Abstract
Ceramide has been shown to cause anoikis, a subtype of apoptosis due to inadequate cell adhesion. However, the underlying mechanism is unclear. Herein, we report that D-e-C6-ceramide (D-e-Cer), via generating sphingosine, disrupts the Golgi complex (GC), which is associated with various cellular effects, including anoikis. Treatment of HeLa cells with D-e-Cer caused cell elongation, spreading inhibition, rounding, and detachment before apoptosis (anoikis). In D-e-Cer–treated cells, glycosylation of β1 integrin in the GC was inhibited, thus its associated integrin receptors failed to translocate to the cell surface. Ceramide treatment also inhibited the reorganization of both microtubule and F-actin cytoskeletons, focal adhesions, and filopodia. These cellular effects were preceded by fragmentation of the Golgi complex. In contrast, L-e-C6-ceramide (L-e-Cer), the enantiomer of D-e-Cer, failed to induce these cellular effects. Mass spectrometric analysis revealed that treatment HeLa cells with D-e-Cer but not L-e-Cer caused a >50-fold increase in the levels of sphingosine, a product of hydrolysis of ceramide. Treatment with D-e-sphingosine and its enantiomer, L-e-sphingosine, caused massive perinuclear vacuolization, Golgi fragmentation, and cell rounding. Together, these results suggest that sphingosine generated from hydrolysis of ceramide causes the GC disruption, leading to various cellular effects.
INTRODUCTION
Ceramide, a metabolite of sphingolipids, has been shown to mediate growth arrest, differentiation, and apoptosis of mammalian cells in response to various stresses (Spiegel and Merrill, 1996; Merrill et al., 1997; Hannun and Obeid, 2002; Levade et al., 2002; Kolesnick and Fuks, 2003). Its pathological accumulation has been implicated in neuron degeneration (Acharya et al., 2003; Bieberich et al., 2003; Cutler et al., 2004). However, the underlying mechanisms are not fully understood.
Recent studies demonstrated that ceramide inhibits cell adhesion and alters the organization of the cytoskeletons in various cell types. Marushige and Marushige (1998) showed that treatment of rat trigeminal neurinoma cells with D-e-ceramide (D-e-Cer), a cell-permeable ceramide, induces cell rounding. Di Bartolomeo and Spinedi (2002) observed that treatment of CHP-100 neuroepithelioma cells with D-e-Cer causes cell detachment and apoptosis, and Z-VAD-fmk, a pan-inhibitor of caspases, inhibits the apoptotic response, but not cell detachment, suggesting that cell detachment occurs independently of or before apoptosis. Feldhaus et al. (2002) revealed that treatment with bacterial sphingomyelinase, which hydrolyzes sphingomyelin of the plasma membrane to generate endogenous ceramide, also impairs cell adhesion of polymorphonuclear neutrophils via inhibiting the F-actin cytoskeleton reorganization. Panigone et al. (2001) demonstrated that up-regulation of prosaposin, a sphingolipid metabolism activator, which activates hydrolysis of complex sphingolipids to release endogenous ceramide(s), inhibits both cell adhesion and growth of breast cancer cells (MCF7). Furthermore, McCaig et al. (2002) showed that treatment with an insulin-like growth factor binding protein enhances the adhesion of tumor epithelial cells (Hs578T) to fibronectin and inhibits ceramide-induced apoptosis. These observations suggest that ceramide causes growth inhibition and apoptosis via inhibiting cell adhesion and cytoskeletal reorganization.
However, the underlying mechanisms by which ceramide inhibits cell adhesion and cytoskeletal activities are unclear. Ceramide has been shown to be targeted to the Golgi complex (GC) (Hanada et al., 2003). Moreover, the GC plays an important role in regulating various cytoskeletal activities (Allan et al., 2002; Stamnes, 2002; Rios and Bornens, 2003), which are in turn closely associated with cell morphology, growth, and survival (Bhalla, 2003). These observations prompted us to investigate whether ceramide modulates cell adhesion and cytoskeletal activities by influencing the structure and function of the GC. We demonstrate that treatment with an exogenous ceramide, D-e-C6-ceramide (D-e-Cer), caused GC fragmentation, which plays an important role in the ceramide-induced morphological changes, defective cell adhesion and spreading, growth arrest, and anoikis. In addition, we also explored the possible mechanism of the ceramide-induced GC fragmentation.
MATERIALS AND METHODS
Materials
N-Hexanoyl-sphingosylphosphorylcholine (C6-sphingomyelin), d-erythro-C6-ceramide (D-e-Cer), l-erythro-C6-Ceramide (L-e-C6-Cer), and d-threo-C6-ceramide (D-t-Cer) were synthesized in the Lipidomics Core at the Medical University of South Carolina. d-erythro-Sphingosine (d-e-Sph) and l-erythro-sphingosine (L-e-Sph) were purchased from Matreya (State College, PA), and d-glucosyl-β1–1′-N-octanoyl-d-erythro-sphingosine (C8, β-D-glucosylceramide) was from Avanti Polar Lipids (Alabaster, AL). Antibodies against α5 integrin, poly(ADP-ribose) polymerase (PARP), and β-actin, and goat IgG conjugated with horseradish peroxidase (HRP) were from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated anti-rabbit and mouse IgG were from Bio-Rad (Hercules, CA). Anti-mouse, -rabbit, and -goat IgGs conjugated with either fluorescein isothiocyanate or rhodamine were from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibodies against β1 integrin, P230 trans-Golgi, and GM130, and plasmids pEYFP-Golgi and pdsRED2-ER were from BD Biosciences (San Jose, CA). Anti-β1 integrin antibody JB1A was from Chemicon International (Temecula, CA). Antibodies against caspase 3, 7, and 9 were from Cell Signaling Technology (Beverly, MA). Anti-giantin antibody was from Covance (Berkeley, CA). N-{12-[(7-Nitro-2–1,3-benzoxadiazol-4-yl)amino]hexanoyl}-d-erythro-sphingosine (NBD-D-e-C6-ceramide), an anti-α-tubulin antibody, Hoechst 33258 dye, and the phalliodin-rhodamine conjugate were purchased from Molecular Probes (Eugene, OR). Opti-MEM and minimal essential medium (MEM) and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, CA). Z-VAD-fmk was from R&D Systems (Minneapolis, MN). Bacterial sphingomyelinase (bSMase) and other unlisted compounds were from Sigma-Aldrich (St. Louis, MO).
Cell Culture and Transfection
The cervical cancer cell line HeLa T-Rex (Invitrogen) was cultured and maintained according to the manufacturer's instructions. HeLa cells were transfected with plasmid DNA by using Lipofectamine 2000 (Invitrogen) as described previously (Mao et al., 2003).
Assessment of Cell Viability
Cell viability was assessed by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT)-based cell growth determination kit (Sigma-Aldrich) according to the manufacturer's instructions. The absorbance at λ595 nm was determined in a plate reader (Molecular Devices, Sunnyvale, CA).
Analysis of Cell Cycle
Cell cycle profiles or apoptosis was analyzed by fluorescence activated cell sorting (FACS) on an FACStar Plus flow cytometer (Becton Dickinson, San Jose, CA) as described previously (Marchesini et al., 2004).
Assessment of Apoptosis
Apoptosis was assessed by 1) staining with Hoechst 33342 for nuclear condensation or fragmentation, 2) staining with annexin-V-rhodamine (Zymed Laboratories, South San Francisco, CA) for the exposure of phosphatidylserine on the cell surface, and 3) FACS analysis for DNA degradation.
Fluorescent and Laser Scanning Confocal Microscopy
Cells were fixed and processed for immunostaining with various antibodies as described previously (Mao et al., 2003). To visualize nuclei and F-actin cytoskeletons, cells were stained with Hoechst 33342 and phalloidin-rhodamine, respectively. The cells were examined under an inverted fluorescent microscope (Nikon Eclipse TE200) or a laser scanning confocal microscope (LSM 510 Meta; Carl Zeiss, Thornwood, NY).
