Abstract
AIM
To study the effects of curcumin on the secretion of interleukin (IL)-6 and IL-8 by corneal limbus epithelial cells.
METHODS
Human corneal limbus epithelial cells were isolated and cultured from donor eyes and irradiated by UVB at different dosages with or without curcumin. MTT test was used for studying the effects of UVB and curcumin on the cell viability. The role of mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) pathways on the UVB-induced secretion of IL-6 and IL-8 were tested by addition of their inhibitors to the culture with or without UVB-radiation. Levels of various signal pathways, IL-6 and IL-8 in the cells and in the conditioned culture medium were measured by ELISA analysis.
RESULTS
UVB at 20 mJ/cm2 or less and curcumin at 20 µmol/L or less did not affect the cell viability of cultured limbus epithelial cells (P>0.05). UVB irradiation at 10 and 20 mJ/cm2 induced a significant increase of secretion of IL-6 and IL-8 and upregulated NF-κB and phosphorylated MAPK pathways of cultured limbus epithelial cells (P<0.05). Various signal pathway inhibitors, including SP600125 (JNK inhibitor), SB203580 (p38 MAPK inhibitor) and BAY11-7082 (NF-κB inhibitor) significantly decreased the UVB-induced secretion of IL-6 and IL-8 secretion (P<0.05). Curcumin at 5-20 µmol/L significantly inhibited UVB-induced secretion of IL-6 and IL-8 by limbus epithelial cells in a dose-dependent manner; while curcumin alone did not affect the secretion of IL-6 and IL-8. The upregulation of NF-κB and MAPK pathways induced by UVB treatment was significantly inhibited by curcumin, suggesting that NF-κB and MAPK pathways are involved in the inhibitory effect of curcumin on UVB-induced production of IL-6 and IL-8.
CONCLUSION
Curcumin may be a promising agent to be explored for the prevention and treatment of pterygium.
Keywords: curcumin, ultraviolet-B, interleukin-6, interleukin-8, corneal limbus epithelial cells, pterygium
INTRODUCTION
Pterygium is a common ocular surface disease. This is a wing-shaped fibrovascular lesion originated from the limbus and covered by epithelial cells. Pterygium can progress to the center of the cornea and causes loss of vision. Previous studies suggested that pterygium is an inflammatory, invasive, and highly vascularized growths, arise from activated limbus epithelial cells[1]–[4]. Epidemiological studies demonstrated that chronic exposure to sunlight, especially ultraviolet (UV) irradiation, is the main cause of pterygium. Chronic UV radiation causes the development of pterygium and the recurrence of pterygium after its surgical excision[4]–[6].
Experimental animal pterygium models have not been established previously[4]. Therefore, an in vitro model has been developed for the investigation of the pathogenesis and treatment of pterygium by using cultured human ocular surface cells from normal tissues or excised pterygium specimens[7]–[10].
Pterygium tissue contains a high level of pro-inflammatory cytokines. UVB irradiation on human epithelial cells or fibroblasts isolated from normal ocular surface tissues or surgical excised pterygium specimens stimulate the expression and secretion of several pro-inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, and IL-8[1],[7]–[10]. This in vitro model has been repeatedly used for studying the pathogenesis of pterygium and for the search of medications that might be used for the prevention and treatment of pterygium[1],[7]–[11].
Chronic inflammatory reaction is involved in the pathogenesis of pterygium[1],[4],[7]–[9]. Up-regulation of various pro-inflammatory factors plays an important role in the pathogenesis of pterygium[7]–[9]. IL-6 is up-regulated in pterygium tissues. This cytokine has a potent pro-inflammatory effect and also stimulates angiogenesis[7]–[8],[12]. IL-8 (CXCL8) attracts neutrophil, T cell and monocytes into the tissues, leads to an inflammatory reaction[13]. IL-8 also induces angiogenesis[13]. All of these effects of these two cytokines lead to the development of inflammatory response and angiogenesis in the pterygium. The expression of IL-6 and IL-8 could be induced by UVB irradiation in normal corneal and pterygium tissues and their various cell components[7]–[9],[14]. Pterygium begins growing from limbus epithelial cells and UVB irradiation also induces inflammatory reactions in these cells earlier than other cell types lined ocular surface[2]–[3]. Therefore, it is appropriate to use cultured limbus epithelial cells as an in vitro model for the investigation of the effects of UVB and various medication on the progress of pterygium
Curcumin (diferuloylmethane), is a β-diketones, a yellow coloring agent extracted from turmeric, has a wide array of pharmacological and biological activities including chemopreventive, chemotherapeutic and anti-proliferative potentials[15]. In vitro study, experimental animal study and clinical trials indicated that curcumin can inhibit inflammation via the decrease of expression of various pro-inflammatory cytokines, chemokines, transcription factors and relevant signal pathways[15]–[19]. Curcumin inhibits UVB-induced expression of IL-6, IL-8 and TNF-α in keratinocytes through the down-regulation of mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) signal pathways[15],[20]–[21]. The effects of curcumin on UVB-induced inflammation in cells from pterygium or normal ocular surface tissues have not been previously reported.
