Abstract
AIM
To investigate the role of CCR7/p-ERK1/2/VEGF signaling in the mouse model of oxygen-induced retinopathy (OIR).
METHODS
Neonatal C57BL/6J mice were evenly randomized into four groups: normoxia, OIR, OIR control (treated with scramble siRNA), and OIR treated (treated with CCR7 siRNA). Normoxia group was not specially handled. Postnatal day 7 (P7) mice in the OIR group were exposed to 75%±5% oxygen for 5d (P7-P12) and then maintained under normoxic conditions for 5d (P12-P17). Mice in the OIR control and OIR treated groups were given injections of scramble or CCR7 siRNA plasmid on P12 before returning to normoxic conditions for 5d (P12-P17). Retina samples were collected from all mice on P17, stained with adenosine diphosphatase (ADPase), and retinal neovascularization (RNV) was assessed. Retinas were also stained with hematoxylin and eosin (H&E) for RNV quantitation. The distribution and expression of CCR7, p-ERK1/2 and vascular endothelial growth factor (VEGF) were assessed via immunohistochemistry, Western blot, and quantitative real-time polymerase chain reaction (qRT-PCR).
RESULTS
High oxygen promoted retinal neovascularization (P<0.05) and increased the number of endothelial nuclei in new vessels extending from the retina to the vitreous body; CCR7 promoted this process (P<0.05). CCR7 and VEGF mRNA were expressed at higher levels in the OIR and OIR control groups than in the normoxia and OIR treated groups. CCR7, p-ERK1/2, and VEGF protein were expressed in the retinas of mice in the OIR and OIR control groups. Intravitreal injection of CCR7 siRNA significantly reduced CCR7, p-ERK1/2, and VEGF expression in the OIR mouse model (all P<0.05). CCR7 significantly enhanced the neovascularization and non-perfusion areas in the OIR group (P<0.05). CCR7 siRNA significantly reduced levels of p-ERK1/2 and VEGF as compared to OIR controls (P<0.05).
CONCLUSION
These results suggest that CCR7/p-ERK 1/2/VEGF signaling plays an important role in OIR. CCR7 may be a potential target for the prevention and treatment of retinopathy of prematurity.
Keywords: chemokine receptor type 7, vascular endothelial growth factor, extracellular signal-regulated kinase, retinal neovascularization, retinopathy of prematurity
INTRODUCTION
C-C chemokine receptor type 7 (CCR7) is mainly expressed in immunocytes such as dendritic cells, naive T cells, B cells, regulatory T cells, memory T cells, and natural killer (NK) cells. CCR7 participates in many physiological and pathological processes in vivo[1]–[2] and mediate cell migration and angiogenesis in many diseases[3].
The extracellular signal-regulated kinase (ERK) signaling pathway participates in the proliferation and angiogenesis of endothelial cells in vitro. The ERK signaling pathway is involved in the release of vascular endothelial growth factor (VEGF) in the retinas of diabetic rats[4]. VEGF is known as the most powerful angiogenesis promoting factor. However, the relationship between CCR7, ERK, and VEGF in retinopathy of prematurity (ROP) has not been illustrated, and the action of CCR7 in retinal neovascularization (RNV) during ROP remains unclear. Therefore, we measured the expression of CCR7, p-ERK1/2, and VEGF in RNV to investigate the role of CCR7/p-ERK1/2/VEGF signaling in the mouse model of oxygen-induced retinopathy (OIR).
MATERIALS AND METHODS
Animals
All experiments were performed in accordance with guidelines set by the Animal Experiment Committee of the Shengjing Hospital of China Medical University, and the study was approved by Shengjing Hospital of China Medical University's Ethics Committee. Mice were housed in a barrier facility with free access to normal food and tap water. They were maintained under standard conditions for lighting (a 12h/12h light/dark cycle), temperature (23°C-25°C), and humidity (50%-60%).
Animal Model
Specific pathogen-free healthy C57BL/6J neonatal mice (China Medical University, Shenyang, China) were evenly randomized into four groups: normoxia, OIR, OIR control (treated with scramble siRNA), and OIR treated (treated with CCR7 siRNA). Mice in the OIR group were handled based on Smith's method[5]. On postnatal day 7 (P7), mice in the the last 3 groups as well as their dams were transferred into a specially made glass case then exposed to 75%±5% oxygen for 5d (P7-P12). The mice in OIR group were returned to room air (normoxic conditions) for 5d (P12-P17). Mice in the last two groups were administered injections of 1 µL scramble or CCR7 siRNA plasmid with a 33-gauge needle attached to a Hamilton syringe on P11, and then returned to normoxic conditionsfor 5d (P12-P17). Mice in the normoxia group were maintained under normoxic conditions for 17d (P0-P17). Mice were humanely euthanized by cervical dislocation on P17, and then their eyeballs were harvested.