Electron Microscopy
HeLa cells grown on Thermonax coverslips were fixed for 30 min in phosphate-buffered saline (PBS) containing 3.7% paraformaldehyde, washed with 0.1 M sodium cacodylate buffer (buffer I) (pH 7.4), and postfixed for 1 h in 1% osmium tetroxide in buffer I. After being rinsed in the buffer I, the cells were dehydrated in a series of ethanol and infiltrated with Epon-Aradite (Ted Pella, Redding, CA). The cell samples were polymerized at 60°C for 2 d before ultrathin sections (∼80 nm) were cut on Reichert Ultracut E microtome and collected on 150 mesh grids. The sections were counterstained with uranyl acetate and lead citrate and examined under an FEI CM120 transmission electron microscope (equipped with a Gatan GIF100 image filter) operating at a beam energy of 120 keV. Images were acquired using a Gatan 1 k × 1 k cooled charge-coupled device (CCD) camera.
Assays of Cell Adhesion
Cell adhesion assay was performed as described previously (Semel et al., 2002). Cells were dislodged by treatment with trypsin-EDTA. After being washed with basal MEM containing 0.5% bovine serum albumin, the cells (105/well) were plated into six-well plates that were pretreated with fibronectin or laminin. After 45-min incubation at 37°C, the nonadherent cells were removed by three washes with PBS. The adherent cells were fixed with 3.7% paraformaldehyde and stained with 0.1% crystal violet, and the absorbance at λ540 nm of the clear cell lysates was determined by the microplate reader. Cell spreading were determined by microscopic analysis after incubation at 37°C for 2 h.
Western Blot Analysis
Western blot analysis was performed as described previously (Mao et al., 2003).
Deglycosylation with Peptide:N-Glycosidase
Treatment of cell lysates with peptide:N-glycosidase F (PNGase F; New England Biolabs, Beverly, MA) was performed according to the instructions of the manufacturer. Briefly, protein samples (1 mg/ml) in 100 mM Tris-HCl, pH 7.4, containing 50 mM β-mercaptoethanol and 0.1% SDS, were boiled for 2 min. After addition of Triton X-100 (0.5%), the samples were incubated with PNGase F (2.5 U/ml) at 37°C for 12 h, and then were treated with the same amount of fresh PNGase F for additional 12 h. Proteins were analyzed by Western blot analysis by using anti-β1 integrin antibody.
Biotinylation of Cell Surface Integrin Subunits
Biotinylation of cell surface proteins was performed as described previously (Salicioni et al., 2004). After being washed with ice-cold PBS three times, cells grown in 10-cm culture plates were treated with the membrane-impermeable biotinylation reagent sulfo-NHS-LC-biotin (0.1 mg/ml) (Pierce Chemical, Rockford, IL), for 15 min at 22°C, according to the manufacturer's instructions. Biotinylation reactions were terminated with glycine (100 mM) in 50 mM Tris-HCl, 150 mM NaCl, pH 7.4, for 15 min at 22°C. After being washed with ice-cold PBS three times, cells were lysed in the lysis buffer, and protein concentrations were determined by the bicinchoninic acid method. Biotinylated cell-surface proteins were precipitated by streptavidin-Sepharose (Amersham Biosciences, Piscataway, NJ) from the cell lysates with the same amount of proteins. The precipitated proteins were separated by SDS-PAGE, and integrin receptors were detected by Western blot analysis.
In Vitro Binding Assay of Integrin Receptors
The binding of α5β1 integrin to fibronectin was assayed by fibronectin affinity chromatography. Fibronectin was coupled to CNB-activated Sepharose-4B beads (Pfizer, New York, NY) to generate fibronectin-Sepharose columns according to the manufacturer's instructions. The same amounts of proteins of different cell lysates were applied to the fibronectin-Sepharose-4B columns (0.5 ml) pre-equilibrated with a binding buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM MgCl2, and 1 mM MnCl2). After the columns were washed with the binding buffer, integrin receptors binding to fibronectin were eluted with 10 mM EDTA in 25 mM Tris-HCl buffer, pH 7.4. Proteins were precipitated by 5% trichloroacetic acid and analyzed by Western blot analysis with the antibody against β1 integrin (J1BA).
Electrospray Ionization/Tandem Mass Spectrometry (ESI-MS/MS) Lipid Analysis Analysis of sphingolipids was performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer, operating in a multiple reaction monitoring positive ionization mode as described previously (Mao et al., 2003).
RESULTS
Treatment with Ceramide Alters Cell Morphology Independently of Apoptosis
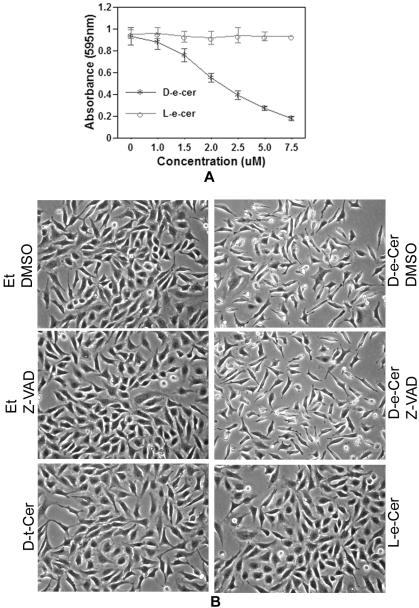
In agreement with previous studies, treatment of HeLa cells with D-e-Cer decreased the number of viable cells in a dose-dependent manner within 24 h (Figure 1A). At 12 h, >90% cells treated with 2.5 μM D-e-Cer underwent drastic morphological changes, including bipolar cell elongation, reduction in cytoplasm, the formation of long cell processes and spindle-like structures, and cell rounding. In contrast, <1% cells treated with the vehicle (ethanol) underwent these morphological changes (Figure 1B). Neither the cell growth arrest nor the morphological alterations were induced by L-e-Cer or D-t-C6-Cer, unnatural stereoisomers of D-e-Cer, even at a concentration as high as 10 μM (Figure 1, A and B), suggesting that the cellular effects of ceramide highly depend on its natural configuration.
Figure 1.
(A) Ceramide treatment causes growth arrest and morphological changes of HeLa cells. HeLa cells grown to a 60% confluence in MEM medium with 10% FBS were treated with various concentrations of D-e-Cer or L-e-Cer for 24 h and were subjected to MTT assays for viable cell number. (B) HeLa cells treated with 2.5 μM D-e-Cer, 10 μM L-e-Cer, 10 μM D-t-Cer, or ethanol (Et) for 12 h in the presence or absence of 50 μM Z-VAD-fmk were imaged under an inverted microscope (Nikon Eclipse TE200) equipped with a CCD camera (Dage MTI 330T-RC). Data represent mean value ± SD of three different experiments performed in duplicate. This figure is representative of at least three independent experiments.
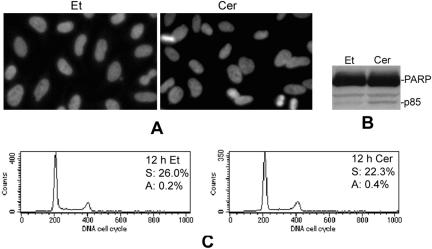
To investigate whether the alterations in cell morphology were a secondary effect to apoptosis, various apoptotic hallmarks were analyzed in cells treated with D-e-Cer for 12 h. Nuclear staining with Hoeschst 33342 revealed neither DNA condensation nor nuclear fragmentation in the ceramide-treated cells (Figure 2A). Western blot analysis indicated that ceramide caused a slight PARP cleavage (Figure 2B). Consistently, FACS analysis revealed that neither DNA degradation nor cell cycle arrest was observed in the ceramide-treated cells (Figure 2C). Furthermore, morphological changes were not inhibited by Z-VAD-fmk, a generic inhibitor of caspases (Figure 1B). These results suggest that the ceramide-induced alterations in cell morphology precede the growth arrest and are independent of cell death.
Figure 2.
Ceramide-induced morphological changes of HeLa cells are independent of apoptosis. HeLa cells treated with the vehicle (Et) or 2.5 μM D-e-Cer (Cer) for 12 h were subjected to nuclear staining with Hoeschst 33342 (A), Western blot analysis of PARP cleavage (B), and FACS analysis (C). The numbers represent the percentage of the cells in the S phase (S) of the cell cycle or apoptotic cells (A) in total cell population. This figure is representative of at least three independent experiments.