The purpose of the present study was to investigate the effects of curcumin on UVB-induced secretion of IL-6 and IL-8 from cultured human limbus epithelial cells and to explore the possibility of using curcumin in the prevention and treatment of pterygium.
MATERIALS AND METHODS
Curcumin
Curcumin (99.5% purity) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Curcumin was dissolved in dimethyl sulfoxide (DMSO) to make a 20 mmol/L stock solution and was added to the medium at different concentrations. Cells were treated with 0.25% DMSO as the control group.
Cell Culture
Limbus epithelial cells were isolated by us (Hu DN) in the Tissue Culture Center, New York Eye and Ear Infirmary from donor eyes supplied by the New York Eye Bank for Sight Restoration (New York, NY, USA). The Eye Bank obtained the donor's consent before the collection of the eyes. The principles outlined in the Declaration of Helsinki (2008) have been followed in the present study. The cornea with limbus and 2 mm wide of sclera were excised from the eyeball, then, the cornea and sclera were excised to leave approximately 1 mm on either side of the limbus. The limbus tissue was washed with Hank's solution (GIBCO, Grand Island, NY, USA) three times and than immersed in a 1.2 U/mL dispase II solution (Sigma) for 2h at 37°C. After the enzymatic dissociation, the limbus epithelial cells were gently scraped by using a iris spatula under the stereo-microscope to isolate the limbus epithelial cells from the Bowman's membrane. Cells were collected and centrifuged. Pellets were resuspended by Ham's F12 nutrient mixture with 10% fetal bovine serum (all from GIBCO), seeded into the culture flask and incubated in a CO2-regulated incubator in humidified 95% air/5% CO2 atmosphere. Seven days later, culture medium were replaced by the defined Keratinocyte-serum free medium (K-SFM, GIBCO). Cells were observed under phase-contrast microscope each day and the culture medium was changed three time a week. After reaching confluence, the limbus epithelial cells were detached using 0.25% trypsin solution (GIBCO), diluted 1:3 and subcultured. Cell cultures in the second passage were used in this study.
Ultraviolet-B Irradiation
Limbus epithelial cells were seeded into 12 well plates and grew to 75% confluence. Cultures were washed and covered with a thin layer of PBS before irradiation to remove potential phototoxic materials in culture medium. Cells were irradiated with various dosages of UVB using 20037 312 UVB bulbs (Staratagene, La Jolia, CA, USA), which emit UVB with a spectral peak at 312 nm. PMA 2100 Data Logging Radiometer (Solar Light, Inc., Genside, PA, USA) was used for monitoring and calibrating the intensity of UVB radiation. After UVB irradiation, cells were washed with PBS and cultured with fresh pre-warmed K-SFM for various periods.
MTT Study
Cell viability of limbus epithelial cells cultured with or without UVB irradiation was tested by the MTT assay. Cells at a density of 5×103 cells/well were seeded into each well in black well 96-well plates (Sigma). After incubation for 24h, cells were irradiated by UVB at 10, 20 and 50 mJ/cm2 as described above. After 24h, 50 µL tetrazolium bromide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 1 mg/mL) (Sigma) was added and cells were cultured at 37°C. Four hours later, the medium was removed and DMSO (100 µL, Sigma) was added. The optical density as the parameter of cell viability was measured at 540 nm with a microplate reader (Multiskan EX, Thermo, Ventana, Finland). Viability of limbus epithelial cells cultured with or without curcumin was also tested by the MTT assay. Briefly, cells were seeded into 96-well plates. After 24h, cells were cultured with or without curcumin at 10, 20 and 50 µmol/L concentrations for 24h and the cell viability was analysis by MTT assay as described above.