Small Interfering RNA
CCR7 and scrambled small interfering RNA (siRNA) was purchased from GenePharma Co. Ltd. (Shanghai, China). The sequence of CCR7 siRNA used is 5′-GAAGUGCAUACACCGAGAC-3′, and its efficacy has been previously demonstrated[6].
Observation of Retinal Neovascularization
Eyeballs were fixed with 4% paraformaldehyde for 3h. The cornea and lens were removed, then the entire retina was dissected and radial cut into four quadrants. Retinas were stained with magnesium-activated adenosine diphosphatase (ADPase). ADPase stained retinal flat mounts were carefully imaged with an optical microscope (Olympus Corporation, Tokyo, Japan). Images were carefully examined to assess the severity of neovascularization with Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA, USA).
Quantification of Retinal Neovascularization
To quantify preretinal neovascular cells, retinal structures were analyzed on 6-µm H&E stained sections. Eyeballs were fixed with 4% paraformaldehyde for 24h, then embedded in paraffin. Whole eyes were sagittally cut into serial sections (6-µm thick) through the cornea and parallel to the optic nerve, and then stained with H&E, dehydrated, vitrified, mounted, observed, and imaged with a light microscope (Olympus B201, Olympus Corp., Tokyo, Japan). Three blinded researchers counted the cells.
Immunohistochemistry
Eyeballs were fixed with 4% paraformaldehyde then dehydrated with ethanol and xylene. Deparaffinized the eye tissue sections. For heat-induced antigen retrieval, antigen retrieval buffers were prepared with citric acid and sodium citrate. Sections were incubated with rabbit anti-CCR7 polyclonal antibody (1:200; Abcam, Cambridge, UK), rabbit anti-p-ERK1/2 (1:150; Cell Signaling Technology, Boston, MA, USA), and rabbit anti-VEGF polyclonal antibody (1:100; Proteintech, Chicago, IL, USA) overnight at 4°C. Sections were then incubated with biotinylated secondary antibody (1:1000; Zhongshan Jinqiao Biotechnology Co. Ltd., China) and the avidin-biotinylated peroxidase complex was activated. Primary antibody was replaced with PBS for negative controls. The peroxidase reaction was developed with horseradish peroxidase labeled avidin/streptomycin, and sections were colored with dimethyl amino-azo-benzene (DAB), dehydrated with alcohol, and sealed with neutral gum. Images were digitally captured using an Olympus B201 optical microscope.
Quantitative Real-time Reverse Transcriptional Polymerase Chain Reaction
RNA was extracted from retinal samples with Trizol (Invitrogen Corp., Carlsbad, CA, USA). Reverse transcription into cDNA was performed with the reverse transcriptase kit TAKARA 047A (PrimeScript RT Reagent kit-Perfect Real-Time; Takara Bio, Otsu, Japan) according to the manufacturer's instructions. Primers were designed and purchased from Sangon Biotech Co. Ltd. (Shanghai, China); β-actin served as a normalizing control. The sequences of primers used are shown in Table 1. The 2−ΔΔCt method was used to determine relative quantification of gene expression[7].
Table 1. Primer sequences for qRT-PCR.
| Gene | Primer sequences (5′-3′) | Product length (bp) | Tm (°C) | |
| β-actin | Forward | CCTCCTCCTGAGCGCAAGTA | 117 | 55 |
| Reverse | GATGGAGGGGCCGGACT | |||
| CCR7 | Forward | AACGGGCTGGTGATACTGAC | 139 | 55 |
| Reverse | AGGACTTGGCTTCGCTGTAG | |||
| VEGF | Forward | CAACTTCTGGGCTCTTCTCG | 144 | 55 |
| Reverse | CCTCTCCTCTTCCTTCTCTTCC | |||
qRT-PCR: Quantitative real-time reverse transcriptional polymerase chain reaction; CCR7: C-C chemokine receptor type 7; VEGF: Vascular endothelial growth factor; Tm: Temperature; bp: Base pair.
