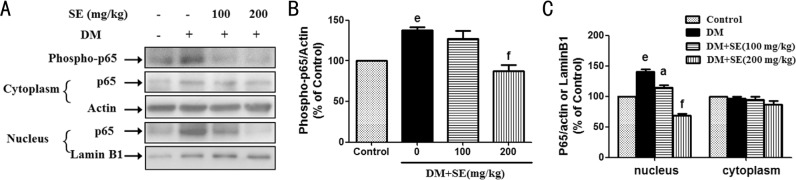
As shown in Figure 4A, 4B, retinal expression of phosphorylated NFκBp65 was increased in STZ-induced diabetic mice, whereas SE (200 mg/kg) reduced such increase (P<0.001). The expression of nuclear NFκBp65 was increased in retinas from STZ-induced diabetic mice (P<0.001), but there was no significant change in the expression of cytosolic NFκBp65 (Figure 4A, 4C). Further, SE (100, 200 mg/kg) reduced the increased nuclear expression of NFκBp65 in STZ-induced diabetic mice (P<0.05, P<0.001) (Figure 4A, 4C).
Figure 4. SE abrogated the activation of NFκB signaling pathway in retinas from STZ-induced diabetic mice.
A: SE reduced the increased expression of phosphorylated NFκBp65 and its subsequent nuclear translocation, representative blots for phosphorylated NFκBp65, nuclear and cytosolic NFκBp65, actin and Lamin B1, and the results represent at least three independent experiments; B: The quantitative densitometric analysis of phosphorylated NFκBp65, and the results are presented as percentage of control (n=3); C: The quantitative densitometric analysis of nuclear and cytosolic NFκBp65, and the results are presented as percentage of control (n=3-4). eP<0.001 compared to control; aP<0.05, fP<0.001 compared to DM.
Herba Scutellaria Barbata Inhibited the Activation of Microglia Cells in Diabetic Mice
Ionized calcium-binding adapter molecule 1 (Iba1) is an often used biomarker for microglia[22]. As shown in Figure 5A, 5B, Iba1 expression was increased in retinas from STZ-induced diabetic mice, whereas SE (200 mg/kg) reduced such increase (P<0.001). Next, we observed the expression of Iba1 in retinas from STZ-induced diabetic mice by using Iba1 immunofluorescence staining assay. As shown in Figure 5C, the number of Iba1-positive microglia cells was increased in ganglion cell layer (GCL) and inner plexiform layer (IPL) in retinas from STZ-induced diabetic mice than from normal control mice. Moreover, such increase was reduced in SE (100, 200 mg/kg)-treated mice.
Figure 5. SE abrogated the activation of retinal microglia cells in STZ-induced diabetic mice.
A: SE reduced the elevated protein expression of Iba1, representative blots for Iba1 and actin, and the results represent three independent experiments; B: The quantitative densitometric analysis of Iba1, and the results are presented as percentage of control (n=3); C: Retinal expression of Iba1 in STZ-induced diabetic mice, the representative pictures of retinal immunofluorescence staining of Iba1 and DAPI, and also the merge of Iba1- and DAPI-stained images are shown at the left (scale bars: 20 mm), the enlarged representative pictures of retinal Iba1-stained images are shown at the right (scale bars: 5 mm), red arrows indicate microglia cells. eP<0.001 compared to control; fP<0.001 compared to DM.
