Abstract
AIM
To develop a critical pathway for primary open angle glaucoma (POAG) diagnosis intended to be efficient, to unify criteria, reduce resource use and minimize costs to the health system.
METHODS
We performed a systematic search on PubMed, Cochrane, Embase and ClinicalTrials.org databases and classified the quality of evidence from level I through III.
RESULTS
A critical pathway was designed by setting a key-decision step by step model on the basis of the best current evidence.
CONCLUSION
A critical pathway, evidence-based guideline, may be a useful tool intended to reduce costs while maintaining or even improving the quality of care for diagnosing a highly prevalent pathology such as open angle glaucoma.
Keywords: glaucoma, diagnosis, critical pathway, guideline
INTRODUCTION
Glaucoma affects more than 60 million people and represents the second leading cause of blindness worldwide. Primary open angle glaucoma (POAG) is the most frequent type of glaucoma, affecting almost 45 million people over the globe. Blindness caused by glaucoma affects 8.4 million people nowadays, and this number is estimated to rise up to 11 million people for the year 2020[1]–[2].
Prompt diagnosis and treatment of ocular hypertension (OH) and glaucoma can diminish the permanent visual field loss and optic nerve head (ONH) damage observed in these patients[2]–[4]. This highlights the significance of reckon on with an effective diagnosis strategy that allows to optimize resources and, mainly, gain time. Glaucoma is commonly diagnosed using several ancillary tests and in fact there is no unique screening method that has proven to be totally effective by its own[2]. This explains several situations of delayed diagnosis seen on these patients, which finally leads to permanent and irreversible visual loss.
Critical pathway guidelines have emerged as improving proposals for making decisions, applied for both diagnosis and treatment of specific pathologies. Particularly, critical pathways have been successfully applied for emergency pathologies in order to optimize time and resources, unifying medical criteria for making decisions[5]. Considering the high prevalence of POAG and its irreversible consequence, we thought to be necessary to have a step by step model of taking decisions on the basis of the best current evidence.
The purpose of this paper is to develop a critical pathway for POAG diagnosis intended to be efficient, to unify criteria, reduce resource use and minimize costs to the health system.
MATERIALS AND METHODS
Critical pathway was designed by setting a key-decision step by step model on the basis of the current evidence. We performed a systematic search on PubMed, Cochrane, Embase and ClinicalTrials.org databases using the following search terms: “primary open angle glaucoma”, “critical pathway”, “ocular hypertension”, “IOP”, “glaucoma epidemiology”, “glaucoma diagnosis”, “gonioscopy”, “visual field in glaucoma”, “pachymetry”, “glaucoma progression”.
The aim of a critical pathway is to reach efficiency, to minimize treatment variability among clinicians, to shorten times and to minimize the resources and costs. Therefore, critical review and proper classification of the quality of the evidence is crucial to support this model. Thus, articles were classified according to their scientific quality from level I through III following the guidelines of the Preferred Practice Patterns of the American Academy of Ophthalmology[3],[6]. Briefly, level I included evidence obtained from at least one properly conducted, well designed, randomized and controlled clinical trial, as well as Meta-analysis including this type of studies. Level II, well-designed, controlled trials without randomization and cohort or control-case studies. Finally, level III included descriptive studies, case reports and expert consensus.
Inclusion criteria for critical pathway were patients that reach to ophthalmologic examination for the first time and also those patients followed up for other ocular pathologies different than POAG. Exclusion criteria were patients with established diagnosis of POAG, patients under glaucoma treatment or patients with other types of glaucoma different that POAG.
