Abstract
AIM
To determine which IIRC scheme was used by retinoblastoma centers worldwide and the percentage of D eyes treated primarily with enucleation versus globe salvaging therapies as well as to correlate trends in treatment choice to IIRC version used and geographic region.
METHODS
An anonymized electronic survey was offered to 115 physicians at 39 retinoblastoma centers worldwide asking about IIRC classification schemes and treatment patterns used between 2008 and 2012. Participants were asked to record which version of the IIRC was used for classification, how many group D eyes were diagnosed, and how many eyes were treated with enucleation versus globe salvaging therapies. Averages of eyes per treatment modality were calculated and stratified by both IIRC version and geographic region. Statistical significance was determined by Chi-square, ANOVA and Kruskal-Wallis tests using Prism.
RESULTS
The survey was completed by 29% of physicians invited to participate. Totally 1807 D eyes were diagnosed. Regarding IIRC system, 27% of centers used the Children's Hospital of Los Angeles (CHLA) version, 33% used the Children's Oncology Group (COG) version, 23% used the Philadelphia version, and 17% were unsure. The rate for primary enucleation varied between 0 and 100% and the mean was 29%. By IIRC version, primary enucleation rates were: Philadelphia, 8%; COG, 34%; and CHLA, 37%. By geographic region, primary enucleation rates were: Latin America, 57%; Asia, 40%; Europe, 36%; Africa, 10%, US, 8%; and Middle East, 8%. However, systemic chemoreduction was used more often than enucleation in all regions except Latin America with a mean of 57% per center (P<0.0001).
CONCLUSION
Worldwide there is no consensus on which IIRC version is used, systemic chemoreduction was the most frequently used initial treatment during the study period followed by enucleation and primary treatment modality, especially enucleation, varied greatly with regards to IIRC version used and geographic region.
Keywords: retinoblastoma, oncology, retina, enucleation, chemotherapy, intra-arterial chemotherapy, ophthalmic artery chemosurgery, cancer
INTRODUCTION
The International Intraocular Retinoblastoma Classification (IIRC) was developed to more accurately describe clinical responses to systemic chemotherapy in cases of intraocular disease as the Reese-Ellsworth system had been designed to describe responses to lateral photon irradiation thirty years previously[1]. In this system, retinoblastoma is graded “A” through “E”, with group A eyes having limited disease and therefore best prognosis and group E eyes having extensive disease and a poorer prognosis when managed with primary systemic chemotherapy. However, multiple versions of the IIRC with different criteria under the same “A” to “E” classification scheme exist including the Philadelphia[2], Children's Hospital of Los Angeles (CHLA)[3], and Children's Oncology Group (COG) versions[4]. Furthermore, while authors frequently stratify their globe salvage rates by IIRC classifications, the majority of papers in the literature fail to identify which IIRC version was used[5]–[24]. Taken together, outcome analysis of apparently similarly grouped eyes becomes difficult.
This problem is particularly salient for group D retinoblastoma eyes. Under the IIRC system, group D eyes are those with non-discrete growth or non-localized seeding with a minimum distance from the tumor depending on the version used. Specifically, the Philadelphia system defines D eyes as tumors with “subretinal and/or vitreous seeds >3 mm from the tumor margin occupying ≤50% of the globe”[2], while the COG system includes in its criteria the presence of vitreous and/or subretinal seeding in addition to subretinal fluid and also increases the radius to >6 mm[4]. The CHLA version defines D eyes as any tumor with “exophytic or endophytic qualities, diffuse/extensive vitreous or subretinal seeding, and/or retinal detachment in >1 quadrant[3]”. Based on these definitions, it is clear that a spectrum of disease severity exists across IIRC versions for group D eyes. Thus, although alternative treatment options including systemic, intra-arterial, intravitreal and periocular chemotherapy seemingly offer excellent globe salvage rates without higher rates of metastasis or secondary cancers[25]–[28] outcome comparison across the literature is challenging, especially when the IIRC version used is not specified. On review of the literature, only three of twenty papers reporting on outcomes for eyes classified using the IIRC specified which version was used in the text[20],[23]–[24]. In addition, because most data on globe-salvaging techniques come from large retinoblastoma centers, it is currently unclear if they are being widely adopted on an international scale[26]–[31].