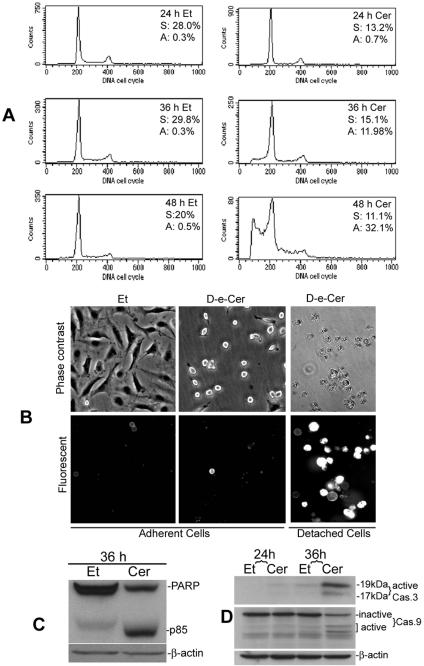
Long-term Treatment with Ceramide Causes Growth Arrest and Apoptosis
On the other hand, cells treated with D-e-Cer for a prolonged period (within 24 h) were arrested at the G1 phase of the cell cycle but did not show significant apoptosis, as revealed by FACS analysis (Figure 3A). Further treatment (>36 h) with D-e-Cer caused substantial cell detachment from the culture vessels, and the detached but not adherent cells, stained positive with annexin-V-rhodamine (Figure 3B). FACS analysis showed that ceramide treatment for the extended period caused a substantial increase in the sub-G0/G1 cell population as well as cell cycle arrest at both the G1 and G2/M phase (Figure 3A). Western blot analysis revealed that, compared with the vehicle control, D-e-Cer also caused substantial PARP cleavage (Figure 3C). Moreover, both caspase 9 and 3 were activated robustly in the D-e-Cer–treated cells, but only slightly in the vehicle-treated cells (Figure 3D). These results suggest that ceramide treatment for a prolonged period causes cell detachment and anoikis, a subtype of apoptosis due to cell detachment.
Figure 3.
Long-term treatment with ceramide causes growth arrest and apoptosis. HeLa cells treated with 2.5 μM D-e-Cer or Et were subjected to FACS analysis at 24, 36, and 48 h (A), annexin-V-rhodamine staining and microscopic analysis at 36 h (B), Western blot analysis of PARP cleavage (C), and Western blot analysis of caspase 9 and 3 activation of (D) at 36 h. p85, the 85-kDa cleaved fragment of PARP; Cas. 9, caspase 9; and Cas. 3, caspase 3. This figure is representative of at least three independent experiments.
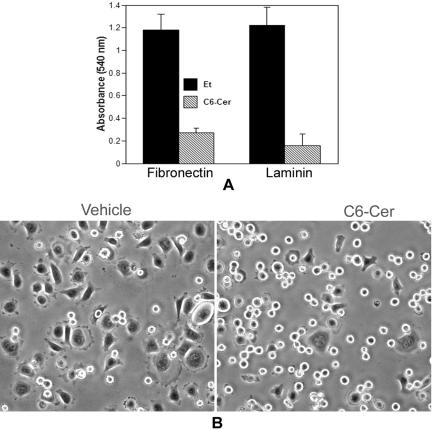
Treatment with Ceramide Suppresses Integrin-mediated Cell Adhesion to and Spreading on Extracellular Matrix
Microscopic observations revealed that the ceramide-treated cells exhibited a tendency to retreat from and lose contact with culture vessels, suggesting that ceramide may cause defects in cell adhesion and spreading. To investigate whether ceramide inhibits cell adhesion to and spreading on extracellular matrix (ECM), the same number of viable HeLa cells treated with D-e-Cer (2.5 μM) or vehicle for 16 h were plated onto six-well plates coated with fibronectin (10 μg/ml) and laminin (20 μg/ml). Compared with the control cells, adhesion of the ceramide-treated cells to fibronectin or laminin was significantly inhibited (Figure 4A). The vehicle-treated cells completely spread on the fibronectin-coated surface within 45 min, whereas ceramide-treated cells failed to do so (Figure 4B). These results suggest that ceramide inhibits cell adhesion to and spreading on ECM.
Figure 4.
Ceramide inhibits integrin-mediated cell adhesion to and spreading on extracellular matrices. The same number of HeLa cells treated with D-e-Cer (2.5 μM) or vehicle control for 16 h as described in Figure 1 was plated onto six-well plates coated with fibronectin (10 μg/ml) and laminin (20 μg/ml) and incubated at 37°C for 45 min. (A) After removal of nonadherent cells by wash with PBS, the number of adherent cells was determined as described under Materials and Methods. (B) Cell morphology was assessed by microscopic analyses. Data represent mean value ± SD of three independent experiments performed in duplicate. This figure is representative of at least three independent experiments.
Treatment with Ceramide Inhibits Glycosylation of β1 Integrin in the GC and Trafficking of β1 Subunit-containing Integrin Receptors to the Cell Surface
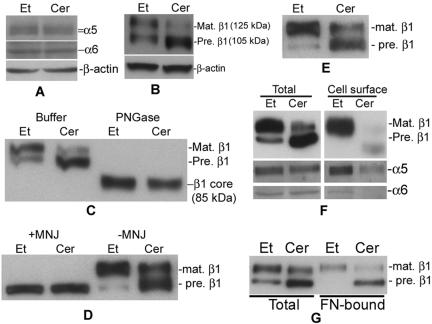
Integrin receptors play a critical role in cell adhesion to ECM (DeMali et al., 2003). To investigate whether ceramide inhibits the function of integrin receptors, first we analyzed the effects of D-e-Cer on expression of α5β1 and α6β1 integrin receptors, the major integrin receptors in HeLa cells that specifically bind to fibronectin and laminin, respectively. By Western blot analysis, it was found that the total expression levels of α5, α6, and β1 integrin subunits were unaltered by treatment with D-e-Cer (Figure 5A). However, treatment with D-e-Cer altered the ratio between two β1 integrin isoforms, reducing the one (125 kDa) with a smaller electrophoretic mobility but concomitantly accumulating the other (105 kDa) with a greater mobility (Figure 5B). These two β1 integrin isoforms, in terms of molecular mass, correspond to the β1 integrin precursor and mature form (Zheng et al., 1994; Veiga et al., 1995), respectively. Therefore, treatment with ceramide inhibited the maturation of β1 integrin.
Figure 5.
Treatment with D-e-Cer inhibits glycosylation of β1 integrin in the GC and trafficking of its associated integrin receptors to the cell surface. (A and B) HeLa cells treated with 2.5 μM D-e-Cer (Cer) or Et for 16 h were lysed, and the cell lysates were subjected to Western blot analysis by using antibodies against α5, α6, and β1 integrin subunits. (C) The above-mentioned cell lysates were treated with PGNase F before Western blot analysis. (D) HeLa cells also were treated with D-e-Cer or Et overnight in the presence of MNJ (200 μg/ml) before Western blot analysis. (E) HT1080 cells were treated with 5 μM D-e-Cer or Et and were subjected to Western blot analysis by using anti-β1 integrin antibody. HeLa cells treated with D-e-Cer or Et for 16 h were biotinylated. Total biotinylated cell surface proteins were precipitated by streptavidin-agarose beads and were subjected to Western blot analysis with the antibody against β1, α5, or α6 integrin subunit (F). (G) The lysates from the above-mentioned cells were subjected to in vitro fibronectin binding assays as described under Materials and Methods. The above-mentioned data are representative of the results from at least three experiments. Mat., mature; Pre., precursor. This figure is representative of at least three independent experiments.
It has been reported that the β1 integrin polypeptide (core) is partially glycosylated in the endoplasmic reticulum (ER) to form the precursor, which is further glycosylated in the GC to yield the mature form (Hotchin and Watt, 1992; Bellis et al., 1999). To determine whether the difference in the electrophoretic mobility between these two β1 integrin isoforms is due to a different degree of their glycosylation, cell lysates were treated with PGNase F, which completely removes oligosaccharide chains from glycoproteins. Western blot analysis revealed that treatment with PGNase F converted both the β1 integrin precursor and mature form to a polypeptide (86 kDa) with a greater electrophoretic mobility, which corresponds to the β1 integrin core in the term of molecular mass (Figure 5C), suggesting that these two β1 integrin isoforms are glycosylated to a different extent and that ceramide inhibits the glycosylation of the β1 integrin precursor.
