Abstract
Background
The occurrence of persistent acute kidney injury (pAKI) following transcatheter aortic valve implantation (TAVI) has serious implications.
Methods
There were 1540 patients undergoing and surviving TAVI included in the nationwide SWEDEHEART registry between 2008 and 2015. Creatinine was measured at baseline and discharge, and those with baseline estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 or dialysis were excluded. pAKI was defined by and encompassing theValve Academic Research Consortium-2 (VARC-2) criteria: increase in serum creatinine concentration ≥26.5 μmol/L or increase by ≥50% (1.5×), or start of in-hospital dialysis until hospital discharge. Logistic regression analysis was used to find baseline factors associated with pAKI. Adjusted Cox regression analysis was used to assess the association of pAKI with mortality. Median follow-up was 1.8 years (IQR 0.7–3.0).
Results
pAKI occurred in 6.1% (n=94) of the patients (71.3% male). These patients had higher creatinine level (117±50 vs 100±35 mmol/L, p<0.001), but similar baseline eGFR (59±21 vs 56±23 mL/min/1.73 m2, p=0.18) and received higher contrast volume (129 mL ±89 vs 110 mL ±78, p=0.027). On multivariable logistic regression analysis, pAKI was predicted by eGFR (OR 0.88, 95% CI (0.79 to 0.98), p=0.019), male gender (OR 2.68, 95% CI (1.63 to 4.38), p<0.001) and apical access (OR 2.23, 95% CI (1.35 to 3.69), p=0.002), whereas contrast volume/10 mL (OR 1.02, 95% CI (1.00 to 1.05), p=0.052) did not reach statistical significance. Mortality at 1 year/end of follow-up was 10.4%/26.9%. pAKI was associated with a doubled risk of death (HR 2.04, 95% CI (1.49 to 2.81), p<0.001).
Conclusion
Persistent AKI after TAVI occurs in 6.1% and is associated with a doubled long-term mortality. Special efforts to avoid AKI should be taken, especially among vulnerable patients.
Keywords: Aortic stenosis, renal function, acute kidney injury, transcatheter aortic valve implantation
Key questions.
What is already known about this subject?
Currently high-risk patients with several comorbidities are accepted for treatment of severe symptomatic aortic stenosis with transcatheter aortic valve implantation (TAVI). Acute kidney injury has in several small single-centre studies and randomised trials been implied as a predictor of worse outcome.
What does this study add?
In a nationwide unselected patient population undergoing TAVI, a worsening of renal function at the time of discharge occurred in 6.1% of patients and was associated with a doubled short-term and long-term mortality, independent of baseline renal function. Predictors of the occurrence of a persistent worsening of renal function were baseline renal function, male gender and apical access site, whereas used contrast volume did not reach significance.
How might this impact on clinical practice?
Prognosis after TAVI can be further improved by avoiding worsening of renal function. Prevention of worsening renal function should focus on both pre-existing renal function, gender and factors associated with access site.
Introduction
Transcatheter aortic valve implantation (TAVI) has become an established method for treating severe symptomatic aortic stenosis in patients who are inoperable or have high surgical risk. Hence, these patients admitted for TAVI have substantial comorbidities, which make them more vulnerable to a large number of periprocedural and postprocedural complications. In previous studies, up to 66% of patients have had at least a moderately reduced renal function.1–3 After the TAVI procedure, a further decline in renal function is common, affecting 15%–40% of all patients, and have shown to be predictive of several-fold increased short-term and long-term mortality.2 4–6 The definition of worsening renal function has varied over time, but currently the most commonly used definition for patients undergoing valve implantations is the Valve Academic Research Consortium-2 (VARC-2) criteria, where already a mild (26 mmol/L) increase in creatinine within 7 days identifies patients with acute kidney injury (AKI).7
The predictors of AKI and the association with outcome have to some extent been examined.1 8 These studies though have mainly related to a renal function decline within the first 24 hours or 7 days to outcome, and have not examined the association if AKI persists until discharge. In these studies it is suggested that several potential important mechanisms contribute to the development of AKI, such as lower renal function at baseline, larger contrast volume use, red blood cell transfusion and higher logistic EuroSCORE.9
The aims of the present study were to understand what is the meaning of an AKI that persists until the time of discharge. This differs from previous studies that mainly focus on AKI that is identified during the first 7 days of the TAVI procedure. In addition, we wanted to identify the predictors of persistent decline in renal function. We also wanted to evaluate how AKI in the different renal function groups was associated with outcome. Finally, we wanted to assess whether persistent AKI was associated with short-term and long-term mortality.