Given these observations, the primary aims of this study were to 1) determine which IIRC scheme was used by retinoblastoma centers worldwide, 2) determine the percentage of D eyes treated primarily with enucleation versus globe salvaging therapies, 3) correlate trends in treatment choice to IIRC version used and geographic region.
SUBJECTS AND METHODS
A 39-question survey was sent via email to 115 ophthalmologists at 39 retinoblastoma centers worldwide using the Survey Monkey website (surveymonkey.com). Institutional review board (IRB) approval at our institution was obtained prior to survey distribution (IRB # WA0064-15). Survey recipients were known to be involved in childhood retinoblastoma management and were included based on their involvement in retinoblastoma care, conferences and forums.
Participants were asked to record data for group D eyes diagnosed in their center between January 1, 2008 and December 31, 2012. They were asked to record: the IIRC scheme used, the total number of group D eyes, and the number of eyes primarily treated with enucleation, intra-arterial chemotherapy, bridging chemotherapy followed by intra-arterial chemotherapy, systemic chemoreduction, external beam radiation, or brachytherapy. No personal or institutional data were collected, although all participants were asked to provide the country in which their practice is located. Relevant data from our own center, Memorial Sloan Kettering was also collected, entered into the electronic survey and included in the overall study data. A copy of the survey can be found as supplemental material to this article.
Survey results were aggregated in an Excel (Version 14.6.6) spreadsheet generated by the Survey Monkey website, which listed responses from each survey participant anonymously. For surveys that were returned incomplete, participants' responses were included in data analysis pertaining to the questions answered and excluded from data analysis for questions omitted. Data regarding brachytherapy were excluded given an exceedingly small sample size (10 eyes total, representing only 0.6% of total eyes).
The percentage of total D eyes treated with enucleation and other globe salvaging therapies was calculated along with mean and median values per center for each treatment modality. Data pertaining to each treatment modality were then grouped by IIRC version and geographic region and the same values were calculated. The Chi-square, one-way ANOVA and Kruskal-Wallis tests were used to determine statistical significance using Prism 6.
RESULTS
The survey was completed in entirety by 25% of physicians invited to participate in the survey and incompletely by an additional 8% of physicians. These responses were gathered from throughout the United States (11), Asia (7), Europe (4), Latin America (4), Africa (2), and the Middle East (1). Nine survey participants did not list their center's location. Totally 1807 D eyes were reported. Regarding IIRC system, 27% of centers used the CHLA version, 33% used the COG version, 23% used the Philadelphia version, and 17% were unsure which version they used. Within the United States, 36% of centers used the Philadelphia version, 36% used the Children's Oncology Group version, 18% used the Children's Hospital of Los Angeles Version and 9% did not know which version they used.
The rate for primary enucleation varied between 0 and 100% across centers. Of the aggregate 1807 D eyes reported, 27% were primarily enucleated. The mean percentage of eyes primarily enucleated per center was 29%, while the median was 17%. These values were derived by first calculating the percentage of D eyes primarily enucleated in each center and then calculating the mean and median of this data set. Twenty-six percent of centers did not primarily enucleate any eye and 26% of centers primarily enucleated greater than 50% of eyes. The two largest centers to participate in the study (≥300 eyes) enucleated less than 20% of eyes overall.
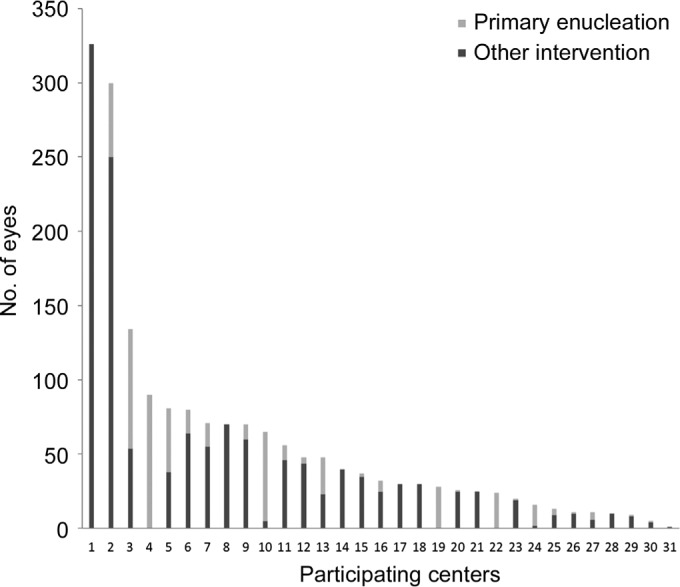
Systemic chemotherapy was the most commonly used treatment, administered to 53% of all group D eyes reported (P<0.0001). The mean percentage of eyes treated with systemic chemotherapy per center was 57%, while the median was 60%. The use of systemic chemotherapy was followed by other globe-salvaging techniques as follows: intra-arterial chemotherapy (16%), bridging plus intra-arterial chemotherapy (4%) and external beam radiation (1%) (P<0.0001). The number of eyes treated with enucleation versus globe-sparing techniques for each participating center is depicted in Figure 1. This figure includes any participant who at minimum answered how many group D eyes were diagnosed and how many of these group D eyes were primarily enucleated.