To determine whether the inhibition of glycosylation of β1 integrin indeed occurs in the GC, we treated cells with 1-deoxymannojirimycin (MNJ), which specifically blocks protein glycosylation in the GC without affecting that in the ER (Hotchin and Watt, 1992). Western blot analysis showed that treatment with MNJ also converted the mature isoform of β1 integrin to its precursor that exhibited the same electrophoretic mobility as the β1 integrin isoform accumulated by treatment with D-e-Cer (Figure 5D), suggesting that ceramide indeed inhibits the glycosylation of β1 integrin in the GC.
To determine whether the ceramide-induced inhibition of the glycosylation of β1 integrin is a cell type-specific or general effect, we investigated the glycosylation status of β1 integrin in HT1080 cells treated with D-e-Cer and the control vehicle, respectively. Western blot analysis revealed that the glycosylation of β1 integrin precursor was significantly inhibited by treatment with D-e-Cer, compared with the vehicle control (Figure 5E), suggesting that the ceramide-induced inhibition of the glycosylation of β1 integrin is cell type independent. Together, these results demonstrate that D-e-Cer blocks the maturation of β1 integrin subunit by inhibiting its glycosylation in the GC.
The maturation of β1 integrin has been shown to be important for its transport to the cell surface and/or binding to ECM ligands. To test whether D-e-Cer inhibits transport of β1 integrin to the cell surface, cell surface proteins were biotinylated and precipitated with streptavidin-agarose beads, which bind to biotinylated proteins specifically. The biotinylated proteins were subjected to Western blot analysis by using the antibody against β1 integrin. As shown in Figure 5F, the mature β1 integrin but not the precursor was transported to the cell surface in the vehicle-treated cells. The levels of the mature β1 integrin on the cell surface in the D-e-Cer–treated cells were significantly decreased compared with the vehicle-treated cells. Consistently, the cell surface α5 and α6 integrins, which have been shown to be transported to the cell surface after binding to β1 integrin in cells (Schwartz et al., 1995), also were substantially decreased in the D-e-Cer–treated cells (Figure 5F). These results suggest that treatment with D-e-Cer inhibits the trafficking of β1 subunit-associated integrin receptors to the cell surface.
The inhibition of the β1 intergin glycosylation has been shown to be associated with the decrease in its ligand binding ability as well (Akiyama et al., 1989). To investigate whether treatment with D-e-Cer also causes a decrease in the fibronectin binding ability of β1 subunit-containing integrin receptors, HeLa cells were lysed with Triton X-100 after treatment with D-e-Cer or vehicle for 12 h, and the cell lysates were applied to fibronectin affinity columns. After washing, integrin receptors bound to the affinity columns were eluted with EDTA, and the eluants were subjected to Western blot analysis with anti-β1 integrin antibody. As shown in Figure 5G, both the mature β1 integrin and its precursor bound to the fibronectin affinity column, suggesting that the ceramide-induced inhibition of the glycosylation has no effect on the ligand binding ability of β1 subunitassociated integrin receptors.
Treatment with Ceramide Disrupts the GC and Blocks Protein Trafficking from the ER to Golgi
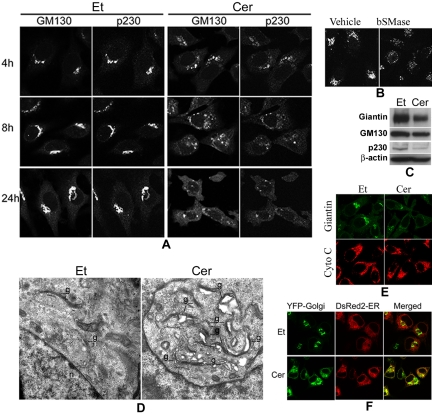
The above-mentioned results showed that treatment with D-e-Cer inhibited the glycosylation of the β1 integrin subunit in the GC, leading to an accumulation of the β1 integrin precursor that is glycosylated in the ER. This could arise if treatment with D-e-Cer blocks transport of β1 integrin precursor from the ER to the GC, inhibits the Golgi-localized enzymes responsible for the glycosylation of β1 integrin, or disturbs the structure and function of the GC. We first investigated the effects of ceramide on the structure of the GC. Cells treated with D-e-Cer or vehicle were immunostained with antibodies against GM130 and p230-Trans, resident proteins of the cis-and trans-Golgi, respectively. Confocal microscopic analyses revealed that treatment with D-e-Cer significantly altered the localization of both GM130 and p230-Trans to the GC in a time-dependent manner (Figure 6A). At 4 h, both proteins were localized in fragmented or dispersed perinuclear vesicular structures in the D-e-Cer–treated cells, whereas they were localized to the compacted ribbon-shaped GC in the vehicle-treated cells. At 8 h, GM130 was localized in more dispersed perinuclear vesicular structures as well as in small punctate structures scattered throughout the cytoplasm, and p230-Trans was predominantly localized in small punctate structures throughout the cytoplasm in the D-e-Cer–treated cells. At 24 h, both GM130 and p230-Trans were in the punctate structures throughout the cytoplasm in the ceramide-treated cells. In contrast, GM130 and p230-Trans remained in distinct juxtanuclear Golgi cisternae in the vehicle-treated cells throughout the entire time course. Treatment of HeLa cells with bacterial sphingomyelinase, which has been shown to generate endogenous ceramide via hydrolysis of sphingomyelin on the plasma membrane (Luberto and Hannun, 1998), also caused a dislocation of GM130 to the fragmented vesicular structures (Figure 6B).
Figure 6.
Treatment with D-e-ceramide causes the fragmentation of the Golgi complex and inhibits protein exit from the ER. (A) HeLa cells treated with D-e-Cer or Et for various time durations were colabeled with antibodies against GM130 and p230-Trans before confocal microscopic analyses. (B) Two hours after treatment with 0.4 U/ml bSMase or the vehicle (glycerol), HeLa cells were stained with the anti-giantin antibody and analyzed by confocal microcopy. (C) Sixteen hours posttreatment with D-e-Cer or Et, expression of giantin, GM130, and p230-Trans was determined by Western blot analysis with their corresponding antibodies. (D) HeLa cells treated with Et or 2.5 μM D-e-Cer for 1 h were fixed and subjected to electronic microscopic analysis. g, Golgi complex; n, nucleus. (E) Note the formation of vacuoles closely associated with the partially ruptured Golgi complex in the D-e-Cer–treated cells. HeLa cells treated with D-e-Cer (2.5 μM) or Et for 4 h before coimmunostaining with antibodies against giantin and cytochrome C (Cyto C), respectively. (F) Twelve hours post-transfection with plasmid pEYFP-Golgi and pDsRed2-ER, HeLa cells were treated with D-e-Cer or vehicle for 6 h. The live cells were imaged under the confocal microscope. This figure is representative of multiple independent experiments.
Western blot analysis showed that, compared with the vehicle-treated cells, expression of GM130, giantin, and p230-Trans was slightly inhibited in the D-e-Cer–treated cells (Figure 6C), suggesting that the ceramide-induced inhibition of the localization of these proteins to the GC is not primarily due to the inhibition of expression of these proteins.
We then investigated the effect of D-e-Cer treatment on the ultrastructure of the GC by electron microscopy. At as early as 1 h, treatment of HeLa cells with 2.5 μM D-e-Cer caused dilation of the GC cisternae, formation of large vacuoles, and separation of cistern stacks (Figure 6D), suggesting that treatment with D-e-Cer causes a profound impact on the structure of the GC.
We then investigated whether treatment with D-e-Cer alters the structure of mitochondria, which have been shown to be an important target of ceramide (Richter and Ghafourifar, 1999; Muriel et al., 2000). By immunostaining with an antibody against cytochrome C, we demonstrated that, compared with treatment with the vehicle, 4-h treatment of HeLa cells with D-e-Cer had no significant effect on morphology of mitochondria, although the GC was severely fragmented (Figure 6E), suggesting that D-e-Cer has an insignificant effect on the integrity of mitochondria at least at the early time points.