Western Blot
We added 500 µmol/L RIPA Lysis Buffer (Sigma-Aldrich Co. St Louis, MO, USA) and 5 µmol/L phenylmethanesulfonyl fluoride (Sigma-Aldrich Co. St Louis, MO, USA) to the retinas. For retinal samples in which p-ERK1/2 was to be tested, we also added 5 µmol/L protein phosphatase inhibitor. We then determined protein concentration using the BCA method. Membranes were then incubated with horse radish peroxidase-conjugated secondary antibody (1:2000; Zhongshan Jinqiao Biotechnology Co. Ltd., Beijing, China) for 2h. Signals were detected with enhanced chemiluminescence Azure c300 chemiluminescent Western blot imaging system (Azure Biosystems, Inc., USA). The ratio between the optical densities of the protein of interest and GAPDH in each same sample was calculated to determine relative protein content.
Statistical Analysis
Statistical analysis of all data was performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). All data are represented as mean±standard deviation (SD). Statistical significance was evaluated by one-way analysis of variance (ANOVA) with the least significant difference post hoc analysis. P<0.05 was considered statistically significant.
RESULTS
Quantitation of Retinal Neovascularization
We examined the retinal vasculature in the normoxia, OIR, OIR control, and OIR treated groups using ADPase in retinal flat mounts at P17 (Figure 1). No abnormal blood vessels were observed in the retinas of the normoxia group. Two layers of retinal vessels were evenly distributed in the retina, the superficial blood vessels were well formed, and the deep blood vessel formed a polygon mesh pattern. The blood vessels in OIR and OIR control groups showed non-perfusion areas and neovascularization. The ratio of new blood vessel area to total retinal area was higher in the OIR treated (0.34±0.04), OIR (0.62±0.08), and OIR control groups (0.60±0.05) than in the normoxia group (0.25±0.01; all P<0.05). In contrast, retinas in the OIR treated group (0.34±0.04) developed less severe neovascular tufts and regions of non-perfusion as compared to the OIR and OIR control groups (both P<0.05), which demonstrates a strong inhibitory effect of CCR7 siRNA on RNV in the OIR treated group. No significant difference was detected between the OIR and OIR control groups (P>0.05).
Figure 1. Inhibitory effect of CCR7 siRNA on RNV in the OIR model.
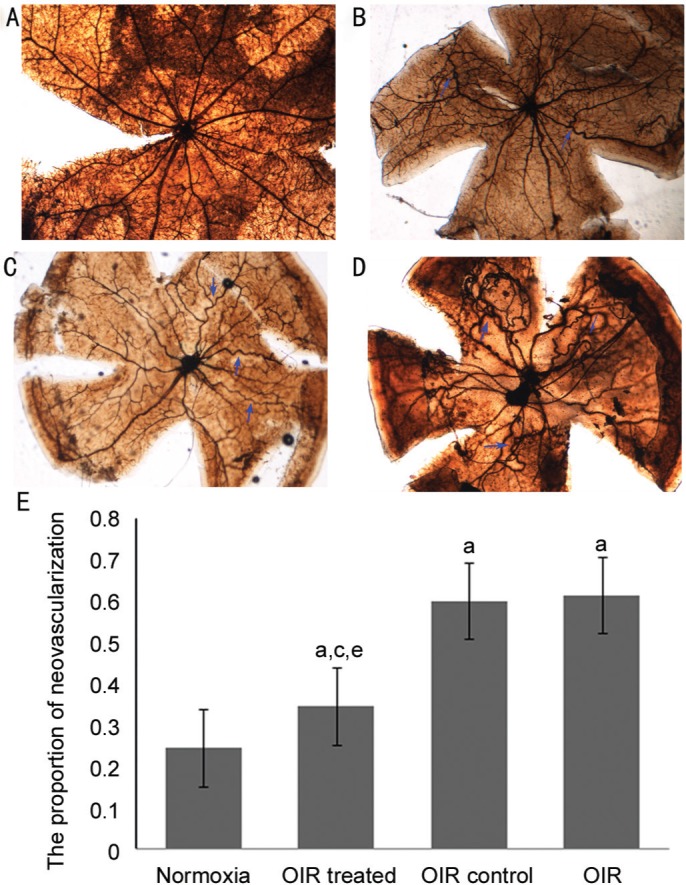
Representative retinal angiographs from the eyes of mice in the normoxia (A), OIR (B), OIR control (C), and OIR treated groups (D). The results of statistical analysis are illustrated in (E). The blue arrows indicate neovascularization (magnification: ×100). Data are shown as mean±SD (n=15). aP<0.05 vs normoxia group, cP<0.05 vs OIR group, and eP<0.05 vs OIR control group.