Figure 1. Treatment choice by center for all eyes reported.
When stratifying enucleation rates by IIRC system, centers using the Philadelphia version had both the greatest number of group D eyes diagnosed and the lowest percent of eyes primarily enucleated overall (P<0.0001) and per center (P=0.03) during the study period. Comparison to centers using the CHLA and COG version are shown in Table 1. When analyzed by geographic region, the primary enucleation rates of group D eyes were lowest in the Middle East (8%) and US (8%) (P<0.0001). Enucleation rates for each geographic region from this survey study are shown in Table 2 and compared to publishedenucleation rates per region in Table 3. Data from papers cited elsewhere in this article that only provided a combined enucleation rate for groups D and E eyes were omitted from Table 3.
Table 1. Comparison of group D eyes diagnosed and enucleated when stratified by IIRC version.
| Country | Total D eyes diagnosed | % D eyes primarily enucleated (P<0.0001) | Mean % D eyes primarily enucleated per center (P=0.03) |
| Philadelphia | 801 | 8 | 8 |
| CHLA | 473 | 37 | 27 |
| COG | 274 | 34 | 26 |
| Unknown | 259 | 63 | 62 |
CHLA: Children's Hospital of Los Angeles; COG: Children's Oncology Group.
Table 2. Comparison of group D eyes diagnosed and enucleated when stratified by geographic region.
| Country | Total D eyes diagnosed | % D eyes primarily enucleated (P<0.0001) | Median % D eyes primarily enucleated per center (P=0.03) |
| USA | 952 | 8 | 0 |
| Middle East | 12 | 8 | 1 |
| Africa | 96 | 10 | 7 |
| Europe | 75 | 36 | 7 |
| Asia | 394 | 40 | 31 |
| Latin America | 190 | 58 | 49 |
Table 3. Comparison of enucleation rates observed in our survey study versus published data.
| Country | Our survey enucleation rate (%) | Published enucleation rate (Avg) | Range | Dates |
| USA[24],[32]–[33] | 8 | 13 | 6-18 | 2000-2013 |
| Middle East[8] | 8 | 43 | 43 | 2001-2007 |
| Africa[5],[11],[23] | 10 | 96 | 91-100 | 2006-2011 |
| Europe[10],[18] | 36 | 58 | 33-82 | 1995-2011 |
| Asia[6],[7],[9],[13]–[14],[22] | 40 | 55 | 15-77 | 1997-2014 |
| Latin America[21] | 58 | 75 | 75 | 2007-2014 |
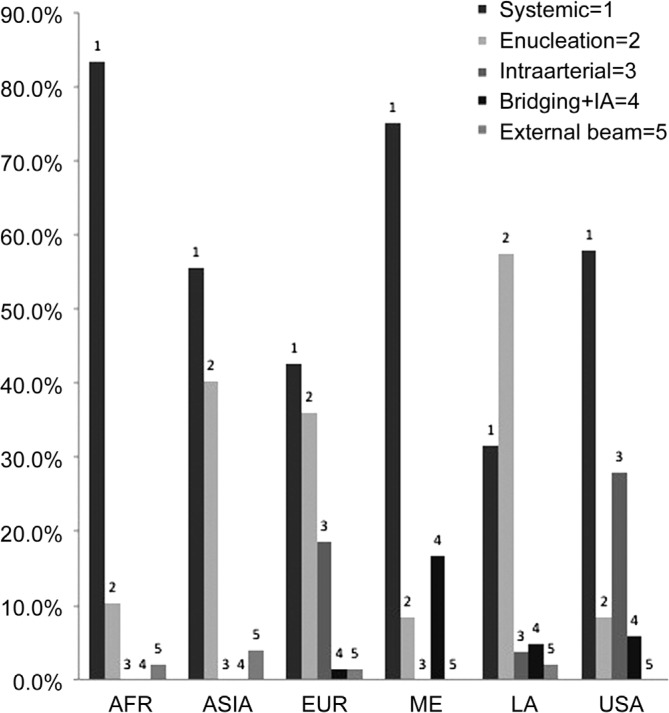
With regards to globe sparing therapies, systemic chemotherapy was used most frequently in all geographic regions except Latin America, which used enucleation more frequently (P<0.0001). Rates for systemic chemotherapy as primary treatment modality were: Africa, 83%, Middle East, 75%; US, 58%; Asia, 56%; Europe, 43% and Latin America, 32%. The use of intra-arterial chemotherapy as primary treatment was only reported in the US (28%), Europe (19%) and Latin America (4%). Bridging chemotherapy in combination with intra-arterial chemotherapy was reported in the Middle East (17%), US (6%), Latin America (5%) and Europe (1%). External beam radiotherapy was used least frequently and reported in Asia (4%), Africa (2%), Latin America (2%), and Europe (1%) (P<0.0001). These rates are compared in Figure 2.
Figure 2. Comparison of treatment choice stratified by geographic region.
AFR: Africa; EUR: Europe; ME: Middle East; LA: Latin America; IA: Intra-arterial.
DISCUSSION
Although the IIRC system was developed to better describe outcomes for intraocular retinoblastoma managed with systemic chemotherapy differing versions of this scheme make it challenging to compare success among centers, especially if the IIRC version is not specified. Based on our review of the literature and our survey data, there is no apparent consensus regarding which version of the IIRC is used both worldwide and within the United States. Moreover, a significant percentage of institutions do not even know which system they are using. This makes critical review of the literature regarding retinoblastoma eyes grouped by IIRC version difficult.