Treatment with D-e-Cer inhibits the glycosylation of β1 integrin in the GC, leading to the accumulation of the β1 integrin precursor, which is glycosylated in the ER, suggesting that the GC fragmentation may block protein trafficking from the ER to the GC. To test this, we transiently transfected HeLa cells with plasmid constructs directing expression of a yellow fluorescent protein (YFP)-Golgi carrying a Golgi targeting sequence and red fluorescent protein (pdsRED2-ER) carrying an ER targeting sequence, respectively. Twelve hours post-transfection, the cells were treated with D-e-Cer for 6 h. In the vehicle-treated cells, the neosynthesized YFP-Golgi and dsRED2-ER were localized to the ribbon-shaped GC and the ER network, respectively (Figure 6E). However, in the D-e-Cer–treated cells, YFP-Golgi was blocked in an ER-like structure in which dsRED2-ER was colocalized, suggesting that D-e-Cer prevents the ER exit of Golgi proteins. This explains why D-e-Cer inhibits the glycosylation of β1 integrin in the GC but enhances that in the ER. Together, the above-mentioned results suggest that treatment with D-e-Cer causes the fragmentation and disruption of the GC, thus blocking protein trafficking from the ER to the GC.
Treatment with Ceramide Alters the Rearrangement of Microtubule and F-Actin Cytoskeletons and Inhibits the Formation of Focal Adhesions and Filopodia
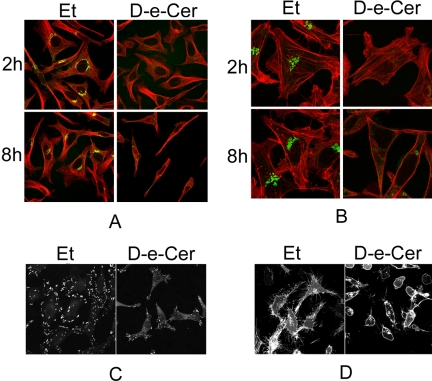
The integrity of the GC has been shown to be essential for the reorganization of the microtubule cytoskeleton and for cell polarity and morphology (Alvarez and Sztul, 1999). To investigate whether ceramide treatment has any effect on the reorganization of the microtubule cytoskeleton, the microtubule network was analyzed by immunostaining with an anti-α-tubulin antibody. Confocal microscopy revealed that, compared to the vehicle-treated cells, the microtubule network in cells treated with D-e-Cer for 2 h lacked major aberrations although the GC was severely fragmented. However, the microtubules in cells treated with D-e-Cer for a prolonged period (>8 h) were randomly distributed, whereas those in the vehicle-treated cells were organized in a polarized manner (Figure 7A), suggesting that D-e-Cer impairs the reorganization of microtubules post-GC fragmentation.
Figure 7.
Ceramide alters the arrangement of the microtubule and F-actin cytoskeletons and inhibits the formation of focal adhesions and filopodia. After being treated with 2.5 μM D-e-Cer or Et for different times, HeLa cells were stained with antibodies against α-tubulin (red) and giantin (green) (A), phalloidin-rhodamine (red) and giantin (green) (B), or paxillin (C) before confocal microscopic analyses. Note that the vehicle-treated cells contain abundant filopodia that are significantly reduced in the ceramide-treated cells. (D) After 24 h transfection with the plasmid pEYFP-Mem, which directs expression of enhanced yellow fluorescent protein (EYFP) targeting to the plasma membrane, HeLa cells were treated for D-e-Cer or vehicle control for 12 h before confocal microscopic analysis. Note that filopodia were significantly inhibited in the D-e-Cer–treated cells. This figure is representative of at least three independent experiments.
The integrity of the GC has been shown to be essential for the reorganization of the F-actin cytoskeleton as well (Alvarez and Sztul, 1999). To investigate whether treatment with D-e-Cer has any effect on this cellular event, cells were labeled with phalloidin-rhodamine, which specifically binds to the F-actin cytoskeleton. Confocal microscopy showed that, at 2 h, both vehicle and D-e-Cer treated cells formed strong polarized F-actin fiber bundles although the GC was fragmented in the ceramide-treated cells. At 8 h, the formation of F-actin fiber bundles was significantly inhibited in the D-e-Cer– but not vehicle-treated cells (Figure 7B), suggesting that D-e-Cer also causes the disorganization of the F-actin cytoskeleton post-GC fragmentation.
The F-actin stress fibers are associated with focal adhesions that play a critical role in cell adhesion and spreading (Parsons et al., 2000; Palazzo and Gundersen, 2002). Therefore, we analyzed the effect of D-e-Cer on focal adhesions by immunostaining with anti-paxillin antibody. Treatment with D-e-Cer significantly reduced the number and size of focal adhesions (Figure 7C). Moreover, the distribution of focal adhesions was altered by treatment with D-e-Cer. Focal adhesions were mainly found at the ends of the ceramide-treated cells, whereas they were distributed at the entire perimeter of the control cells. These results suggest that D-e-Cer has a profound inhibitory effect on focal adhesions.
The reorganization of the F-actin cytoskeleton plays an important role in the formation of filopodia, which in turn is associated with cell spreading (Small et al., 2002). Therefore, we investigated whether treatment with D-e-Cer inhibits the formation of filopodia. To visualize filopodia in living cells, we transfected HeLa cells with a plasmid construct pEYFP-Mem that directs the expression of a plasma membrane-bound fluorescent protein (YFP-Mem). By confocal microscopy, we observed that the control cells formed abundant filopodia, which were barely detectable in the ceramide-treated cells (Figure 7D). These results suggest that D-e-Cer systematically inhibits the organization of both microtubule and F-actin cytoskeletons and cytoskeleton-associated cellular events.
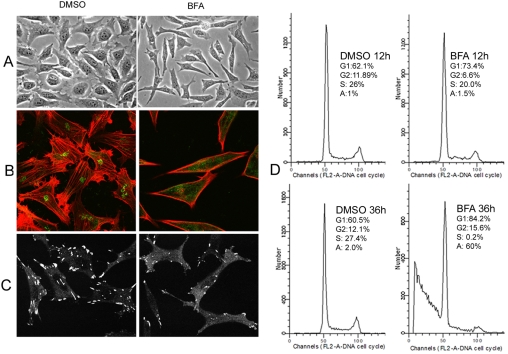
Disruption of the Golgi Complex via Brefeldin A (BFA) Causes Similar Cellular Effects to Treatment with Ceramide
The above-mentioned results clearly demonstrated that the GC fragmentation precedes all other cellular effects in response to treatment with D-e-Cer. However, it remains unknown whether the GC fragmentation is the cause of other cellular effects. If the GC fragmentation is responsible for the other cellular effects, disruption of the GC via different mechanisms would lead to similar cellular effects. To test this, we treated HeLa cells with BFA, which disrupts the GC by fusing it to the ER. Similar to D-e-Cer, BFA caused drastic morphological changes (Figure 8A), inhibition of the reorganization of the F-actin cytoskeleton (Figure 8B), and focal adhesions (Figure 8C). Short-term (<12 h) BFA treatment caused cell cycle arrest of HeLa cells, whereas prolonged treatment (>36 h) caused both cell cycle arrest and apoptosis (Figure 8D). These results suggest that the disruption of the GC is the cause of other cellular effects in response to treatment with D-e-Cer.
Figure 8.
Brefeldin A causes the similar effects to ceramide treatment. (A) HeLa cells were treated with dimethyl sulfoxide (DMSO) or 100 nM BFA for 8 h and were photographed under the inverted microscopy. These cells were fixed and co-stained with the anti-giantin (green) and phalloidin-rhodamine (red) (B) or stained with the anti-paxillin antibody (C) before confocal microscopy. HeLa cells treated with DMSO or BFA for various times were subjected to FACS analysis (D). G1, G1 phase, G2, G2 phase, S, S phase; A, apoptosis. This figure is representative of at least three independent experiments.
Sphingosine Causes the GC Fragmentation
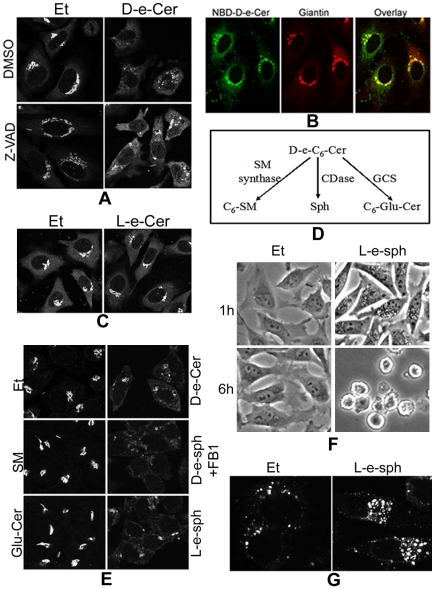
Disassembly of organelles, including the GC, has been shown in some situations to be a secondary consequence of apoptosis (Walker et al., 2004). The above-mentioned results showed that the ceramide-induced GC fragmentation in HeLa cells occurred as early as 1 h post-ceramide treatment, whereas the ceramide-induced apoptosis occurred at least 20 h later, indicating that the ceramide-induced GC fragmentation is before and independent of apoptosis. This was confirmed by confocal microscopic analyses showing that the GC fragmentation was not inhibited by Z-VAD-fmk (Figure 9A).