High Performance Liquid Chromatography Analysis of Herba Scutellaria Barbata
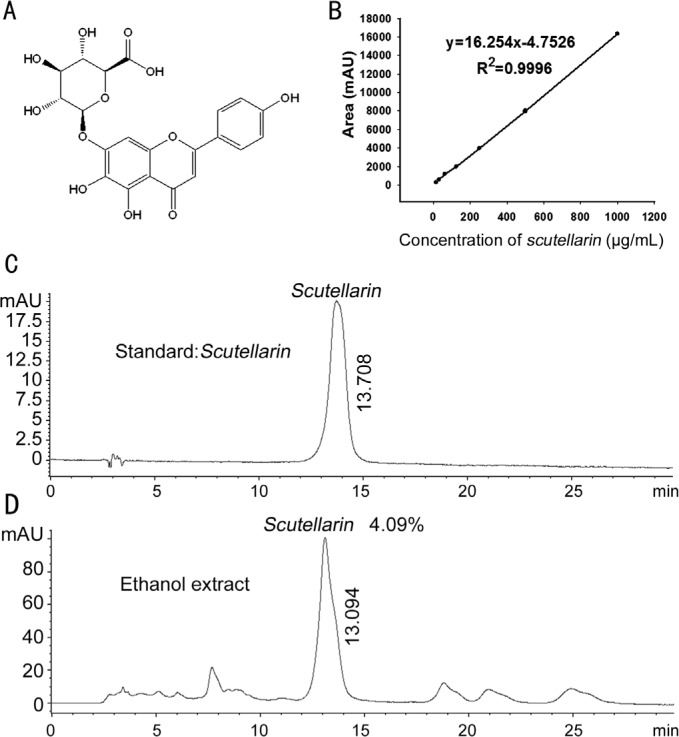
Scutellarin is the main compound in SE, and it is also the chemical marker used by the Chinese pharmacopoeia for evaluating the quality of SE[23]. The chemical structure of scutellarin was shown in Figure 6A. The HPLC chromatograms and calibration curve of scutellarin were shown in Figure 6B–6D, and the HPLC results demonstrated that the amount of scutellarin in SE was 4.09%.
Figure 6. HPLC analysis of the content of scutellarin in SE.
A: The chemical structure of scutellarin; B: The calibration curve of scutellarin; C: HPLC chromatogram of scutellarin; D: HPLC chromatogram of scutellarin in SE.
DISCUSSION
The increased retinal blood vessel permeability due to the breakdown of BRB is the hallmark for NPDR, which is the early stage of DR[4]–[5]. In this study, we found that SE reduced the increased BRB leakage in STZ-induced diabetic mice. This is the first study demonstrated that SE had protection against NPDR, which indicates the potential application of SE for the treatment of DR.
The BRB is div ided into an inner and an outer BRB. The inner BRB is composed by TJ between retinal capillary endothelial cells and the outer BRB is formed by TJ between retinal pigment epithelial cells[4],[24]. Both inner and outer BRB TJ proteins are mainly composed of occludins, claudins, ZO proteins, and junctional adhesion molecules (JAMs)[25]. ZO-1, occludin, claudin-1, claudin-5 and claudin-19 are the main reported BRB TJ proteins, and they are all reported to be critical for maintaining the integrity of BRB[26]. In this study, we found that the mRNA and protein expression of claudin-1 and claudin-19 was both reduced in retinas from STZ-induced diabetic mice, and such decrease was reversed by SE. The results imply that SE restored BRB dysfunction by up-regulating the expression of claudin-1 and claudin-19. However, there was no alternation in the mRNA expression of occludin and ZO-1, and retinal mRNA expression of claudin-5 was weakly increased in STZ-induced diabetic mice. A variety of reports demonstrated the reduced expression of occludin and claudin-5 in retinas from STZ-induced diabetic rats[27]–[29]. As for ZO-1, some reports showed the decreased expression of retinal ZO-1[27]–[28], whereas others demonstrated that there was no alteration in retinal ZO-1 expression in STZ-induced diabetic rats[29]. However, we got the different results in STZ-induced diabetic mice in this study. We think that different species applied in this experiment and the duration of maintaining diabetes may be the reason for acquiring such different results.
The increased interaction between leukocytes and retinal endothelial cells plays an important role in the early stage of DR, which initiates retinal inflammation and leads to the increased leukostasis and permeability of BRB[30]. ICAM-1 plays a critical role in regulating the adhesion of leukocytes to endothelial cells, and the increased ICAM-1 expression has been reported to be related with the development of DR[31]. TNF-α and IL-1β, two well-known pro-inflammatory cytokines, have also been found to be implicated in the pathogenesis of DR[32]–[33]. In this study, we found that SE decreased retinal expression of ICAM-1, TNF-α and IL-1β, and also reduced the increased serum TNF-α and IL-1β levels in STZ-induced diabetic mice. All those effects will contribute to SE-induced the reduction in retinal leukostasis and inflammation during the development of DR.