RESULTS
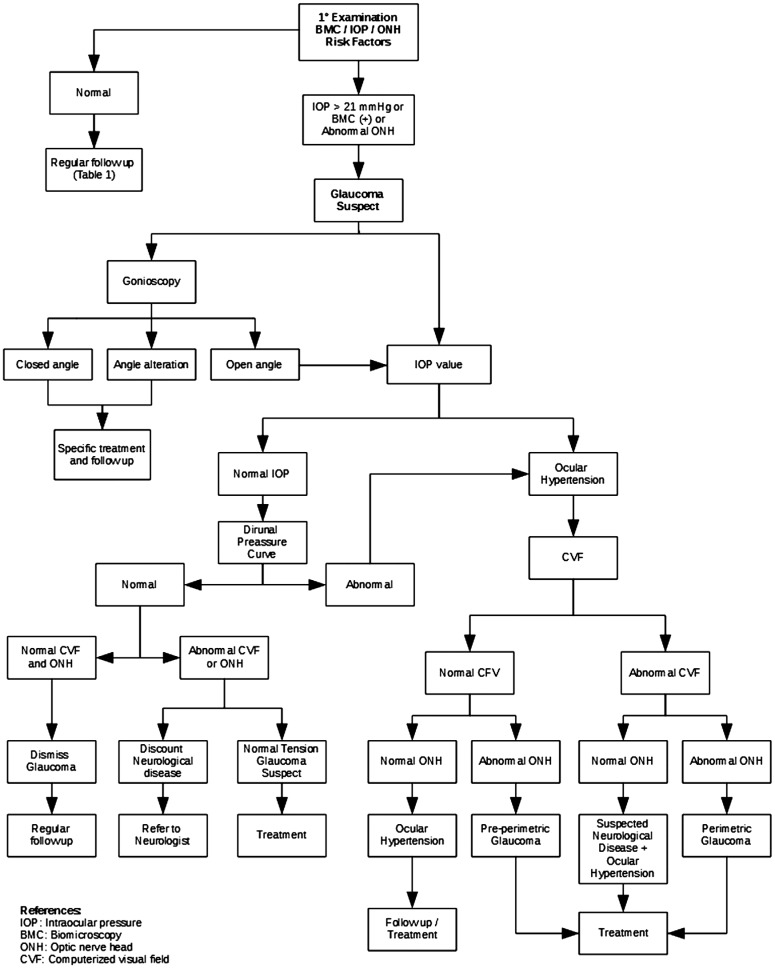
A total of 37 papers were selected that meet our standards of inclusion criteria. In all, we found 9 level I, 14 level II and 14 level III papers, basing each step of the pathway on the highest quality evidence possible. The critical pathway is shown in Figure 1.
Figure 1. Critical pathway for POAG diagnosis.
Roughly, first clinical examination should be focused on detecting glaucoma suspect patients that are going to be studied throughout the pathway. Patients that do not fulfill criteria for glaucoma suspect could be regularly followed up according to Table 1. Gonioscopy is a key element for splitting decision while it aims to differentiate open anterior chamber angle from other types of glaucoma like closed angle or patients with other angle alterations. After OH is confirmed, qualitative and quantitative analysis of ONH and retinal nerve fiber layer (RNFL) plus abnormalities in computerized visual field (CVF) represents major key steps for making decisions through the diagnosis process. This step by step model of making decisions proposed by the critical pathway ensure prompt diagnosis for POAG patients and reduce costs to the health system.
Table 1. Regular follow up.
| Age (a) | With risk factors | Without risk factors (a) |
| ≥65 | 6-12mo | 1 |
| 55-64 | 1-2d | 1-3 |
| 40-54 | 1-3d | 2-4 |
| <40 | 2-4d | 5-10 |
DISCUSSION
Even though some guidelines for open angle glaucoma diagnosis have been published, we believe that applying a step by step model is the best way for unify medical criteria and to reach cost-effectiveness.