Figure 9.
Sphingosine generated from hydrolysis of ceramide may cause the GC fragmentation via vacuolization. HeLa cells were preincubated with dimethyl sulfoxide (DMSO) or Z-VAD-fmk (50 μM) for 1 h before addition of Et or 2.5 μM D-e-Cer to medium. (B) Sixteen hours later, the cells were fixed and immunostained with the anti-giantin antibody before confocal microscopy (A). Thirty min after incubation with 2.5 μM NBD-C6-cer, HeLa cells were fixed and labeled with the anti-giantin antibody before confocal microscopy. (C) HeLa cells were treated with Et or 10 μM L-e-C6-ceramide (L-e-Cer) for 16 h before immunostaining with giantin and confocal microscopy. (D) Metabolism of ceramide. C6-Glu-Cer, C6-glucosylceramide; CDase, ceramidase; GCS, glucosylceramide synthase; SM, sphingomyelin. (E) HeLa cells were treated with Et, 20 μM D-e-C6-SM, or 20 μM D-e-C8-Glu-Cer for 16 h, and 2.5 μM D-e-sphingosine (D-e-Sph), 2.5 μM L-e-sphingosine (L-e-sph), or 2.5 μM D-e-Cer for 4 h before immunostaining with the anti-giantin antibody and confocal microscopy. (F) HeLa cells treated with Et or 2.5 μM L-e-Sph for 1 h or 6 h were fixed and photographed under a inverted microscope. Note the massive perinuclear vacuolization in the cells treated with L-e-sph for 1 h and total cell rounding within 6 h. The above-mentioned cells treated with Et or L-e-sph for 1 h were stained with the anti-LAMP1 antibody before confocal microscopy (G). Note that the perinuclear vacuoles stained positive with the anti-LAMP1, indicating the lysosomal origin. This figure is representative of at least three independent experiments.
We then investigated the mechanism of the ceramide-induced GC fragmentation. It has been shown that exogenous cell-permeable ceramide is preferentially targeted to the GC (Lipsky and Pagano, 1985), and so is the endogenously generated ceramide (Hanada et al., 2003). To determine whether this organelle-specific targeting of D-e-Cer also applies to HeLa cells, we labeled cells with 2.5 μM NBD-D-e-C6-ceramide, a fluorescent analog of D-e-C6-ceramide. Confocal microscopy demonstrated that NBD-D-e-C6-ceramide was mainly transported to the perinuclear ribbon-like structure where giantin resided (Figure 9B), suggesting that exogenously applied D-e-Cer is favorably transported to the GC in HeLa cells.
One would argue that, as a lipophilic molecule, D-e-Cer, when accumulated in a membranous organelle, may disrupt the structure of the organelle simply by altering the physiochemical properties of the membrane. To test this possibility, we investigated whether the D-e-Cer enantiomer L-e-Cer, which possesses similar physiochemical properties except for stereospecificity, causes GC fragmentation. Treatment of HeLa cells for a prolonged period (16 h) with L-e-Cer at an elevated concentration (10 μM) failed to alter the morphology of the GC as revealed by immunostaining with giantin (Figure 9C), suggesting that the lipophilic property of D-e-Cer is not accountable for the GC fragmentation.
Unlike D-e-C6-Cer, L-e-Cer is a nonmetabolizable stereoisomer. We speculated that further metabolism of D-e-Cer may be required for the induction of the GC fragmentation. By mass spectrometric analyses, we found that treatment of HeLa cells with 2.5 μM D-e-Cer, but not L-e-Cer, caused a >50-fold increase in the levels of sphingosine, a metabolite of ceramide hydrolysis by a ceramidase (Table 1). In addition to sphingosine, ceramide can be converted to sphingomyelin and glycosylceramide via the action of sphingomyelin synthase and glucosylceramide synthase, respectively (Figure 9D). Treatment of HeLa cells with 10 μM C6-sphingomyelin or C8-glucosylceramide for a prolonged period (>16 h) failed to induce the GC fragmentation (Figure 9E). In contrast, treatment of HeLa cells with 2.5 μM D-e-sphingosine in the presence of 50 μM fumonisin B1 (FB1), which prevents sphingosine from being incorporated into ceramide by inhibiting ceramide synthase, rapidly induced the GC fragmentation (Figure 9E). Treatment of HeLa cells with L-e-sphingosine, the nonmetabolizable stereoisomer of D-e-sphingosine, was as potent as D-e-sphingosine in inducing the GC fragmentation (Figure 9E). These results suggest that sphingosine generated from hydrolysis of ceramide may cause the GC fragmentation. In addition, treatment of HeLa cells with L-e-sphingosine caused the massive formation of perinuclear vacuoles within 1 h and complete cell rounding within 6 h (Figure 9F). The vacuoles stained positive with an antibody against LAMP1, a lysosomal marker (Figure 9G), indicating that they were originated from lysosomes. These results suggest that sphingosine generated in the GC may cause the GC fragmentation via vacuolization.
Table 1.
Sphingosine generation from ceramide stereoisomers
| Treatment | Et | D-e-Cer | L-e-Cer |
|---|---|---|---|
| Sph (pmol/μmol Pi) | 26.5 ± 3.4 | 1424.0 ± 67 | 44.2 ± 5.6 |
Sphingolipids extracted from HeLa cells treated with Et, 2.5 μM D-e-Cer, or 2.5 μM L-e-Cer for 1 h were analyzed for contents of total sphingosine (Sph) (D-e-Sph plus L-e-Sph) in cells by ESI-MS/MS. Samples were normalized to total phospholipids (Pi). Data represent mean value ± SD of three independent experiments performed in triplicate.
DISCUSSION
Ceramide has been shown to cause various cellular effects, including growth inhibition, apoptosis, morphological changes, cell rounding and detachment, and inhibition of the cytoskeletal organization. The question is whether these different cellular effects are interconnected and have a common cause. In this study, we clearly demonstrated that these cellular effects are a consequence of the same cellular event, the disruption of the GC.
Ceramide-induced Disruption of the GC Is Responsible for the Morphological Changes
By Western blot analysis, we found that treatment with D-e-Cer significantly inhibited the glycosylation of the β1 integrin subunit in the GC, leading to the accumulation of the β1 integrin precursor that is glycosylated in the ER. This observation prompted us to investigate whether treatment with ceramide alters the structure and function of the GC. Through the combination of immunostaining and confocal microscopy, we demonstrated that treatment of HeLa cells with d-e-Cer caused redistribution of all examined Golgi resident proteins, including giantin, GM130, and p230-Trans, to the fragmented and dilated vesicular structures at the early time points. These dilated vesicles dispersed into tiny vesicles throughout the cytoplasm at the late time points. Western blot analyses revealed that treatment with D-e-Cer slightly affected expression of these Golgi-resident proteins, suggesting that the delocalization of the Golgi marker proteins is not primarily due to the inhibition of the expression of these proteins. By electron microscopy, we found that treatment with ceramide caused dilation of the GC cisternae, formation of large vacuoles, and separation of the cisternal stacks due to vacuolization. Rosenwald and coworkers demonstrated that treatment of Chinese hamster ovary cells with D-e-Cer causes the formation of enlarged vesicles as well (Rosenwald and Pagano, 1993). Together, these results suggest that treatment with D-e-Cer disrupts the structure of the GC via vacuolization.
The integrity of the GC is important for many cytoskeletal activities. Alvarez and Sztul (1999) showed that prolonged treatment with BFA, which disrupts the GC by fusing the Golgi membranes to the ER membranes, alters the organization of the both F-actin and microtubule cytoskeletons, leading to drastic changes in cell morphology. We observed the similar effects of BFA in HeLa cells, including the inhibition of the reorganization of the F-actin cytoskeleton and focal adhesions as well as drastic morphological changes. Treatment with D-e-Cer also inhibited the reorganization of both microtubule and F-actin cytoskeletons and the formation of focal adhesions and filopodia and caused drastic changes in cell morphology. All of these inhibitory effects occurred post-GC fragmentation. Therefore, the disruption of the GC, via whatever mechanisms, would lead to the similar inhibitory effects on cytoskeletal activities and cell morphology.