Effects of Ultraviolet-B and Curcumin on IL-6 and IL-8 Secretion
Limbus epithelial cells were seeded into 12-well plates at the density of 2×105 cells per well. After cultured for 24h, curcumin at the final concentrations of 5, 10, and 20 µmol/L was added to the culture medium. One hour later, culture medium was withdrawn, washed and cultured with PBS and irradiated with UVB at 20 mJ/cm2 as described above. Immediately after irradiation, PBS was replaced by fresh culture medium. Cells were cultured for 24h. Conditioned culture media were collected and centrifuged to remove dead cells and debris. The supernatants were stored at -70°C until analysis. Tests were performed in triplicate.
Study of p38 Mitogen-activated Protein Kinase and c-Jun N-terminal Kinase Levels in Cultured Limbus Epithelial Cells
Limbus epithelial cells (1×106) were seeded into 6-well plates. Curcumin was added to the culture to obtain a final concentrations at 20 µmol/L 24h later. Cultures were irradiated with UVB (20 mJ/cm2) 1h later. Cells were harvested 24h later and protein was extracted. c-Jun N-terminal kinace (JNK) and p38 MAPK ELISA kits (with sensitivity at 0.8 U/mL) (Biosource, Camarillo, CA, USA) were used for the measurement of phosphorylated JNK and p38 MAPK levels in protein extracted from collected cells according to the manufacturer's instructions, respectively. The results (in triplicate) were expressed as percentages of the control, which were the cultures not treated by curcumin and UVB.
Study of NF-κB Levels in Nuclear Extracts from Cultured Limbus Epithelial Cells
Limbus epithelial cells were seeded and treated as described above. Cells were scraped 30min after the treatment of UVB. Nuclear fraction was obtained by the treatment of collected cells with hypotonic buffer (BioSource). Nuclear extracts were obtained by the treatment of nuclear fraction with cell extraction buffer (BioSource). NF-κB ELISA kit (Invitrogen) was used for the measurement of NF-κB levels according to the manufacturer's instructions. The results (in triplicate) were expressed as percentages of the control, which were the cultures not treated by curcumin and UVB.
Effects of Mitogen-activated Protein Kinase and NF-κB Inhibitors on UVB-induced Secretion of IL-6 and IL-8
Cells (0.2×106 cells/well) were seeded into multi-well plates (12 well). Signal pathway inhibitors were added 24h later [JNK inhibitor: SP600125; p38 MAPK inhibitor: SB203580; NF-κB inhibitor: BAY11-7082 (Calbiochem, the concentrations of the MAPK inhibitors and NF-κB inhibitor were 10 µmol/L and 5 µmol/L, respectively)] and cultured for 1h. Cells were then treated by UVB as described above. After 24h, conditioned medium was collected, centrifuged and stored at deep freezer.
Measurement of IL-6 and IL-8 Protein Levels
Enzyme-linked immunosorbent assay (ELISA) was used for the measurement of IL-6 and IL-8 protein levels in the supernatant of cultured limbus epithelial cells. Quantikine IL-6 ELISA kit and IL-8 ELISA kit (R&D Systems, Minneapolis, MN, USA) were used to determine the levels of IL-6 or IL-8 according to the manufacturer's instruction. The optical density of the ELISA samples was measured at 450 nm and corrected by 540 nm using a microplate reader (Multiskan EX, Thermo, Ventana, Finland). The amounts of IL-6 and IL-8 (pg/mL) were calculated from a standard curve. The sensitivity of the assay for IL-6 and IL-8 was 0.7 pg/mL and 3.5 pg/mL, respectively. Tests were performed in triplicate.
Statistical Analysis
Each experiment was replicated 3 times and the data were presented as mean±standard deviation (SD). A Student's t-test was performed to assess the significance of difference between the means of the tested group and the controls. Values of P<0.05 were considered statistically significant. All data analysis was performed using specific software (SPSS 19.0, SPSS Inc., Chicago, IL, USA).
RESULTS
MTT Study
Cell viability of cultured limbus epithelial cells irradiated by 10-20 mJ/cm2 UVB were not affected by UVB irradiation (P>0.05 as compared with the controls). Cells irradiated with UVB at 50 mJ/cm2 showed a significant decrease of cell viability (P<0.05) (Figure 1A). Therefore, UVB at 20 mJ/cm2 or less were used in the following experiments. Curcumin at 10-20 µmol/L did not affect the cell viability (P>0.05 as compared with the controls). However, viability of cells treated with 50 µmol/L curcumin showed a slight but significant decrease as compared with cells not treated with curcumin (P<0.05) (Figure 1B). Therefore, curcumin at 20 µmol/L or less were used in the following experiments.