Qualitative of Retinal Neovascularization
H&E stained retina sections are shown in Figure 2. Preretinal neovascular cells were nearly absent from retinas in the normoxia group (Figure 2A) but more abundant in the OIR (Figure 2D) and OIR control (Figure 2C) groups than in the OIR treated (Figure 2B) group, demonstrating an inhibitory effect of CCR7 siRNA on RNV in the OIR treated group.
Figure 2. Effect of CCR7 siRNA on pre-RNV in mice with OIR.
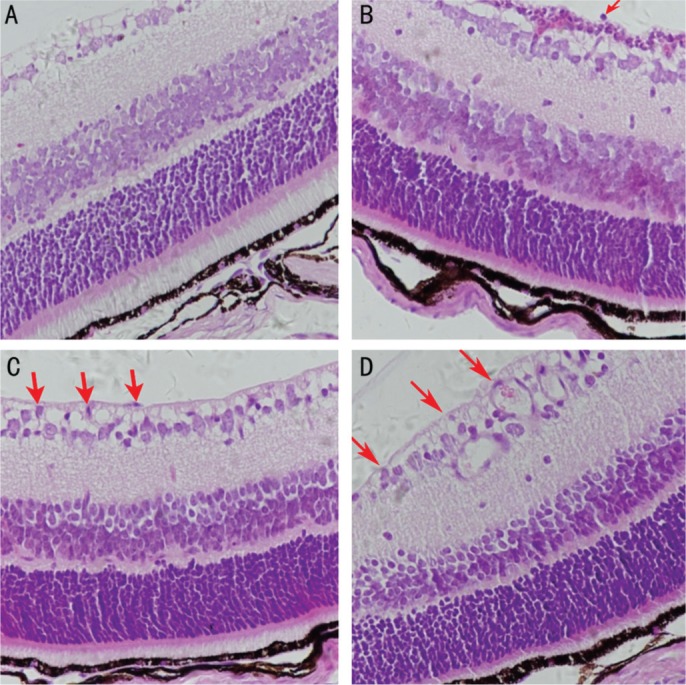
Images shown are of representative retinal sections from the normoxia (A), OIR treated (B), OIR control (C), and OIR (D) groups. The red arrows indicate preretinal neovascular cells (magnification: ×400).
CCR7/p-ERK1/2/VEGF Signaling in the Oxygen-induced Retinopathy Mouse Model
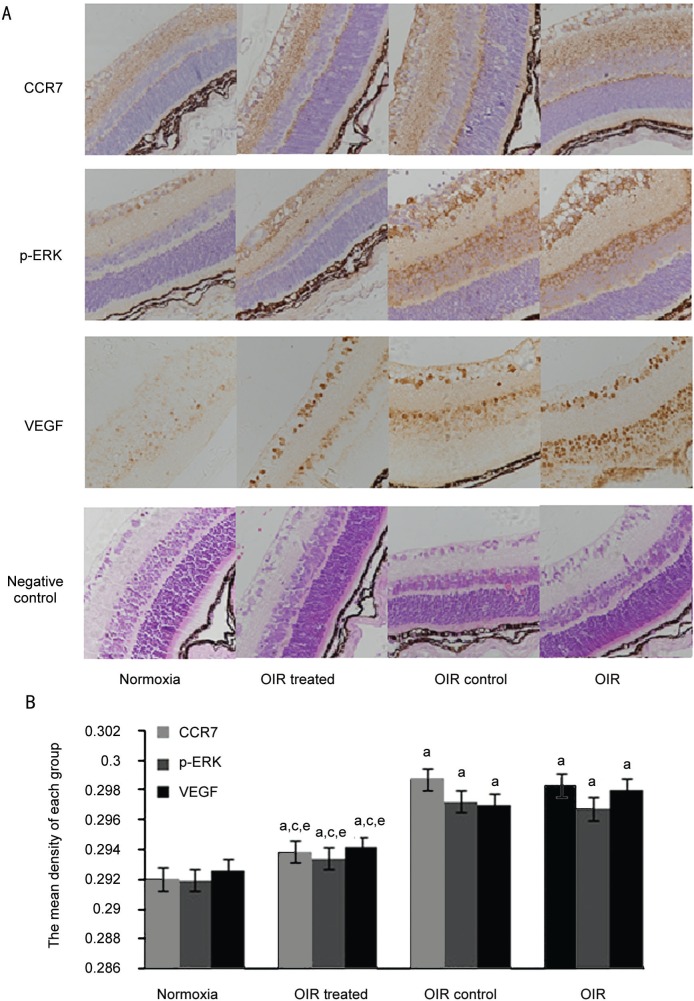
Immunohistochemistry was analyzed with Nis Elements BR3.0 (Nikon Instruments Inc., Japan) (Figure 3). The mean density of CCR7, p-ERK1/2, and VEGF was low in the normoxia and OIR treated groups, but high in the OIR and OIR control groups (all P<0.05). These results indicate that hypoxia induced the expression of CCR7, p-ERK1/2, and VEGF, and that reducing CCR7 could inhibit the expression of p-ERK1/2, and VEGF.