The Ceramide-induced Cell Detachment Is Due to Block of Translocation of Integrin Receptors to the Cell Surface
Feldhaus et al. (2002) showed that the increased generation of endogenous ceramide causes a decrease in the cell surface levels of integrin receptors, although the underlying mechanism was unclear. We demonstrated that treatment with D-e-Cer inhibited the maturation of β1 integrin by blocking its glycosylation in the GC, leading to the accumulation of the β1 integrin precursor, an immature form of β1 integrin. We further showed that the immature form of β1 integrin failed to reach the cell surface, thus leading to a substantial decrease in the cell surface levels of β1 integrin. Consistently, the cell surface levels of the α5 and α6 integrin subunits, which have been shown to bind to β1 integrin subunit before being transported to the cell surface, also were significantly decreased, indicating that the ceramide-induced disruption of the GC causes a decrease in the cell surface levels of β1 subunit-containing integrin receptors. The β1 integrin precursor (immature form)-containing receptors also have been shown to be incapable of binding to their ECM ligands (Akiyama et al., 1989). However, we found that the immature form of β1 integrin was capable of binding to fibronectin in vitro. Integrin receptors at the cell surface rather than those inside cells are functional in binding to ECM. Therefore, the decrease in the cell surface levels of integrin receptors is at least in part responsible for the defective cell adhesion.
By transient expression of a fluorescent protein (YFG-Golgi) tagged with a Golgi targeting sequence, we demonstrated that YFG-Golgi was trapped in the ER in the cells treated with D-e-Cer, whereas it is correctly targeted to the GC in the control cells, suggesting that D-e-Cer causes a defect in protein trafficking from the ER to the GC. This explains why treatment with D-e-Cer inhibits the glycosylation of β1 integrin in the GC, but not in the ER.
Inadequate Cell Adhesion and Spreading Are Associated with the Ceramide-induced Cell Growth Arrest and Apoptosis
Aszodi et al. (2003) showed that the deletion of β1 integrin subunit causes a defect in cell adhesion and spreading, leading to an inhibition of G1 progression and increased apoptosis of chondrocyte, suggesting that integrin receptor-mediated cell adhesion is critical for cell cycle progression and survival. In agreement, we demonstrated that the ceramide-induced defective cell adhesion and spreading were accompanied with cell cycle arrest and anoikis. The cell cycle arrest occurred postalterations in cell morphology and inhibition of cell spreading. Furthermore, apoptosis did not occur until cell detachment was initiated. These results suggest that apoptosis of the ceramide-treated cells is caused by inadequate cell adhesion and spreading. Apoptosis resulting from inadequate cell adhesion or cell detachment, termed anoikis, plays a critical role in regulating tissue homeostasis, especially for epithelium and endothelium (Grossmann, 2002; Michel, 2003). Most studies focus on the late events of anoikis— how cell death is executed postcell detachment. However, much remains unknown about signaling processes that initiate loss of a cell's contact with its surrounding ECM. This study suggests that ceramide or its metabolites may serves as an intrinsic signal for initiation of anoikis.
Fragmentation of the Golgi Complex Is Caused by Increased Hydrolysis of Ceramide
Ceramide is synthesized de novo in the ER and is transported to the GC where it is incorporated into various complex sphingolipids. Recently, a ceramide transfer protein (CERT) that directs translocation of ceramide from the ER to the GC has been identified (Hanada et al., 2003). Endogenous ceramides synthesized in the ER fail to translocate to the GC in a CERT mutant cell line. Exogenously applied NBD-D-e-Cer, a fluorescent analog of ceramide, has been shown to be transported to the GC in wild-type cells, but fails to do so in the CERT-deficient cells. In agreement, in this study, we found that exogenously applied NBD-D-e-Cer was favorably transported to and was enriched in the GC. It has been suggested that ceramide can physically perturb biological membranes due to its lipophilic feature (van Blitterswijk et al., 2003). However, we demonstrated that L-e-Cer, the enantiomer of D-e-Cer, which shares the similar physiochemical properties with D-e-Cer, is without any effect even at the elevated concentrations, suggesting that its lipophilic property is not responsible for the disruption of the GC.
The ceramide-induced GC fragmentation is highly dependent on its stereospecificity. We demonstrated that only D-e-Cer with a naturally occurring configuration induced the GC fragmentation. Compared with D-e-Cer, other ceramide stereoisomers are poor substrates for most metabolic enzymes of ceramide (Venkataraman and Futerman, 2001). Indeed, we found that treatment with D-e-Cer, but not L-e-Cer, caused a substantial increase in the levels of sphingosine, a metabolite of ceramide hydrolysis catalyzed by a ceramidase. Addition of exogenous D-e-sphingosine to HeLa cells, in the presence of FB1, which blocks the conversion of sphingosine to ceramide, potently caused the GC fragmentation and cell rounding. L-e-sphingosine, the nonmetabolizable enantiomer of D-e-sphingosine, induced the GC fragmentation as potently as D-e-sphingosine. Treatment with exogenous sphingosine caused the formation of a large number of perinucluear vacuoles originated from lysosomes, suggesting that, like many other organic amines (Morissette et al., 2004), sphingosine has the ability to cause vacuolization when trapped in an acidic organelle. Because D-e-Cer is enriched in the GC, sphingosine generated from D-e-Cer is very likely to be in the same compartment. The GC is an acidic organelle, so the accumulation of sphingosine in this organelle would form vacuoles. By electron microscopy, we could demonstrate the vacuolization in the GC in the D-e-Cer–treated cells. These observations suggest that sphingosine derived from hydrolysis of ceramide is very likely to be responsible for the GC fragmentation and its total disruption.
In conclusion, we provide ample evidence to support our hypothesis that the disruption of the structure and function of the GC is responsible for morphological changes, growth arrest, cell adhesion defects, and anoikis in response to treatment with D-e-Cer. These cellular effects are caused by the increased hydrolysis of D-e-Cer, probably through generating sphingosine. This study presents several novel findings on the mechanisms by which D-e-Cer induces cellular effects, thus point new directions for future studies of cellular responses to sphingolipids.
Acknowledgments
This work is supported in part by the Department of Defense/Hollings Cancer Center grant GC3532-03-42153 (to C. M.); National Institutes of Health grants R01 CA104834 (to C. M.), P20RR017677(to L.M.O.), and P01 CA097132 (to Y.A.H.); and Veterans Administration merit award (to L.M.O.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0594) on January 12, 2005.