Figure 1. Effects of UVB and curcumin on viability of corneal limbus epithelial cells.
A: UVB at 10-20 mJ/cm2 did not affect the viability of human limbus epithelial cells as determined by MTT test which was significantly affected by UVB at 50 mJ/cm2; B: Curcumin at 5-20 µmol/L did not affect the viability of human limbus epithelial cells as determined by MTT test, whereas the cell viablity was significantly affected by curcumin at 50 µmol/L. Error bars reveal the means±standard deviation in triplicate tests. aP<0.05 as compared with the cells not treated with UVB and curcumin.
Effects of UVB on IL-6 and IL-8 Secretion
Cultured human limbus epithelial cells showed relatively low levels of constitutive secretion of IL-6 and IL-8 (Figure 2). UVB irradiation at 5, 10 and 20 mJ/cm2 induced a dose-dependent increase of IL-6 and IL-8 secretion by cultured limbus epithelial cells (Figure 2). IL-6 and IL-8 secretion levels in cells irradiated with 10 and 20 mJ/cm2 UVB were significantly greater than those in cells not treated with UVB (P<0.05). UVB at 20 mJ/cm2 caused an increase of IL-6 and IL-8 secretion to 5.8- and 4.7-fold of cells not irradiated, respectively (Figure 2).
Figure 2. Effects of UVB on secretion of IL-6 and IL-8 by cultured corneal limbus epithelial cells.
A: UVB irradiation at 10 and 20 mJ/cm2 induced a significant increase of IL-6 secretion and IL-8 secretion by cultured limbus epithelial cells; B: The increase of IL-8 secretion. was measured after the same management in (A). Error bars reveal the means±standard deviation in triplicate tests. aP<0.05 as determined by ELISA analysis.
Effects of Curcumin on UVB-induced IL-6 and IL-8 Secretion
Curcumin did not affect the constitutive secretion of IL-6 and IL-8 in cultured human limbus epithelial cells (Figure 3). Curcumin at 5, 10 and 20 µmol/L dose-dependently inhibited UVB-induced IL-6 and IL-8 secretion. Both IL-6 and IL-8 secretion in cells treated with UVB and curcumin at 5-20 µmol/L was significantly decreased as compared with the positive controls (cells treated with UVB alone, P<0.05) (Figure 3). IL-6 and IL-8 secretion levels in cells treated with UVB and 20 µmol/L curcumin showed no significant difference from those in the negative controls (cells not treated with UVB, P>0.05), indicating that curcumin at this dosage can completely block UVB-induced secretion of these two pro-inflammatory cytokines in cultured limbus epithelial cells.
Figure 3. Effects of curcumin on UVB-induced secretion of IL-6 and IL-8 in corneal limbus epithelia cells.
A: Curcumin alone (20 µmol/L) did not affect the secretion of IL-6 and IL-8 while curcumin at 5 µmol/L (U+C5), 10 µmol/L (U+C10) and 20 µmol/L (U+C20) significantly inhibited the UVB-induced secretion of IL-6 and IL-8 by corneal limbus epithelial cells in a dose-dependent manner. Error bars reveal the means±standard deviation in triplicate tests. aP<0.05 as compared with cells irradiated with UVB alone.
Effects of Ultraviolet-B on NF-κB and Phosphorylated Mitogen-activated Protein Kinase Levels
UVB treatment induced significant increase of phosphorylated JNK and p38 MAPK levels in limbus epithelial cells (P<0.05) (Figure 4). UVB treatment also induced significant increase of NF-κB levels in nuclear extracts of the limbus epithelial cells (P<0.05) (Figure 4).
Figure 4. Effects of curcumin on UVB-induced expression of NF-κB and phosphorylated JNK and p38 MAPK levels in corneal limbus epithelia cells.

UVB irradiation (20 mJ/cm2) induced a significant increase of NF-κB and phosphorylated JNK and p38 MAPK levels of cultured limbus epithelial cells (aP<0.05), as compared with cells without curcumin and UV irradiation. Curcumin at 20 µmol/L (UVB+C) significantly decreased UVB-induced expression of NF-κB and phosphorylated JNK and p38 MAPK levels in corneal limbus epithelial cells (cP<0.05). Error bars reveal the means±standard deviation in triplicate tests.