Figure 3. Hypoxia-induced CCR7 expression is mediated through the p-ERK1/2-VEGF pathway in the OIR mouse model.
A: Protein expression of CCR7, p-ERK1/2, and VEGF was determined by immunohistochemistry (magnification: ×400); B: The mean density of CCR7, p-ERK1/2, and VEGF in each group. aP<0.05 vs normoxia group, cP<0.05 vs OIR group, and eP<0.05 vs OIR control group.
Expression of C-C Chemokine Receptor Type 7 and Vascular Endothelial Growth Factor
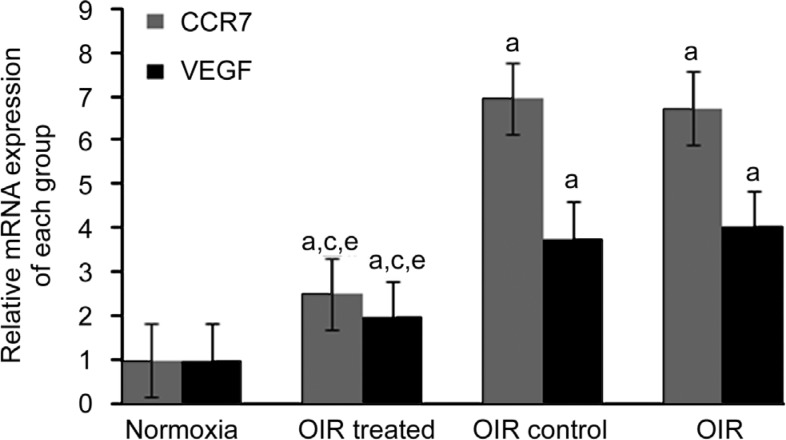
The relative expression of CCR7 mRNA as compared to the normoxia group was 2.51±0.04 (OIR treated), 6.95±0.75 (OIR control), and 6.72±0.77 (OIR) and the expression of VEGF was 1.97±0.04 (OIR treated), 3.78±0.29 (OIR control), and 4.04±0.16 (OIR). CCR7 and VEGF mRNA levels in the OIR treated group were decreased as compared to the OIR and OIR control groups (all P<0.05). Additionally, the expression of these mRNAs in the OIR and OIR control groups was increased as compared to the normoxia group (all P<0.05). No significant difference was detected between the OIR and OIR control groups (all P>0.05) (Figure 4).
Figure 4. CCR7 siRNA inhibited RNV through inhibition of the expression of VEGF in the OIR mouse model.
The mRNA expression of CCR7 and VEGF was determined by qRT-PCR. Data are shown as mean±SD (n=10). aP<0.05 vs normoxia group, cP<0.05 vs OIR group, and eP<0.05 vs OIR control group.
CCR7/p-ERK1/2/VEGF Signaling Activity in Oxygen-induced Retinopathy Mice
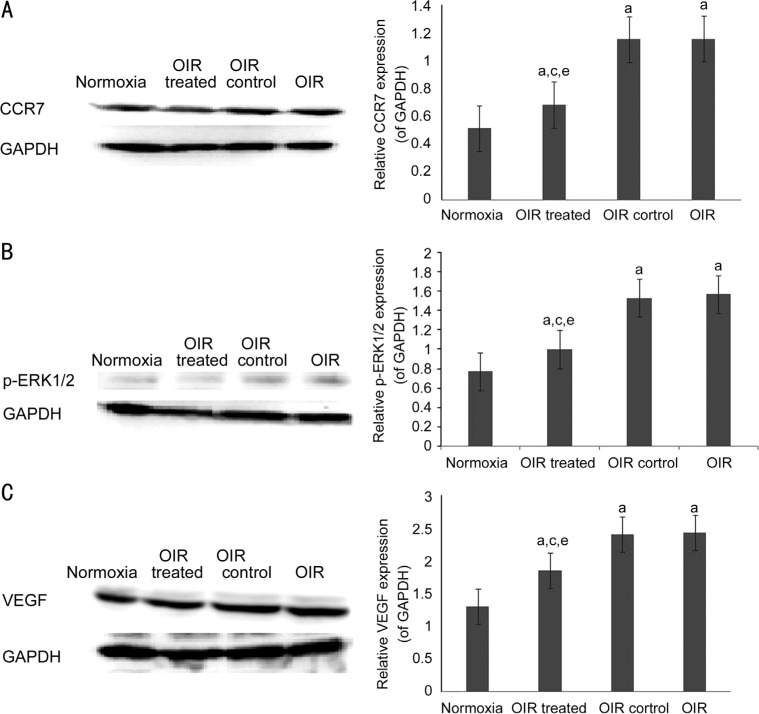
The relative protein quantity of CCR7 was 0.51±0.01, 0.68±0.02, 1.16±0.01, and 1.16±0.03 in the normoxia, OIR treated, OIR control, and OIR groups; p-ERK1/2 was 0.77±0.05, 1.00±0.08, 1.53±0.07, and 1.57±0.05; VEGF was 1.30±0.04, 1.85±0.03, 2.41±0.05, and 2.44±0.04, respectively. As compared to the OIR control group, CCR7, p-ERK1/2, and VEGF protein levels were decreased in the OIR treated group (all P<0.05). Additionally, the expression of each of these proteins was significantly increased in the OIR and OIR control groups as compared to the normoxia group (all P<0.05), whereas no significant difference was detected between the OIR and OIR control groups (all P>0.05) (Figure 5).