Initial examination should include all the components of the comprehensive eye examination. Because screening methods for glaucoma in general population are not cost-effective, only patients considered glaucoma suspect should be studied deeper[2]–[3]. For instance, glaucoma suspect patients are those with biomicroscopy abnormalities, high intraocular pressure (IOP) levels, abnormal appearance of the ONH and those with family history of glaucoma. Besides that, older age, type 2 diabetes, myopia, thinner central cornea, corneal hysteresis (CH) and corneal resistance factor, pseudoexfoliation material, low ocular perfusion pressure, African ancestry and Latino ethnicity are also known risk factors for glaucoma and should be assessed as well[3],[6]–[13]. Patients that not fulfill criteria to be considered glaucoma suspect are considered normal and can be followed up according to the regime showed in Table 1.
During first ophthalmological examination, detailed biomicroscopy and pupillary dilatation should be performed looking for anterior segment abnormalities, with special consideration in the presence of pseudoexfoliation syndrome (PXFS). PXFS is known to be a strong independent risk factor for glaucoma progression in patients with OH[11]. Recent studies showed that after a mean of 8.7y without treatment, glaucoma conversion rate was twice as high in patients with OH and PXFS as in control patients (OH without PXFS)[12]. Exfoliative glaucoma (XFG) affects up to 6 million people worldwide and is known to have higher IOP levels and greater 24h IOP fluctuations, with worse response to medical and surgical treatment and worse overall prognosis[13].
Older age and ethnicity are well-known risk factors for glaucoma. Several epidemiological studies demonstrated a direct relationship between increasing age and the higher prevalence of glaucoma[3],[6]. In addition to this, POAG is three-fold times more frequent among Africans Americans and Hispanics ancestry compared with non-Hispanic Whites[1],[14]. Furthermore, blindness from glaucoma reaches six times more Africans Americans than Caucasian Americans patients, although there is no clear evidence if this difference lies in the individual predisposition or it represents difficulties in the access to the health system[15].
Family history is another risk factor for glaucoma. For instance, the Rotterdam Eye Study, the Baltimore Eye Survey and the Los Angeles Latino Eye Study demonstrated that individuals with first-degree relatives with confirmed POAG have higher odds of having glaucoma[6],[10],[14]. Interestingly, the odds of developing glaucoma increases as does the number of relatives with confirmed POAG.
High IOP level is a major risk factor for glaucoma. Several population-based studies provided strong evidence to consider IOP as a key element for glaucomatous optic nerve damage progression[11],[15]–[17]. Because there is great inter-individual variation in the susceptibility of the optic nerve to IOP-related damage, defining an IOP cutoff measure for screening and diagnosing glaucoma remains arbitrary. Nevertheless, most studies support the fact that IOP levels higher than 21 mm Hg represent a higher risk for glaucoma development and so it seems reasonable to accept that value as standard cutoff[6],[16]–[17].
Measurement of central corneal thickness (CCT) is an important element for glaucoma diagnosis because it represents not only an independent risk factor but also it helps the interpretation of IOP readings when corneal thickness is too distant from normal values[3],[6],[10],[18]. Population-based studies showed that Latinos or African Americans, whom mean CCT is 546 and 534 µm respectively, have higher glaucoma prevalence than Caucasian Americans or Asians with mean CCT of 556 and 552 µm, respectively. Hence, CCT is considered an independent risk factor for glaucoma development[18]–[19]. On the other hand, IOP readings could be under or overestimated when CCT are too thin or too thick, and despite some correcting-formulas have been published, there are still none universally accepted. Finally, because IOP readings from Goldmann applanation tonometry (GAT) depends on corneal resistance to indentation and stiffness, patients with abnormal corneas like those following keratorefractive surgery, keratoconus or Fuchs endoteliopathy should be measured by methods less influenced by corneal thickness like pneumotonometry. However, it should be noted that pneumotonometry measurements are known to overestimate IOP readings when compared to GAT and those overestimations are higher as the IOP level increases[19].
CH and corneal resistance factor, two previously unmeasured corneal biomechanical characteristics, have gained importance referring to glaucoma diagnosis and follow-up. It has been suggested that changes in corneal biomechanical factors are associated with the development and progression of glaucoma, especially in normal tension glaucoma[10],[20]–[21]. Recent studies demonstrated that low CH is directly associated with progressive glaucomatous optic neuropathy[20]. Thus, CH can be used not only for screening and diagnosis purpose but also as one of the prognostic factors for glaucoma progression, independent of corneal thickness or IOP.