Effects of Curcumin on UVB-induced Expression of NF-κB and Phosphorylated Mitogen-activated Protein Kinase
UVB-induced increase of phosphorylated JNK and p38 MAPK levels and NF-κB levels in limbus epithelial cells was significantly reduced by the treatment of curcumin (P<0.05) (Figure 4).
Effects of Mitogen-activated Protein Kinase and NF-κB Inhibitors on UVB-induced IL-6 and IL-8 Secretion
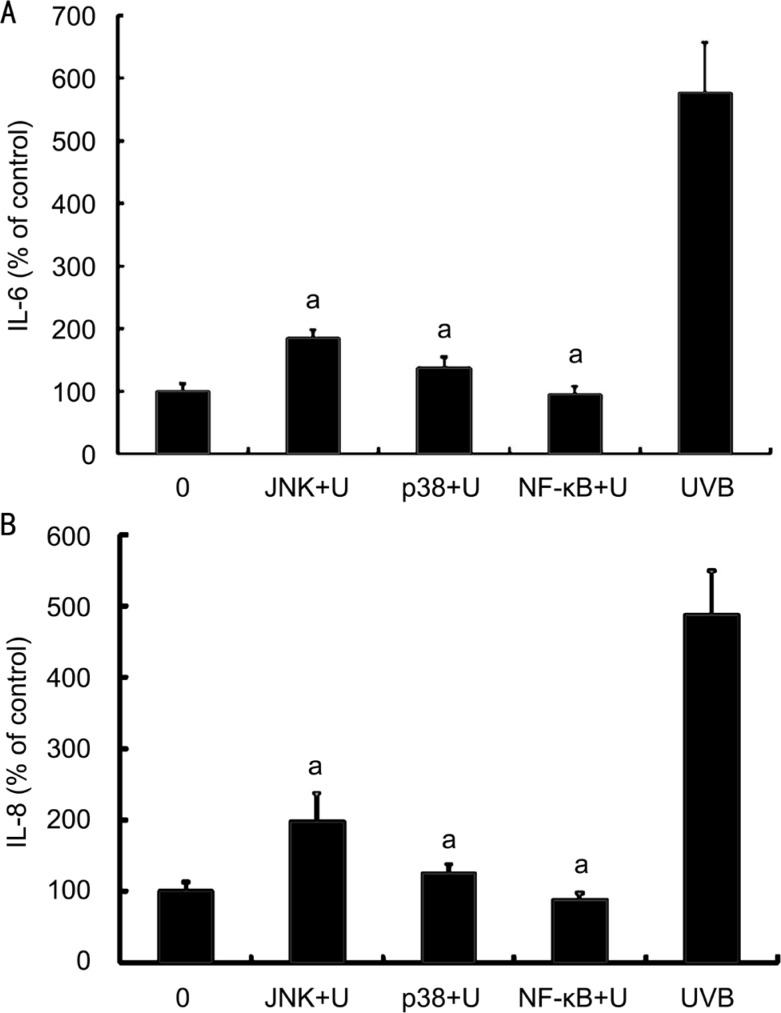
JNK inhibitor, p38 MAPK inhibitor and NF-κB inhibitor significantly decreased the UVB-induced secretion of IL-6 secretion by cultured human limbus epithelial cells (P<0.05, as compared with cells irradiated with 20 mJ/cm2 UVB) (Figure 5A). Secretion of IL-6 in cells treated with UVB and JNK or p38 MAPK inhibitor still slightly higher than that in cells not treated with UVB (P<0.05); while secretion of IL-6 in cells treated with UVB and NF-κB inhibitor did not differ from cells not treated with UVB (P>0.05) (Figure 5A). These results suggested that NF-κB inhibitor can completely block the UVB-induced secretion of IL-6 in limbus epithelial cells, while JNK or p38 MAPK inhibitor only had a partially blocking effect.
Figure 5. Effects of MAPK and NF-κB inhibitors on UVB-induced secretion of IL-6 and IL-8 by corneal limbus epithelial cells.