With regards to primary treatment choice for group D retinoblastoma, enucleation has historically been the standard of care for patients with advanced disease. However, over the last three decades, major centers decreased their rates of enucleation in favor of globe-salvaging techniques[24],[29]–[30]. Further refinements of chemotherapy delivery methods, especially recent series using intra-arterial chemotherapy, have shown increased rates of ocular salvage even in cases of advanced tumors[25]–[28]. Given this emerging data, it is interesting to compare practice patterns between international retinoblastoma centers as it is currently unknown whether new forms of treatment are available or widely utilized in developing countries.
Based on our survey study, enucleation rates varied greatly among centers, with 27% of group D eyes treated primarily with enucleation overall. Systemic chemotherapy was the most popular primary treatment choice for 53% of group D eyes. Intra-arterial chemotherapy, both alone and with bridging chemotherapy, and external beam radiation were less popular as initial treatment choice. The utilization of intra-arterial chemotherapy may reflect the study period, which only extended to 2012. As this technique gains popularity with time and is explored by other centers its utilization will likely increase. This is supported by a study by Grigorovski et al[31] that reports three-quarters of international centers regard intra-arterial chemotherapy as first-line treatment for advanced unilateral eyes. Participants were not asked to differentiate between bilateral versus unilateral disease in this survey. In addition, the survey did not elicit which treatment modalities were available to each participant at the time of diagnosis. Further survey studies are necessary to evaluate what factors contributed to the enucleation rates reported.
When stratifying data by IIRC version, centers using the Philadelphia version diagnosed the greatest number of D eyes and primarily enucleated the fewest eyes. This may be because in comparison to the COG and CHLA IIRC version, the Philadelphia version has the lowest cutoff for distance of subretinal and/or vitreal seeds away from the tumor and no mention of subretinal fluid or retinal detachment[2]–[4]. Thus, some eyes classified under the Philadelphia version as D eyes may have been considered C eyes under an alternate scheme. In addition, the Philadelphia version is the only classification scheme to include a definitive size cutoff for the tumor, setting it at ≤50% of the globe for group D eyes. This means that eyes with larger tumors above this cutoff would automatically be classified as group E under the Philadelphia scheme while the same eyes may be considered group D under COG or CHLA classification depending on their other qualities. Taken together, it follows that eyes containing less advanced tumors may have been more comfortably treated with globe-salvaging strategies by clinicians, thus possibly explaining the lowest percentage of enucleations in eyes classified as group D under the Philadelphia version. If a significant difference in ocular survival across IIRC version is confirmed by future studies, it might support standardization of the IIRC system.