Patients with axial myopia are prone to develop glaucoma. Several large cross-sectional epidemiological studies showed evidence that persons with myopia have a higher prevalence of POAG than normal patients. Hypothetically, patients with axial myopia have weaker scleral support, which finally increases ONH susceptibility to glaucomatous ONH damage[6],[22]–[24].
Gonioscopy represents a key-decision test for glaucoma suspect patients. With gonioscopy, physicians would differentiate patients with open anterior chamber angle that will continue throughout the pathway from alternative diagnosis like angle-closure glaucoma and those patients with structural abnormalities like angle recession, peripheral anterior synechiae, angle neovascularization, pigment dispersion, and inflammatory precipitates who will need specific treatments and follow up[25].
Patients with several risk factors for glaucoma but constantly normal IOP readings during medical examinations should be further assessed. Several ocular, hemodynamic and neurohormonal factors determine circadian variations in IOP readings in normal patients and, even more, these variations where found to be greater in patients with suspected or confirmed glaucoma[26]–[27]. Measurements made with GAT during office hours capture the IOP at specific moments and therefore do not reflect IOP fluctuations over a 24h period. Thus, glaucoma suspect patients with IOP levels below 21 mm Hg need to be studied with a Diurnal Pressure Curve (DPC) in order to detect, if any, IOP peaks during the entire day[27]. Rebound tonometry as developed by iCare (Tiolat, Helsinki, Finland) emerged as a complement for self-monitor IOP, and clinical studies have demonstrated high agreement between GAT and iCare results[28]–[31]. The instrument is easy to use for IOP measurements at home, and the results are reliable after a short period of instruction and practice. Thus, iCare represents a good alternative for IOP readings after office when DPC is not practicable. In case DPC is normal (no IOP peaks are detected) and patients have no visual field or ONH alterations, glaucoma diagnosis is dismissed. If a patient has a normal DPC but there are signs of visual field or ONH alterations, normal tension glaucoma and/or neurological disease should be ruled out. Finally, those patients with abnormal DPC have confirmed OH and should continue through the critical pathway.
CVF evaluation with static treshold perimetry remains the gold standard test for glaucoma detection and follow up[2],[6]. It is important to mention that testing strategies should be individualized to patients degree of vision loss by using specific programs that evaluate the central threshold sensitivity at 24 degrees, 30 degrees, and 10 degrees, and by varying stimulus size[6],[32]. On the other hand, ONH and RNFL evaluation with both qualitative and quantitative imaging test should be performed[2],[33]. Stereoscopic disc photograph is the preferred method for evaluation and follow up, whereas confocal scanning laser ophthalmoscopy, optical coherence tomography (OCT), and scanning laser polarimetry showed similar results to detect glaucomatous patients[33]–[37]. Nevertheless, physicians should be encouraged to always use computer-based quantitative imaging test as complementary tests and evaluate the patients full context after making decisions.
The next step for splitting decisions in a glaucoma suspect patient relies on the results of both CVF and ONH. Those patients with normal CVF and ONH are considered OH patients and the decision for treating and/or follow up depends on several factors that are beyond the purpose this pathway. By the other side, those patients with OH, normal CVF but abnormal ONH are diagnosed as pre-perimetric glaucoma and should be treated[6].
In the case of patients with high IOP readings and abnormal CVF but non glaucomatous optic nerve damage should be referred to neurologist to rule out neurological diseases. Finally, patients with OH, abnormal visual field and alteration of ONH and RNFL are diagnosed as perimetric glaucoma and should be treated[35],[37].
Even though there is no universally accepted consensus regarding glaucoma diagnosis due to its multifactorial etiology and clinical presentation, we believe that this evidence-based step by step model of making decisions improves the quality of medical attention and unify criteria.