Figure 5. CCR7 siRNA decreases the protein expression of p-ERK1/2 and VEGF.
CCR7 (A), p-ERK1/2 (B), and VEGF (C) protein expression was determined by Western blot. Data are shown as mean±SD (n=10). aP<0.05 vs normoxia group, cP<0.05 vs OIR group, eP<0.05 vs OIR control group.
DISCUSSION
Chemokines are chemical induction factors for specific G-protein coupled receptors (GPCRs). Chemokines belong to a family of cytokines, are secreted by different cell types, and play a role in chemotaxis. They play an important role in both normal and abnormal physiological processes. CCR7 is a member of the chemokine receptor family. CCR7 expression on the cell surface combined with activation by its high affinity ligands, CCL19 and CCL21, can promote integrin aggregation, thereby activating the coupled G-protein in the cytoplasm. This activation quickly induces Ca2+ mobilization and stimulates the mitogen activated protein kinase (MAPK) and focal adhesion kinase (FAK) pathways, thereby activating protein kinase C and downstream guanosine triphosphatase (GTP)-binding tyrosine kinases. This stimulates signal transduction in a variety of ways, recombinants the bone scaffold protein in cells, cause target cell migration and produce efficient chemotaxis. A variety of stimulating factors such as growth factors, cytokines, viruses, GPCR ligands, and oncogenic proteins can activate this pathway[8].
CCL19/21-CCR7 signaling plays an important role in the initiation, development, and metastasis of many tumor types[9]–[11]. CCR7 mediates angiogenesis in different tumor microenvironments as well as metastasis-mediated angiogenesis[12]. Pathological angiogenesis that occurs during thymic hyperplasia in myasthenia gravis may be related abnormal recruitment of vascular endothelial cells by CCL21[13].
CCL19[14] and CCL21[15] expression was detected in vascular endothelial cells in the synovial tissue of patients with rheumatoid arthritis (RA). CCL19 and CCL21 induce the formation of potent angiogenic factors in macrophages and RA fibroblasts[16]. In RA, CCR7 and its ligands regulate the formation of new blood vessels through different signaling pathways[17]. Furthermore, CCR7 and its ligands mediate cell migration and angiogenesis in many additional diseases[3]. Our data show that CCR7 promotes retinal neovascularization in OIR, and that CCR7 inhibition reduced this retinal neovascularization. We thus conclude that CCR7 promotes RNV in OIR.
ERK is a serine/threonine protein that was isolated and identified by Boulton et al[18]. The ERK signaling pathway plays a key role in transducing mitogenic cell signals[18]. ERK signals is the downstream of the three stages of MAPK signaling reactions, that is, the Ras-Raf-MEK cascade. The Ras-Raf-MEK-ERK pathway is one of the most important signal transduction pathways involved in cell growth, development, division, migration, metabolism, apoptosis, and other physiological processes in vivo[19]–[20]. Phosphorylated ERK can convert extracellular stimuli into intracellular reactions, promoting the phosphorylation and activation of multiple transcription factors in the nucleus, thereby enhancing transcriptional activity. ERK promotes tumorigenesis by mediating extracellular matrix degradation, adhesion, and migration of tumor cells as well as angiogenesis[19]–[21]. Various growth factors, ions, and hydrogen peroxide can activate the ERK pathway through phosphorylation. The ERK signaling pathway has been demonstrated to promote proliferation and angiogenesis of endothelial cells in vitro. In RNV diseases, the present study indicates that the ERK signaling pathway may be involved in the development and progression of diabetic retinopathy by regulating angiogenesis-related growth factors. The results of our study show that in the group with high RNV, p-ERK1/2 expression increased; in the normoxiaand OIR treated groups, p-ERK1/2 expression and RNV decreased synchronously. It can thus be inferred that p-ERK1/2 promotes RNV in OIR.