With respect to geographic region, the lowest enucleation rates were found in the US (8%) and Middle East (8%). Higher enucleation rates were reported in Europe, Asia and Latin America. The US enucleation rate of 8% is consistent with published data as shown in Table 3. However, all other primary enucleation rates calculated from our survey data were lower than those found in the literature, including the worldwide average per center (29% from our survey versus 57% from the literature). Many variables may explain this finding such as under representation of regions by participation of a limited number of centers (the Middle East was represented by one center and Africa by 2 centers) and overrepresentation by the USA which had one of the lowest enucleation rates, or difference in management patterns between the published (including late 1990s and early 2000s) and study (2008-2012) time periods. In addition, little data on enucleation rates for group D eyes exist for comparison to our results. We cannot generalize the results of the few studies from a limited number of centers to an entire region and this may also account for inconsistencies between our data and reported enucleation rates as displayed in Table 3. An alternate hypothesis is that inherent bias in survey studies exists and centers with low enucleation rates may be more likely to respond to a survey regarding enucleation than centers with high rates. However, some published data suggest that web-based surveys eliminate some of this bias likely due to anonymity[32].
Despite significant advances in the understanding and treatment of retinoblastoma, there is limited consensus about how to classify and treat advanced tumors. Few centers report which IIRC version they use, and when stated, the discrepancies among versions make comparing results across the literature difficult for readers. Regardless of IIRC version used, however, and the success of globe sparing techniques such as intra-arterial chemotherapy, the use of enucleation as primary treatment for group D retinoblastoma is still widespread and variable worldwide. We look forward to future studies on larger cohorts with further diversity by region that confirm our results.
Acknowledgments
The abstract for this paper was presented at the ARVO 2016 Annual Meeting, sponsored by The Association for Research in Vision and Ophthalmology on May 3, 2016.
We thank all those who participated in this survey study.
Foundation: Supported in part by grants NIH/NCI Cancer Center Support Grant P30 CA008748.
Authors' Contributions: Scelfo C, Jenkins T: concept, data collection, writing; Francis JH: concept, critical revisions; Marr B: critical revisions; Abramson DH: concept, data collection, writing, critical revisions; Khetan V, Pe'er J: revisions, data collection; Shields CL, Munier F, Berry J, Harbour JW, Yarovoy A, Lucena E, Murray TG, Bhagia P, Paysse E, Tuncer S, Chantada GL, Moll AC, Ushakova T, Plager DA, Ziyovuddin I, Leal CA, Materin MA, Ji XD, Cursino JW, Polania R, Kiratli H, All-Ericsson C, Kebudi R, Honavar SG, Vishnevskia-Dai V, Epelman S, Daniels AB, Ling JD, Traore F, Ramirez-Ortiz MA: data collection.
Conflicts of Interest: Scelfo C, None; Francis JH, None; Khetan V, None; Jenkins T, None; Marr B, None; Abramson DH, None; Shields CL, None; Pe'er J, None; Munier F, None; Berry J, None; Harbour JW, None; Yarovoy A, None; Lucena E, None; Murray TG, None; Bhagia P, None; Paysse E, None; Tuncer S, None; Chantada GL, None; Moll AC, None; Ushakova T, None; Plager DA, None; Ziyovuddin I, None; Leal CA, None; Materin MA, None; Ji XD, None; Cursino JW, None; Polania R, None; Kiratli H, None; All-Ericsson C, None; Kebudi R, None; Honavar SG, None; Vishnevskia-Dai V, None; Epelman S, None; Daniels AB, None; Ling JD, None; Traore F, None; Ramirez-Ortiz MA, None.
REFERENCES
- 1.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, Shields JA. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Mashayekhi A, Demirci H, Meadows AT, Shields JA. Practical approach to management of retinoblastoma. Arch Ophthalmol. 2004;122(5):729–735. doi: 10.1001/archopht.122.5.729. [DOI] [PubMed] [Google Scholar]
- 3.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1) doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Just Diagnosed Staging. Children's Oncology Group Website. [Accessed on March 23, 2016]. Available at https://www.childrensoncologygroup.org/index.php/newlydiagnosedwithretinoblastoma. September 2011.