A: Various signal pathway inhibitors, including SP600125, JNK inhibitor (JNK); SB203580, p38 MAPK inhibitor (p38) and BAY11-7082, NF-κB inhibitor (NF-κB) significantly decreased the UVB-induced secretion of IL-6 by cultured limbus epithelial cells; B: The secretion of IL-8 was measured after the same management in (A). Error bars reveal the means±standard deviation in triplicate tests. aP<0.05 as compared with cells irradiated with UVB alone.
DISCUSSION
The effects of UVB on the production of IL-6 and IL-8 of limbus epithelial cells have not been reported previously. In the present study, UVB induced significantly increase of secretion of IL-6 and IL-8 by cultured limbus epithelial cells. This is consistent with previously reports, which described the UVB-induced IL-6 and IL-8 by other types of ocular surface epithelial cells, such as normal corneal epithelial cells or pterygium epithelial cells[7]–[9].
The signal pathways involved in UVB-induced production of IL-6 and IL-8 by corneal epithelial cells, pterygium epithelial cells and keratinocytes include MAPK and NF-κB pathways[7]–[9],[20]–[21]. UVB may directly activate the NF-κB in the cytoplasm. Activated NF-κB translocates to the nuclei and work as a transcription factor for stimulating the expression of various pro-inflammatory cytokines, including IL-6 and IL-8[21]. Alternatively, UVB may first activate MAPK signal pathway. The activated MAPKs can translocate into the nucleus and phosphorylates the target transcription factors, such as NF-κB. Our study suggested that curcumin inhibited the UVB-induced expression of IL-6 and IL-8 via MAPK and NF-κB signal pathways. This result is consistent with the previous reports that UVB-induced production of IL-6 and IL-8 is relevant to the activation of MAPK and NF-κB signal pathways in epithelial cells from normal cornea or pterygium specimens and in keratinocytes[7]–[9],[20]–[21].
The present study revealed that curcumin at low and non-lethal dosages (5-20 µmol/L) does not affect the constitutive secretion of IL-6 and IL-8 by limbus epithelial cells, but curcumin at the same dosages can significantly inhibits UVB-induced secretion of IL-6 and IL-8 in limbus epithelial cells. This results are consistent with the results found in previous studies that curcumin can inhibit UVB-induced production and secretion of IL-6 and IL-8 in keratinocytes[20]–[21].
Curcumin is a natural anti-inflammatory compound with a long history of use, has been used as a remedy for the treatment and prevention of inflammatory diseases[16]–[19]. Aggarwal et al[16] reported that more than 60 clinical trials had been conducted for studying the efficacy and safety of curcumin. In addition to these studies, another 35 clinical trials have evaluated the efficacy of curcumin. Curcumin was found to be effective in the treatment of various chronic inflammatory diseases including psoriasis, inflammatory bowel diseases and different types of arthritis, such as rheumatoid arthritis and osteoarthritis[16],[18]. It has even been demonstrated to be effective in the treatment of other severe diseases such as Alzheimer's[22], cystic fibrosis[23], and AIDS[24]. Clinical trials also demonstrated that curcumin is a safe, nontoxic remedy, can be given orally and is quite safe and affordable[15]–[19].
The main limitation of curcumin is its insolubility in aqueous systems. Various attempts to enhance the bioavailability and efficacy of curcumin have been reported by the encapsulation of curcumin into liposomes, phospholipid complexes or nanoparticle. The primary aim of these studies is to achieve increased solubilization of curcumin and, at the same time, to protect curcumin against inactivation by hydrolysis[13],[25]. Therefore, in the future, curcumin can be used not only as an oral nutrition supplement, but also can be used locally, such as the eye drops for the treatment of various ocular inflammatory diseases.
The present study found that curcumin at low and safe dosages significantly inhibits the UVB-induced secretion of IL-6 and IL-8 by cultured limbus epithelial cells; but does not affect the constitutive secretion of IL-6 and IL-8. These results suggest that curcumin may be a promising agent to be explored for the prevention of progress of pterygium, and also may be used for the prevention of recurrence of pterygium after its surgical excision.
Acknowledgments
Conflicts of Interest: Chao SC, None; Hu DN, None; Roberts J, None; Shen X, None; Lee CY, None; Nien CW, None; Lin HY, None.