References
- Acharya, U., Patel, S., Koundakjian, E., Nagashima, K., Han, X., and Acharya, J. K. (2003). Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science 299, 1740-1743. [DOI] [PubMed] [Google Scholar]
- Akiyama, S. K., Yamada, S. S., and Yamada, K. M. (1989). Analysis of the role of glycosylation of the human fibronectin receptor. J. Biol. Chem. 264, 18011-18018. [PubMed] [Google Scholar]
- Allan, V. J., Thompson, H. M., and McNiven, M. A. (2002). Motoring around the Golgi. Nat. Cell Biol. 4, E236-E242. [DOI] [PubMed] [Google Scholar]
- Alvarez, C., and Sztul, E. S. (1999). Brefeldin A (BFA) disrupts the organization of the microtubule and the actin cytoskeletons. Eur. J. Cell Biol. 78, 1-14. [DOI] [PubMed] [Google Scholar]
- Aszodi, A., Hunziker, E. B., Brakebusch, C., and Fassler, R. (2003). Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis, S. L., Newman, E., and Friedman, E. A. (1999). Steps in integrin beta1-chain glycosylation mediated by TGFbeta1 signaling through Ras. J. Cell. Physiol. 181, 33-44. [DOI] [PubMed] [Google Scholar]
- Bhalla, K. N. (2003). Microtubule-targeted anticancer agents and apoptosis. Oncogene 22, 9075-9086. [DOI] [PubMed] [Google Scholar]
- Bieberich, E., MacKinnon, S., Silva, J., Noggle, S., and Condie, B. G. (2003). Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J. Cell Biol. 162, 469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, R. G., Kelly, J., Storie, K., Pedersen, W. A., Tammara, A., Hatanpaa, K., Troncoso, J. C., and Mattson, M. P. (2004). Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc. Natl. Acad. Sci. USA 101, 2070-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali, K. A., Wennerberg, K., and Burridge, K. (2003). Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15, 572-582. [DOI] [PubMed] [Google Scholar]
- Di Bartolomeo, S., and Spinedi, A. (2002). Ordering ceramide-induced cell detachment and apoptosis in human neuroepithelioma. Neurosci. Lett. 334, 149-152. [DOI] [PubMed] [Google Scholar]
- Feldhaus, M. J., Weyrich, A. S., Zimmerman, G. A., and McIntyre, T. M. (2002). Ceramide generation in situ alters leukocyte cytoskeletal organization and beta 2-integrin function and causes complete degranulation. J. Biol. Chem. 277, 4285-4293. [DOI] [PubMed] [Google Scholar]
- Grossmann, J. (2002). Molecular mechanisms of “detachment-induced apoptosis-anoikis.” Apoptosis 7, 247-260. [DOI] [PubMed] [Google Scholar]
- Hanada, K., Kumagai, K., Yasuda, S., Miura, Y., Kawano, M., Fukasawa, M., and Nishijima, M. (2003). Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803-809. [DOI] [PubMed] [Google Scholar]
- Hannun, Y. A., and Obeid, L. M. (2002). The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277, 25847-25850. [DOI] [PubMed] [Google Scholar]
- Hotchin, N. A., and Watt, F. M. (1992). Transcriptional and post-translational regulation of beta 1 integrin expression during keratinocyte terminal differentiation. J. Biol. Chem. 267, 14852-14858. [PubMed] [Google Scholar]
- Kolesnick, R., and Fuks, Z. (2003). Radiation and ceramide-induced apoptosis. Oncogene 22, 5897-5906. [DOI] [PubMed] [Google Scholar]
- Levade, T., Malagarie-Cazenave, S., Gouaze, V., Segui, B., Tardy, C., Betito, S., Andrieu-Abadie, N., and Cuvillier, O. (2002). Ceramide in apoptosis: a revisited role. Neurochem. Res. 27, 601-607. [DOI] [PubMed] [Google Scholar]
- Lipsky, N. G., and Pagano, R. E. (1985). A vital stain for the Golgi apparatus. Science 228, 745-747. [DOI] [PubMed] [Google Scholar]
- Luberto, C., and Hannun, Y. A. (1998). Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J. Biol. Chem. 273, 14550-14559. [DOI] [PubMed] [Google Scholar]
- Mao, C., Xu, R., Szulc, Z. M., Bielawski, J., Becker, K. P., Bielawska, A., Galadari, S. H., Hu, W., and Obeid, L. M. (2003). Cloning and characterization of a mouse endoplasmic reticulum alkaline ceramidase: an enzyme that preferentially regulates metabolism of very long chain ceramides. J. Biol. Chem. 278, 31184-31191. [DOI] [PubMed] [Google Scholar]
- Marchesini, N., Osta, W., Bielawski, J., Luberto, C., Obeid, L. M., and Hannun, Y. A. (2004). Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J. Biol. Chem. 279, 25101-25111. [DOI] [PubMed] [Google Scholar]
- Marushige, Y., and Marushige, K. (1998). Alterations in focal adhesion and cytoskeletal proteins during apoptosis. Anticancer Res. 18, 301-307. [PubMed] [Google Scholar]
- McCaig, C., Perks, C. M., and Holly, J. M. (2002). Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J. Cell Sci. 115, 4293-4303. [DOI] [PubMed] [Google Scholar]
- Merrill, A. H., Schmelz, E. M., Dillehay, D. L., Spiegel, S., Shayman, J. A., Schroeder, J. J., Riley, R. T., Voss, K. A., and Wang, E. (1997). Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142, 208-225. [DOI] [PubMed] [Google Scholar]
- Michel, J. B. (2003). Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler. Thromb. Vasc. Biol. 23, 2146-2154. [DOI] [PubMed] [Google Scholar]
- Morissette, G., Moreau, E., C-Gaudreault, R., and Marceau, F. (2004). Massive cell vacuolization induced by organic amines such as procainamide. J. Pharmacol. Exp. Ther. 310, 395-406. [DOI] [PubMed] [Google Scholar]
- Muriel, M. P., Lambeng, N., Darios, F., Michel, P. P., Hirsch, E. C., Agid, Y., and Ruberg, M. (2000). Mitochondrial free calcium levels (Rhod-2 fluorescence) and ultrastructural alterations in neuronally differentiated PC12 cells during ceramide-dependent cell death. J. Comp. Neurol. 426, 297-315. [DOI] [PubMed] [Google Scholar]
- Palazzo, A. F., and Gundersen, G. G. (2002). Microtubule-actin cross-talk at focal adhesions. Sci. STKE 2002, PE31. [DOI] [PubMed] [Google Scholar]
- Panigone, S., Bergomas, R., Fontanella, E., Prinetti, A., Sandhoff, K., Grabowski, G. A., and Delia, D. (2001). Up-regulation of prosaposin by the retinoid HPR and its effect on ceramide production and integrin receptors. FASEB J. 15, 1475-1477. [DOI] [PubMed] [Google Scholar]
- Parsons, J. T., Martin, K. H., Slack, J. K., Taylor, J. M., and Weed, S. A. (2000). Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606-5613. [DOI] [PubMed] [Google Scholar]
- Richter, C., and Ghafourifar, P. (1999). Ceramide induces cytochrome c release from isolated mitochondria. Biochem. Soc. Symp. 66, 27-31. [DOI] [PubMed] [Google Scholar]
- Rios, R. M., and Bornens, M. (2003). The Golgi apparatus at the cell centre. Curr. Opin. Cell Biol. 15, 60-66. [DOI] [PubMed] [Google Scholar]
- Rosenwald, A. G., and Pagano, R. E. (1993). Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J. Biol. Chem. 268, 4577-4579. [PubMed] [Google Scholar]
- Salicioni, A. M., Gaultier, A., Brownlee, C., Cheezum, M. K., and Gonias, S. L. (2004). Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J. Biol. Chem. 279, 10005-10012. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A., Schaller, M. D., and Ginsberg, M. H. (1995). Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 11, 549-599. [DOI] [PubMed] [Google Scholar]
- Semel, A. C., Seales, E. C., Singhal, A., Eklund, E. A., Colley, K. J., and Bellis, S. L. (2002). Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J. Biol. Chem. 277, 32830-32836. [DOI] [PubMed] [Google Scholar]
- Small, J. V., Stradal, T., Vignal, E., and Rottner, K. (2002). The lamellipodium: where motility begins. Trends Cell Biol. 12, 112-120. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and Merrill, A. H. (1996). Sphingolipid metabolism and cell growth regulation. FASEB J. 10, 1388-1397. [DOI] [PubMed] [Google Scholar]
- Stamnes, M. (2002). Regulating the actin cytoskeleton during vesicular transport. Curr. Opin. Cell Biol. 14, 428-433. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk, W. J., van der Luit, A. H., Veldman, R. J., Verheij, M., and Borst, J. (2003). Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369, 199-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga, S. S., Chammas, R., Cella, N., and Brentani, R. R. (1995). Glycosylation of beta-1 integrins in B16–F10 mouse melanoma cells as determinant of differential binding and acquisition of biological activity. Int. J. Cancer 61, 420-424. [DOI] [PubMed] [Google Scholar]
- Venkataraman, K., and Futerman, A. H. (2001). Comparison of the metabolism of L-erythro- and L-threo-sphinganines and ceramides in cultured cells and in subcellular fractions. Biochim. Biophys. Acta 1530, 219-226. [DOI] [PubMed] [Google Scholar]
- Walker, A., Ward, C., Sheldrake, T. A., Dransfield, I., Rossi, A. G., Pryde, J. G., and Haslett, C. (2004). Golgi fragmentation during Fas-mediated apoptosis is associated with the rapid loss of GM130. Biochem. Biophys. Res. Commun. 316, 6-11. [DOI] [PubMed] [Google Scholar]
- Zheng, M., Fang, H., and Hakomori, S. (1994). Functional role of N-glycosylation in alpha 5 beta 1 integrin receptor. De-N-glycosylation induces dissociation or altered association of alpha 5 and beta 1 subunits and concomitant loss of fibronectin binding activity. J. Biol. Chem. 269, 12325-12331. [PubMed] [Google Scholar]