A critical pathway, evidence-based guideline, may be a useful tool intended to reduce costs while maintaining or even improving the quality of care in the diagnosis of a highly prevalent pathology such as open angle glaucoma.
Acknowledgments
We would like to express our deepest gratitude to Dr. Carlos Bantar for his valuable collaboration in the preparation of the manuscript.
Conflicts of Interest: Allocco AR, None; Ponce JA, None; Riera MJ, None; Magurno MG, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed S, Khan Z, Si F, et al. Summary of glaucoma diagnostic testing accuracy: an evidence-based Meta-analysis. J Clin Med Res. 2016;8(9):641–649. doi: 10.14740/jocmr2643w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prum BE, Jr, Lim MC, Mansberger SL, Stein JD, Moroi SE, Gedde SJ, Herndon LW, Jr, Rosenberg LF, Williams RD. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern (®) Guidelines. Ophthalmology. 2016;123(1):P112–P151. doi: 10.1016/j.ophtha.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 4.Gugleta K, Polunina A, Kochkorov A, Waldmann N, Portmann N, Katamay R, Flammer J, Orgul S. Association between risk factors and glaucomatous damage in untreated primary open-angle glaucoma. J Glaucoma. 2013;22(6):501–505. doi: 10.1097/IJG.0b013e3182447d9b. [DOI] [PubMed] [Google Scholar]
- 5.Pearson SD, Goulart-Fisher D, Lee TH. Critical pathways as a strategy for improving care: problems and potential. Ann Intern Med. 1995;123(12):941–948. doi: 10.7326/0003-4819-123-12-199512150-00008. [DOI] [PubMed] [Google Scholar]
- 6.Prum BE, Jr, Lim MC, Mansberger SL, Stein JD, Moroi SE, Gedde SJ, Herndon LW, Jr, Rosenberg LF, Williams RD. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern (®) Guidelines. Ophthalmology. 2016;123(1):P41–P111. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Fallon M, Valero O, Pazos M, Antón A. Diagnostic accuracy of imaging devices in glaucoma: a meta-analysis. Surv Ophthalmol. 2017;pii doi: 10.1016/j.survophthal.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 8.The Advanced Glaucoma Intervention Study (AGIS): 7 The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 9.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP, Los Angeles Latino Eye Study Group Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(2):227–232.e1. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbonaro F, Hysi PG, Fahy SJ, Nag A, Hammond CJ. Optic disc planimetry, corneal hysteresis, central corneal thickness, and intraocular pressure as risk factors for glaucoma. Am J Ophthalmol. 2014;157(2):441–446. doi: 10.1016/j.ajo.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Miglior S, Bertuzzi F. Exfoliative glaucoma: new evidence in the pathogenesis and treatment. Prog Brain Res. 2015;221:233–241. doi: 10.1016/bs.pbr.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Teekhasaenee C, Suwan Y, Supakontanasan W, Tulvatana W, Ritch R. The clinical spectrum and a new theory of pathogenesis of true exfoliation syndrome. Ophthalmology. 2016;123(11):2328–2337. doi: 10.1016/j.ophtha.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Holló G, Katsanos A, Konstas AG. Management of exfoliative glaucoma: challenges and solutions. Clin Ophthalmol. 2015;9:907–919. doi: 10.2147/OPTH.S77570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varma R, Ying-Lai M, Francis BA, Nguyen BB, Deneen J, Wilson MR, Azen SP, Los Angeles Latino Eye Study Group Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111(8):1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6) doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 16.Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, Prum B, Shafranov G, Allen RC, Beck A, AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004;111(4):651–664. doi: 10.1016/j.ophtha.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Melles R, Lin SC. The impact of central corneal thickness on the risk for glaucoma in a large multiethnic population. J Glaucoma. 2014;23(9):606–612. doi: 10.1097/IJG.