REFERENCES
- 1.Solomon A, Li DQ, Lee SB, Tseng SC. Regulation of collagenase, stromelysin, and urokinase-type plasminogen activator in primary pterygium body fibroblasts by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2000;41(8):2154–2163. [PubMed] [Google Scholar]
- 2.Di Girolamo N, Coroneo MT, Wakefield D. UVB-elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK-dependent pathway. Invest Ophthalmol Vis Sci. 2003;44(11):4705–4714. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- 3.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119(5):695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 4.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Taylor HR, West SK, Rosenthal FS, Munoz B, Newland HS, Emmett EA. Corneal changes associated with chronic UV irradiation. Arch Ophthalmol. 1989;107(10):1481–1484. doi: 10.1001/archopht.1989.01070020555039. [DOI] [PubMed] [Google Scholar]
- 6.Sekelj S, Dekaris I, Kondza-Krstonijević E, Gabrić N, Predović J, Mitrović S. Ultraviolet light and pterygium. Coll Antropol. 2007;31(Suppl 1):45–47. [PubMed] [Google Scholar]
- 7.Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43(11):3430–3437. [PubMed] [Google Scholar]
- 8.Di Girolamo N, Wakefield D, Coroneo MT. UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci. 2006;47(6):2430–2437. doi: 10.1167/iovs.05-1130. [DOI] [PubMed] [Google Scholar]
- 9.Nolan TM, Di Girolamo N, Sachdev NH, Hampartzoumian T, Coroneo MT, Wakefield D. The role of ultraviolet irradiation and heparin-binding epidermal growth factor-like growth factor in the pathogenesis of pterygium. Am J Pathol. 2003;162(2):567–574. doi: 10.1016/S0002-9440(10)63850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notara M, Refaian N, Braun G, Steven P, Bock F, Cursiefen C. Short-term UVB-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015;15(3):643–654. doi: 10.1016/j.scr.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Di Girolamo N, Coroneo M, Wakefield D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase-1 expression in ocular epithelial cells after UVB radiation. Am J Pathol. 2005;167(2):489–503. doi: 10.1016/S0002-9440(10)62992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu DN, Chen M, Zhang DY, Ye F, McCormick SA, Chan CC. Interleukin-1β increases baseline expression and secretion of interleukin-6 by human uveal melanocytes in vitro via p38 MAPK/NF-κB pathway. Invest Ophthalmol Vis Sci. 2011;52(6):3767–3774. doi: 10.1167/iovs.10-6908. [DOI] [PubMed] [Google Scholar]
- 13.Hu DN, Bi M, Zhang DY, Ye F, McCormick SA, Chan CC. Constitutive and LPS-induced expression of MCP-1 and IL-8 by human uveal melanocytes in vitro and relevant signal pathways. Invest Ophthalmol Vis Sci. 2014;55(9):5760–5769. doi: 10.1167/iovs.14-14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy M, Kim KH, Harten B, Brown J, Planck S, Meshul C, Edelhauser H, Rosenbaum JT, Armstrong CA, Ansel JC. Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci. 1997;38(12):2483–2491. [PubMed] [Google Scholar]
- 15.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30(2):85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Gupta SC, Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol. 2013;169(8):1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffer M, Schaffer PM, Bar-Sela G. An update on Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care. 2015;18(6):605–611. doi: 10.1097/MCO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 18.Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Bio Factors. 2013;39(1):69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- 19.Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029–3042. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho JW, Park K, Kweon GR, Jang BC, Baek WK, Suh MH, Kim CW, Lee KS, Suh SI. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp Mol Med. 2005;37(3):186–192. doi: 10.1038/emm.2005.25. [DOI] [PubMed] [Google Scholar]
- 21.Grandjean-Laquerriere A, Gangloff SC, Le Naour R, Trentesaux C, Hornebeck W, Guenounou M. Relative contribution of NF-κB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by keratinocytes. Cytokine. 2002;18(3):168–177. doi: 10.1006/cyto.2002.0888. [DOI] [PubMed] [Google Scholar]
- 22.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 23.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glöckner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304(5670):600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 24.Sui Z, Salto R, Li J, Craik C, Ortiz de Montellano PR. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg Med Chem. 1993;1(6):415–422. doi: 10.1016/s0968-0896(00)82152-5. [DOI] [PubMed] [Google Scholar]
- 25.Nardo L, Maspero A, Selva M, Bondani M, Palmisano G, Ferrari E, Saladini M. Excited state dynamics of bis-dehydroxycurcumin carboxylic acid, a water-soluble derivative of the photosensitizer curcumin. J Phys Chem A. 2012;116(37):9321–9330. doi: 10.1021/jp307928a. [DOI] [PubMed] [Google Scholar]