Methods
Study population
The nationwide SWEDEHEART registry includes all consecutive patients undergoing TAVI in all eight centres in Sweden. The TAVI registry is a part of the SWEDEHEART registries, with data storage at Uppsala Clinical Research Center. The registry covers all TAVI procedures in the country since TAVI was started, and new patients are entered online directly after procedures are done. The treating physician enters information including baseline characteristics, laboratory values, echocardiographic and procedural data and complications. A subset of patients have been checked against hospital records with very few inconsistencies. Over 99% of records are complete in all fields. Mortality data are gathered from the national population registry, which is 100% complete for our patients.
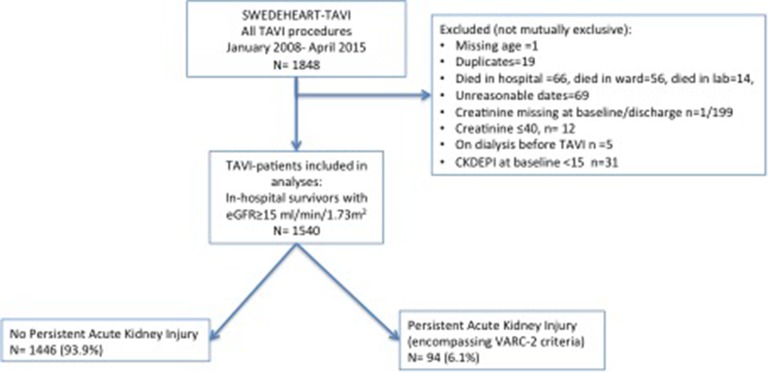
There were 1861 patients undergoing TAVI between 2008 and 2015. A total of 1540 patients were included (figure 1) after excluding patients requiring preoperative dialysis (n=5) as AKI cannot be assessed in these patients. Also, those with a baseline estimated glomerular filtration rate (eGFR) <15 (n=31) were excluded due to their expected high AKI risk.
Figure 1.
Flow chart. eGFR, estimated glomerular filtration rate; TAVI, transcatheter aortic valve implantation; VARC-2, Valve Academic Research Consortium-2.
The decision to intervene on a severe aortic stenosis was taken by a multidisciplinary team consisting of an interventional cardiologist, imaging specialist and cardiothoracic surgeon. The main valve technologies used were the Medtronic CoreValve and the Edwards SAPIEN. A transfemoral approach was the default strategy for all patients. For those treated with the Medtronic CoreValve, some patients were treated via the subclavian or direct aortic approach. For the Edwards SAPIEN devices, a transapical approach was used when a transfemoral route was not possible.
This study protocol was approved by the Ethics Board in Stockholm, Sweden.
Persistent acute kidney injury (pAKI) definition
Creatinine measurement at baseline and at discharge is collected prospectively in the registry. The persistent AKI definition encompassed the VARC-2 definitions,7 with the modification that creatinine at discharge was used and not a peak value within 72 hours to 7days post-TAVI. Urine output data were not registered. pAKI was defined as having at least VARC-2 stage 1 or more. VARC-2 staging was as follows: stage 1: increase in creatinine ≥26 mmol/L, or increased creatinine by ≥50% to ≤199% (1.5–1.99× baseline value); stage 2: creatinine increase by ≥200% to ≤299% (2.0–2.9× baseline value); and stage 3: creatinine increase by ≥300% or requiring dialysis therapy.
Two other pAKI definitions were used for sensitivity analysis: first, a relative ≥25% increase in creatinine from baseline to discharge, or initiation of dialysis therapy; and second, fulfilling the risk ‘R’ criteria in the Risk, Injury, Failure, Loss, ESRD (RIFLE)definition, consisting of either a ≥25% increase in eGFR calculated by the chronic kidney epidemiology collaboration (CKD-EPI) equation10 or a ≥150% increase in creatinine or initiation of dialysis.