Transcription factor NFκB is a key factor involved in inflammation by regulating the expression of pro-inflammatory cytokines such as TNF-α and IL-1β, and ICAM-1[27],[34]. Next, we observed the effects of SE on NFκB activation in retinas from STZ-induced diabetic mice. The results showed that SE reduced the increased phosphorylation of NFκBp65 and its subsequent nuclear translocation, indicating that SE attenuated retinal inflammation in STZ-induced diabetic mice by inhibiting the activation of NFκB signaling pathway.
Previous studies have already demonstrated the activation of retinal microglia cells and its important roles in regulating retinal inflammation during the development of DR[35]. Iba1 is an often used biomarker for microglia[22]. In this study, the results of Western-blot and Iba1 immunofluorescence staining showed that SE reduced the increased activation of microglia cells in STZ-induced diabetic mice, which may contribute to its attenuation on retinal inflammation.
In this study, we can see that SE (200 mg/kg) had better effect than SE (100 mg/kg) in all the experiments except in the result of serum TNF-α content. SE (100 mg/kg) restored the decreased expression of TJ proteins and weakly inhibited retinal inflammation, but did not reduce the increased retinal Iba1 expression. All these results evidenced that SE ameliorated STZ-induced DR in mice.
In conclusion, this study demonstrated that SE prevented BRB breakdown by inhibiting retinal inflammation through abrogating NFκB signaling pathway, restoring the decreased expression of TJ proteins including claudin-1 and claudin-19, and reducing the activation of retinal microglia cells. This study indicates the huge potential application of SE in the treatment of DR.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81173517; No.81322053).
Conflicts of Interest: Mei XY, None; Zhou LY, None; Zhang TY, None; Lu B, None; Ji LL, None.
REFERENCES
- 1.Fantes RJ, Durairaj VD, Oliver SC. Diabetic retinopathy: an update on treatment. Am J Med. 2010;123(3):213–216. doi: 10.1016/j.amjmed.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson CP, Ferris III FL, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 4.Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do carmo A, Ramos P, Reis A, Proenca R, Cunha-vaz JG. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998;67(5):569–575. doi: 10.1006/exer.1998.0546. [DOI] [PubMed] [Google Scholar]
- 6.Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal EC, Manivannan A, Hosoya K, Terasaki T, Cunha-Vaz J, Ambrosio AF, Forrester JV. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48(11):5257–5265. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 8.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Invest Ophthalmol Vis Sci. 2000;41(11):3561–3568. [PubMed] [Google Scholar]
- 9.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158(1):147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 11.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gologorsky D, Thanos A, Vavvas D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediators Inflamm. 2012;2012:629452. doi: 10.1155/2012/629452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao D, Guo Y, Li X, Li X, Li Z, Xue M, Ou Z, Liu M, Yang M, Liu S, Yang S. An aqueous extract of Radix Astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy. Evid Based Complement Alternat Med. 2013;2013:578165. doi: 10.1155/2013/578165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao HS, Shi XY, Wei WB, Wang NL. Effect of the regimen of Gaoshan Hongjingtian on the mechanism of poly (ADP-ribose) polymerase regulation of nuclear factor kappa B in the experimental diabetic retinopathy. Chin Med J (Engl) 2013;126(9):1693–1699. [PubMed] [Google Scholar]