- 5.Waddell KM, Kagame K, Ndamira A, Twinamasiko A, Picton SV, Simmons IG, Johnston WT, Newton R. Clinical features and survival among children with retinoblastoma in Uganda. Br J Ophthalmol. 2015;99(3):387–390. doi: 10.1136/bjophthalmol-2014-305564. [DOI] [PubMed] [Google Scholar]
- 6.Lim FPM, Soh SY, Iyer JV, Tan AM, Swati H, Quah BL. Clinical profile, management, and outcome of retinoblastoma in Singapore. J Pediatr Ophthalmol Strabismus. 2013;50(2):106–112. doi: 10.3928/01913913-20121211-01. [DOI] [PubMed] [Google Scholar]
- 7.Shah PK, Narendran V, Kalpana N. Outcomes of intra- and extraocular retinoblastomas from a single institute in South India. Ophthalmic Genet. 2015;36(3):248–250. doi: 10.3109/13816810.2013.867450. [DOI] [PubMed] [Google Scholar]
- 8.Naseripour M, Nazari H, Bakhtiari P, Modarres-zadeh M, Vosough P, Ausari M. Retinoblastoma in Iran: outcomes in terms of patients' survival and globe survival. Br J Ophthalmol. 2009;93(1):28–32. doi: 10.1136/bjo.2008.139410. [DOI] [PubMed] [Google Scholar]
- 9.Huang D, Zhang Y, Zhang W, Wang Y, Zhang P, Hong L, Zhou Y, Han T, Zhi T. Study on clinical therapeutic effect including symptoms, eye preservation rate, and follow-up of 684 children with retinoblastoma. Eur J Ophthalmol. 2013;23(4):532–538. doi: 10.5301/ejo.5000245. [DOI] [PubMed] [Google Scholar]
- 10.Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol. 2009;93(7):887–890. doi: 10.1136/bjo.2008.142679. [DOI] [PubMed] [Google Scholar]
- 11.ElZomor H, Taha H, Aleieldin A, Nour R, Zaghloul MS, Fawzi M, Kamel A, Alfaar AS. High risk retinoblastoma: prevalence and success of treatment in developing countries. Ophthalmic Genet. 2013;36(3):287–289. doi: 10.3109/13816810.2015.1016241. [DOI] [PubMed] [Google Scholar]
- 12.Li SY, Chen SC, Tsai CF, Sheu SM, Yeh JJ, Tsai CB. Incidence and survival of retinoblastoma in Taiwan: a nationwide population-based study 1998-2011. Br J Ophthalmol. 2016;100(6):839–842. doi: 10.1136/bjophthalmol-2015-307211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn SM, Kim HS, Kim DJ, Lee SC, Lyu CJ, Han JW. Favorable outcome of alternate systemic and intra-arterial chemotherapy for retinoblastoma. Pediatr Hematol Oncol. 2016;33(1):74–82. doi: 10.3109/08880018.2015.1135363. [DOI] [PubMed] [Google Scholar]
- 14.Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014;121(2):517–524. doi: 10.1016/j.ophtha.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Alkofide A, Ayas M, Khafagah Y, Rawashde A, Anas M, Barria M, Siddiqui K, Almesfer S, Alkatan H. Efficacy of vincristine and carboplatin as chemo-reduction for advanced bilateral retinoblastoma, the Saudi experience. Saudi J Ophthalmol. 2013;27(3):193–196. doi: 10.1016/j.sjopt.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okimoto S, Nomura K. Clinical manifestations and treatment of retinoblastoma in Kobe Children's Hospital for 16 Years. J Pediatr Ophthalmol Strabismus. 2014;51(4):222–229. doi: 10.3928/01913913-20140513-01. [DOI] [PubMed] [Google Scholar]
- 17.Akyüz C, Kiratli H, Sen H, Aydin B, Tarlan B, Varan A. Intra-arterial chemotherapy for retinoblastoma: a single-center experience. Ophthalmologica. 2015;234(4):227–232. doi: 10.1159/000439357. [DOI] [PubMed] [Google Scholar]
- 18.Bartuma K, Pal N, Kosek S, Holm S, All-Ericsson C. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Ophthalmol. 2013;92(5):404–411. doi: 10.1111/aos.12282. [DOI] [PubMed] [Google Scholar]
- 19.Kunkele A, Jurklies C, Wieland R, Lohmann D, Bornfeld N, Eggert A, Schulte JH. Chemoreduction improves eye retention in patients with retinoblastoma: a report from the German Retinoblastoma Reference Centre. Br J Ophthalmol. 2013;97(10):1277–1283. doi: 10.1136/bjophthalmol-2013-303452. [DOI] [PubMed] [Google Scholar]