VEGF participates in many physiological and pathological processes in the body, strongly stimulates vascular endothelial proliferation and migration as well as maintenance of vascular integrity[22], increases vascular permeability[23], and promotes angiogenesis[24]–[25]. VEGF acts through both autocrine and paracrine signaling, promoting endothelial cell growth in arteries, veins, and lymphatic vessels by activating specific receptors on vascular endothelial cells[26]. VEGF expression promotes normal physiological vascular growth[27]–[29]. Abnormal VEGF expression can occur under pathological conditions. In ischemic disease, hypoxia can stimulate VEGF expression. The resulting strong induction of endothelial cell mitosis promotes the formation of new blood vessels and improves tissue blood supply. VEGF is a key factor for ocular neovascularization, directly promoting the formation and development of new blood vessels; its expression is closely related to disease severity. In this study, VEGF also played a role in promoting RNV.
CCR7 signal can through the ERK pathway[30]. CCR7 can activate MAPK family proteins including p38, JNK, and ERK1/2 via G(i)-dependent mechanisms[31]. In monocytes, the phosphorylation of ERK1/2, p38, and JNK can be induced by the combination of CCR7 and CCL19, thus playing an important role in cell migration[32]. The expression of CCR7 was positively correlated to p-ERK1/2 in this study. CCR7 inhibition-mediated suppression of p-ERK1/2 signaling has also been shown to be important for the regulation of angiogenesis via VEGF and other pathways. In bovine retinal microvascular endothelial cells in vitro, VEGF stimulated the phosphorylation of ERK1/2 in a dose-dependent manner to promote cell proliferation and endothelial cell formation[33]. In a rat model of ROP, the ERK-VEGF signaling axis has been demonstrated to play a key role in endothelial cell proliferation. VEGF and other growth factors affect cellular functions through the ERK pathway, thereby promoting the transcription and expression of select genes, and in so initiating cell proliferation and differentiation. This signaling pathway plays an important role in cell growth, development, and proliferation[34]. In this study, p-ERK1/2 and VEGF expression was high in the group with high levels of RNV; in the group with less RNV, p-ERK1/2 and VEGF expression was reduced accordingly. Thus, we concluded that p-ERK1/2/VEGF plays a prominent role in the OIR model of RNV.
It has been reported that CCR7 increases VEGF expression in fibroblasts such as synovial cells in RA and osteoarthritis through the p-ERK1/2 signaling pathway, and thereby promotes angiogenesis[17]. CCR7 can also increase VEGF expression in non-small cell lung cancer cells through the p-ERK1/2 signaling pathway, thus promoting tumor angiogenesis[32]. Taken together, all of the above studies have shown that the CCR7/p-ERK1/2/VEGF pathway promotes the production of new blood vessels. In addition, the CCR7-VEGF pathway has been shown to promote RNV[35]. The present study showed that the expression of CCR7 and VEGF in the OIR model was correlated with high expression of CCR7/VEGF, and the expression of CCR7 and VEGF decreased after CCR7 was inhibited.
Considering our data and those of previous reports, the CCR7/p-ERK1/2/VEGF pathway may be important in promoting neovascularization in OIR. We have found a positive correlation between CCR7, p-ERK1/2, and VEGF in the present study. Expression of CCR7 lead to p-ERK1/2 and VEGF expression as well as neovascularization, and CCR7 inhibition suppressed p-ERK1/2 and VEGF expression and neovascularization. Thus, we speculate that CCR7/p-ERK1/2/VEGF signaling plays an important role in ROP.
Vitreous cavity injection is a definitive and effective way to treat RNV; however, siRNA injection has not been applied in RNV treatments. Our research shows that CCR7 siRNA can be an effective part of anti-VEGF therapy. Unfortunately, the delivery of siRNA, toxicity, and off-target effects have not been successfully resolved; these challenges must be addressed in order to use siRNA for gene therapy.
In conclusion, CCR7/p-ERK1/2/VEGF signaling promotes RNV in a mouse model of OIR. CCR7-targeting intervention via siRNA represents a potential anti-angiogenic therapy for RNV of ROP, and may contribute to future therapeutic strategies.