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108(10):1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 20.De Moraes CV, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21(4):209–213. doi: 10.1097/IJG.0b013e3182071b92. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013;120(8):1533–1540. doi: 10.1016/j.ophtha.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CH, Chen RI, Lin SC. Myopia and glaucoma: sorting out the difference. Curr Opin Ophthalmol. 2015;26(2):90–95. doi: 10.1097/ICU.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 23.Miki A, Ikuno Y, Asai T, Usui S, Nishida K. Defects of the lamina cribrosa in high myopia and glaucoma. PLoS One. 2015;10(9):e0137909. doi: 10.1371/journal.pone.0137909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang RT, Singh K. Myopia and glaucoma: diagnostic and therapeutic challenges. Curr Opin Ophthalmol. 2013;24(2):96–101. doi: 10.1097/ICU.0b013e32835cef31. [DOI] [PubMed] [Google Scholar]
- 25.Mallick J, Devi L, Malik PK, Mallick J. Update on normal tension glaucoma. J Ophthalmic Vis Res. 2016;11(2):204–208. doi: 10.4103/2008-322X.183914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Lee EJ, Han JC, Sohn SW, Rhee T, Kee C. The effect of diurnal fluctuation in intraocular pressure on the evaluation of risk factors of progression in normal tension glaucoma. PLoS One. 2016;11(10):e0164876. doi: 10.1371/journal.pone.0164876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautam N, Kaur S, Kaushik S, Raj S, Pandav SS. Postural and diurnal fluctuations in intraocular pressure across the spectrum of glaucoma. Br J Ophthalmol. 2016;100(4):537–541. doi: 10.1136/bjophthalmol-2015-306861. [DOI] [PubMed] [Google Scholar]
- 28.Pahlitzsch M, Brünner J, Gonnermann J, Maier AB, Torun N, Bertelmann E, Klamann MK. Comparison of ICare and IOPen vs Goldmann applanation tonometry according to international standards 8612 in glaucoma patients. Int J Ophthalmol. 2016;9(11):1624–1628. doi: 10.18240/ijo.2016.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi NG, Jones SK, Freedman SF. Icare ONE home tonometry in children with and without known glaucoma. J Glaucoma. 2016;25(2):e66–e69. doi: 10.1097/IJG.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 30.Mudie LI, LaBarre S, Varadaraj V, Karakus S, Onnela J, Munoz B, Friedman DS. The Icare HOME (TA022) Study: performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123(8):1675–1684. doi: 10.1016/j.ophtha.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 31.Borrego Sanz L, Morales-Fernandez L, Martínez de-la-Casa JM, Sáenz-Francés F, Fuentes M, García-Feijóo J. The Icare-Pro rebound tonometer versus the hand-held applanation tonometer in congenital glaucoma. J Glaucoma. 2016;25(2):149–154. doi: 10.1097/IJG.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 32.Collaborative Normal-Tension Glaucoma Study Group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126(4):487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 33.Chong GT, Lee RK. Glaucoma versus red disease: imaging and glaucoma diagnosis. Curr Opin Ophthalmol. 2012;23(2):79–88. doi: 10.1097/ICU.0b013e32834ff431. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, de Boer JF, Chen TC. Diagnostic capability of spectral-domain optical coherence tomography for glaucoma. Am J Ophthalmol. 2012;153(5):815–826.e2. doi: 10.1016/j.ajo.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gracitelli CP, Abe RY, Medeiros FA. Spectral-domain optical coherence tomography for glaucoma diagnosis. Open Ophthalmol J. 2015;9:68–77. doi: 10.2174/1874364101509010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe RY, Gracitelli CP, Medeiros FA. The use of spectral-domain optical coherence tomography to detect glaucoma progression. Open Ophthalmol J. 2015;9:78–88. doi: 10.2174/1874364101509010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98:ii15–9. doi: 10.1136/bjophthalmol-2013-304326. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]