- 20.Palazzi MA, Stephan C, Brandalise SR, AguiarSdos S. Retinoblastoma diagnosis: a proposal based on the experience of Centro InfantilBoldrini, Brazil. Pediatr Hematol Oncol. 2013;30(5):379–385. doi: 10.3109/08880018.2013.775201. [DOI] [PubMed] [Google Scholar]
- 21.Ossandón D, Zanolli M, Pérez V, Rojas T, Quijarro P, Kabalan P, Alvarez D, Varas M. Multidisciplinary management of retinoblastoma: Experience in 37 eyes. Arch Soc Esp Oftalmol. 2015;90(2):55–62. doi: 10.1016/j.oftal.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Shin JY, Kim JH, Yu YS, Khwarg SI, Choung HK, Shin HY, Ahn HS. Eye-preserving therapy in retinoblastoma: prolonged primary chemotherapy alone or combined with local therapy. Korean J Ophthalmol. 2010;24(4):219–224. doi: 10.3341/kjo.2010.24.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Kettani A, Aderdour S, Daghouj G, Knari S, Zaghloul K, Zafad S, Harif M, Benchekroun S. Retinoblastoma: preliminary results of national treatment protocol at Casablanca University Medical Center. J Fr Ophtalmol. 2014;37(2):115–124. doi: 10.1016/j.jfo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Abramson DH, Daniels AB, Marr BP, Francis JH, Brodie SE, Dunkel IJ, Gobin YP. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma. PLoS One. 2016;11(1):e0146582. doi: 10.1371/journal.pone.0146582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abramson DH, Fabius AW, Issa R, Francis JH, Marr BP, Dunkel IJ, Gobin YP. Advanced unilateral retinoblastoma: the impact of ophthalmic artery chemosurgery on enucleation rate and patient survival at MSKCC. PLoS One. 2015;10(12):e0145436. doi: 10.1371/journal.pone.0145436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramson DH, Francis JH, Dunkel IJ, Marr BP, Brodie SE, Gobin YP. Ophthalmic artery chemosurgery for retinoblastoma prevents new intraocular tumors. Ophthalmology. 2013;120(3):560–565. doi: 10.1016/j.ophtha.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Abramson DH, Marr BP, Dunkel IJ, Brodie S, Zabor EC, Driscoll SJ, Gobin YP. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96(4):499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
- 28.Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery) Ophthalmology. 2010;117(8):1623–1629. doi: 10.1016/j.ophtha.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Shields JA, Shields CL, Sivalingam V. Decreasing frequency of enucleation in patients with retinoblastoma. Am J Ophthalmol. 1989;108(2):185–188. doi: 10.1016/0002-9394(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Gündüz K, Günalp I, Yalçındağ N, Unal E, Taçyıldız N, Erden E, Geyik PÖ. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004;111(10):1917–1924. doi: 10.1016/j.ophtha.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Grigorovski N, Lucena E, Mattosinho C, Parareda A, Ferman S, Catala J, Chantada G. Use of intra-arterial chemotherapy for retinoblastoma: results of a survey. Int J Ophthalmol. 2014;7(4):726–730. doi: 10.3980/j.issn.2222-3959.2014.04.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Leahey A, Griffin G, Jabbour P, Shields JA. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina. 2013;33(10):2103–2109. doi: 10.1097/IAE.0b013e318295f783. [DOI] [PubMed] [Google Scholar]
- 33.Berry JL, Jubran R, Kim JW, Wong K, Bababeygy SR, Almarzouki H, Lee TC, Murphree AL. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2012;60(4):688–693. doi: 10.1002/pbc.24303. [DOI] [PubMed] [Google Scholar]