Other variable definitions
Bleeding events consisted of all major bleedings (defined according to VARC-2 as major or higher) occurring either in the procedural laboratory or in the ward. Urgent TAVI was defined if the patient was deemed to require urgent procedure or was in a critical preoperative state. eGFR was estimated with the CKD-EPI equation.10 Creatinine clearance11 was used to calculate contrast volume to creatinine clearance ratio. Data on vital status were obtained by merging the registry with the national population registry.
Statistical analysis
Continuous variables are presented as mean±SD or as median (IQR). Continuous variables were tested for differences with the Student’s two-sample t-test or non-parametric Kruskal-Wallis test. Categorical data are presented as percentages, and comparison between groups was performed by the χ2 test.
First, factors associated with developing persistent AKI were assessed with a univariate logistic regression model. Based on clinical knowledge we included the following variables in the multivariate logistic regression model: age, sex, left ventricular ejection fraction (LVEF), baseline eGFR, contrast volume, chronic obstructive pulmonary disease (COPD), diabetes, known hypertension, prior coronary revascularisation, bleeding complication and procedural access site.
The association between pAKI and mortality was displayed graphically as a Kaplan-Meier curve and compared with the log-rank test. For 30-day mortality, only age, pAKI and gender were used in a logistic regression model. For 1-year and long-term mortality, a Cox regression analysis was performed with the following variables: persistent AKI, gender, LVEF, diabetes mellitus, hypertension, previous coronary revascularisation, peripheral arterial disease and an atrial fibrillation diagnosis.
Finally, on sensitivity analyses we repeated the analyses using the two alternative definitions of pAKI.
Statistical analysis was performed with Stata V.13.1.
Results
Patient, echocardiographic and procedural characteristics
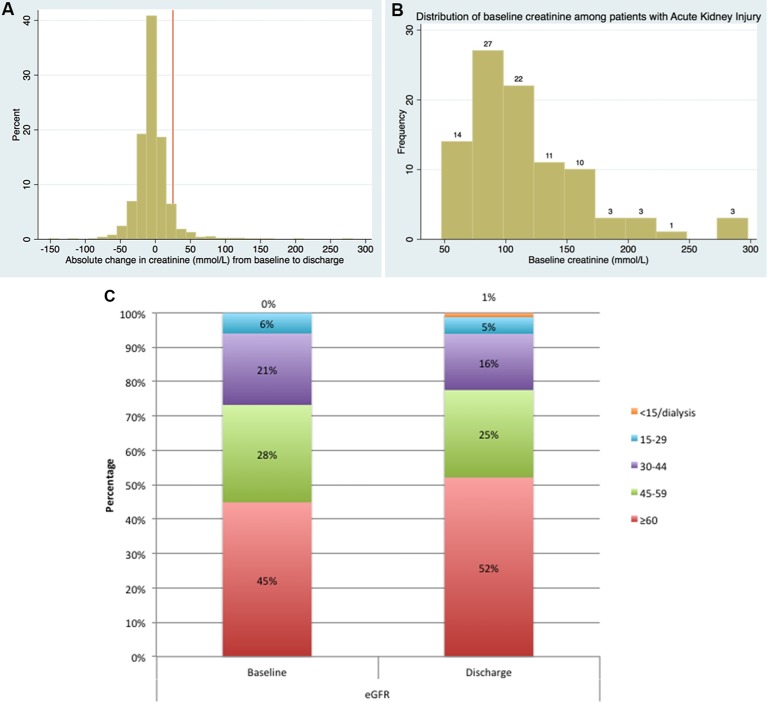
Persistent AKI occurred in 94/1540 (6.1%) patients. The majority of patients (78%) who fulfilled the modified VARC2 criteria increased their creatinine by ≥26 µmol/L (table 1). The change in absolute level of creatinine for all patients is shown in figure 2A, while the baseline creatinine value for patients who developed persistent AKI is shown in figure 2B,C.
Table 1.