- 15.Yu ZY, Gong CY, Lu B, Yang L, Sheng YC, Ji LL, Wang ZT. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J Diabetes Res. 2015;2015:518317. doi: 10.1155/2015/518317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, Li Q, Chen H, Shen A, Cai Q, Lin J, Peng J. Scutellaria barbata D. Don inhibits growth and induces apoptosis by suppressing IL-6 inducible STAT3 pathway activation in human colorectal cancer cells. Exp Ther Med. 2015;10(4):1602–1608. doi: 10.3892/etm.2015.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao GY, Balunas MJ. Current therapeutic role and medicinal potential of Scutellaria barbata in traditional chinese medicine and western research. J Ethnopharmacol. 2016;182:170–180. doi: 10.1016/j.jep.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Dai ZJ, Lu WF, Gao J, Kang HF, Ma YG, Zhang SQ, Diao Y, Lin S, Wang XJ, Wu WY. Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don. BMC Complement Altern Med. 2013;13:150. doi: 10.1186/1472-6882-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye CL, Huang Q. Extraction of polysaccharides from herbal Scutellaria barbata D. Don. (Ban-Zhi-Lian) and their antioxidant activity. Carbohydr Polym. 2012;89(4):1131–1137. doi: 10.1016/j.carbpol.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 20.Wu YF, Chen DF. Anti-complementary effect of polysaccharide B3-PS1 in Herba Scutellariae Barbatae (Scutellaria barbata) Immunopharmacol Immunotoxicol. 2009;31(4):696–701. doi: 10.3109/08923970903095314. [DOI] [PubMed] [Google Scholar]
- 21.Yu ZY, Lu B, Sheng YC, Zhou LY, Ji LL, Wang ZT. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim Biophys Acta. 2015;1850(4):824–831. doi: 10.1016/j.bbagen.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55(7):687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 23.Chinese Pharmacopoeia Commission . Beijing: The medicine science and technology press of China; 2015. Pharmacopeia of the people's republic of China (2015 version) pp. 118–119. [Google Scholar]
- 24.Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol. 1976;41(2):287–327. doi: 10.1007/BF00146764. [DOI] [PubMed] [Google Scholar]
- 25.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Opthalmol. 2011;21(Suppl 6):S3–S9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 26.Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10(2):103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 27.Leal EC, Martins J, Voabil P, Liberal J, Chiavaroli C, Bauer J, Cunha-Vaz J, Ambrosio AF. Calcium dobesilate inhibits the alternations in tight junction proteins and leukocyte adhesion to retinal endothelial cells induced by diabetes. Diabetes. 2010;59(10):2637–2645. doi: 10.2337/db09-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan LL, Yan H. FTY720 attenuates retinal inflammation and protects blood-retinal barrier in diabetic rats. Invest Ophthalmol Vis Sci. 2016;57(3):1254–1263. doi: 10.1167/iovs.15-18658. [DOI] [PubMed] [Google Scholar]
- 29.Tzeng TF, Hong TY, Tzeng YC, Liou SS, Liu IM. Consumption of polyphenol-rich Zingiber Zerumbet Rhizome extracts protects against the breakdown of the Blood-retinal barrier and retinal inflammation induced by diabetes. Nutrients. 2015;7(9):7821–7841. doi: 10.3390/nu7095369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel N. Targeting leukostasis for the treatment of early diabetic retinopathy. Cardiovasc Hematol Disord Drug Targets. 2009;9(3):222–229. doi: 10.2174/187152909789007052. [DOI] [PubMed] [Google Scholar]
- 31.Ugurlu N, Gerceker S, Yulek F, Ugurlu B, Sari C, Baran P, Cagil N. The levels of the circulating cellular adhesion molecules ICAM-1, VCAM-1 and endothelin-1 and the flow-mediated vasodilation values in patients with type 1 diabetes mellitus with early-stage diabetic retinopathy. Intern Med. 2013;52(19):2173–2178. doi: 10.2169/internalmedicine.52.8572. [DOI] [PubMed] [Google Scholar]
- 32.Capitao M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016;117(11):2443–2453. doi: 10.1002/jcb.25575. [DOI] [PubMed] [Google Scholar]
- 33.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88(10):1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Ann Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 35.Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin AT, Allen DM, Cardona AE. The role of microglia in diabetic retinopathy. J Ophthalmol. 2014;2014:705783. doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]