Acknowledgments
Foundation: Supported by the Science and Technology Planning Foundation of Liaoning Province (No.2010225034).
Conflicts of Interest: Yuan LH, None; Chen XL, None; Di Y, None; Liu ML, None.
REFERENCES
- 1.MartIn-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schweickart VL, Raport CJ, Godiska R, Byers MG, Eddy RL, Jr, Shows TB, Gray PW. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics. 1994;23(3):643–650. doi: 10.1006/geno.1994.1553. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y, He LD, Sheng ZJ, Yong MM, Sheng YS, Wei Dong X, Wen Wen T, Ming ZY. Increased CCL19 and CCL21 levels promote fibroblast ossification in ankylosing spondylitis hip ligament tissue. BMC Musculoskelet Disord. 2014;15:316. doi: 10.1186/1471-2474-15-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye X, Xu G, Chang Q, Fan J, Sun Z, Qin Y, Jiang AC. ERK1/2 signaling pathways involved in VEGF release in diabetic rat retina. Invest Ophthalmol Vis Sci. 2010;51(10):5226–5233. doi: 10.1167/iovs.09-4899. [DOI] [PubMed] [Google Scholar]
- 5.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 6.Chi BJ, Du CL, Fu YF, Zhang YN, Wang RW. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int J Clin Exp Pathol. 2015;8(10):12533–12540. [PMC free article] [PubMed] [Google Scholar]
- 7.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 8.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105(2):186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 9.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4(12):2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, Thomas DG, Luker GD. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012;31(45):4750–4758. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79(4):639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Sun L, Yin L, Ming J, Zhang S, Luo W, Qiu X. CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour Biol. 2013;34(5):2703–2708. doi: 10.1007/s13277-013-0822-z. [DOI] [PubMed] [Google Scholar]
- 13.Berrih-Aknin S, Ruhlmann N, Bismuth J, Cizeron-Clairac G, Zelman E, Shachar I, Dartevelle P, de Rosbo NK, Le Panse R. CCL21 Overexpressed on lymphatic vessels drives thymic hyperplasia in myasthenia. Ann Neurol. 2009;66(4):521–531. doi: 10.1002/ana.21628. [DOI] [PubMed] [Google Scholar]
- 14.Burman A, Haworth O, Hardie DL, Amft EN, Siewert C, Jackson DG, Salmon M, Buckley CD. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174(3):1693–1700. doi: 10.4049/jimmunol.174.3.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204(1):28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 16.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011;63(10):2884–2893. doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Role of the CCL21 and CCR7 pathways in rheumatoid arthritis angiogenesis. Arthritis Rheum. 2012;64(8):2471–2481. doi: 10.1002/art.34452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of proteins-erine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 19.Yoon S, Seger R. The extracellular signal-regulated kinase:multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24(1):21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 20.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22(17):6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory. Mol Neurobiol. 2001;23(2-3):83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- 22.Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274(15):10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 23.Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996;59(1):100–115. [PubMed] [Google Scholar]
- 24.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, Bazan NG. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci. 2003;44(10):4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Keshet E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, Davissmyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1993;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 27.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114(10):1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 28.Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozaki H, Seo MS, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood JM, Campochiaro PA. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156(2):697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shannon LA, Calloway PA, Welch TP, Vines CM. CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J Biol Chem. 2010;285(50):38781–38787. doi: 10.1074/jbc.M110.152173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riol-Blanco L, Sánchez-Sánchez N, Torres A, Tejedor A, Narumiya S, Corbí AL, Sánchez-Mateos P, Rodríguez-Fernández JL. The chemokine receptor CCR7 activates in dendritic cells two signaling modules that independently regulate chemotaxis and migratory speed. J Immunol. 2005;174(7):4070–4080. doi: 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Liu L, Qiu X, Jiang L, Huang B, Li H, Li Z, Luo W, Wang E. CCL21/CCR7 promotes G2/M phase progression via the ERK pathway in human non-small cell lung cancer cells. PLoS One. 2011;6(6):e21119. doi: 10.1371/journal.pone.0021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003;44(4):1722–1731. doi: 10.1167/iovs.01-1193. [DOI] [PubMed] [Google Scholar]
- 34.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 35.Di Y, Chen XL, Wang AY. Expression and significance of CCR7 and VEGF in retinal endothelial cell under hypoxia. Guoji Yanke Zazhi (Int Eye Sci) 2015;15(3):403–406. [Google Scholar]