Baseline characteristics
| Factor | No acute kidney injury | Persistent acute kidney injury | p Value |
| N | 1446 | 94 | |
| Age (years) | 82 (7) | 80 (7) | 0.051 |
| Gender | 733 (50.7%) | 27 (28.7%) | <0.001 |
| Baseline creatinine (mmol/L) | 100 (35) | 117 (50) | <0.001 |
| eGFR at baseline (mL/min/1.73 m2) | 59 (21) | 56 (23) | 0.18 |
| VARC-2 criteria | |||
| Stage 1: 1.5–1.99× increase or 26 mmol/L in creatinine | 78 (83%) | ||
| Stage 2: 2–2.9× increase in creatinine | 4 (4%) | ||
| Stage 3: ≥3× increase in creatinine or initiation of renal replacement therapy | 12 (13%) | ||
| 4 CKD groups at baseline | 652 (45.1%) | 37 (39.4%) | 0.084 |
| >60 | |||
| 45–59 | 414 (28.6%) | 22 (23.4%) | |
| 30–44 | 298 (20.6%) | 25 (26.6%) | |
| 15–29 | 82 (5.7%) | 10 (10.6%) | |
| Weight (kg) | 74 (15) | 78 (16) | 0.011 |
| Height (cm) | 168 (9) | 170 (10) | 0.011 |
| Body surface area | 1.8 (0.2) | 1.9 (0.2) | 0.003 |
| Diabetes mellitus | 315 (21.8%) | 25 (26.6%) | 0.28 |
| Known hypertension | 1045 (72.3%) | 67 (71.3%) | 0.84 |
| Chronic obstructive pulmonary disease | 282 (19.5%) | 29 (30.9%) | 0.008 |
| Prior cardiac surgery | 418 (28.9%) | 35 (37.2%) | 0.086 |
| Prior PCI | 419 (29.0%) | 27 (28.7%) | 0.96 |
| Prior PCI/CABG | 701 (48.5%) | 51 (54.3%) | 0.28 |
| Prior stroke | 204 (14.1%) | 18 (19.1%) | 0.18 |
| Known peripheral arterial disease | 290 (20.1%) | 24 (25.5%) | 0.20 |
| Atrial fibrillation | 514 (35.5%) | 32 (34.0%) | 0.77 |
| NYHA class | |||
| 1 | 6 (0.4%) | 0 (0.0%) | 0.64 |
| 2 | 99 (6.9%) | 7 (7.4%) | |
| 3 | 1096 (75.9%) | 67 (71.3%) | |
| 4 | 243 (16.8%) | 20 (21.3%) | |
| Logistic EuroSCORE I | 22%(14)14 | 23%(17)17 | 0.41 |
| LVEF % | |||
| ≥50% | 874 (60.5%) | 50 (53.8%) | 0.40 |
| 40%–49% | 251 (17.4%) | 18 (19.4%) | |
| 30%–39% | 198 (13.7%) | 18 (19.4%) | |
| ≤30 | 121 (8.4%) | 7 (7.5%) | |
| Aortic valve area (cm2) | 0.64 (0.2) | 0.67 (0.17) | 0.28 |
| Mean aortic valve gradient (mm Hg) | 49 (16) | 46 (17) | 0.091 |
| Annulus diameter (mm) | 23 (2.7) | 24 (2.5) | 0.009 |
| Systolic pulmonary artery pressure (mm Hg) | 44 (15) | 45 (15) | 0.64 |
| Aortic regurgitation (0–3) | |||
| 0 | 508 (35.5%) | 35 (38.0%) | 0.27 |
| 1 | 757 (52.8%) | 52 (56.5%) | |
| 2 | 137 (9.6%) | 5 (5.4%) | |
| 3 | 31 (2.2%) | 0 (0.0%) | |
| Mitral regurgitation (0–3) | |||
| 0 | 350 (24.6%) | 30 (32.3%) | 0.28 |
| 1 | 821 (57.7%) | 52 (55.9%) | |
| 2 | 223 (15.7%) | 10 (10.8%) | |
| 3 | 29 (2.0%) | 1 (1.1%) | |
| Urgent TAVI | 17 (1.2%) | 0 (0.0%) | 0.29 |
| Make of prosthesis | |||
| Medtronic CoreValve/Evolut R | 771 (53.4%) | 53 (56.4%) | 0.23 |
| Edwards SAPIEN | 604 (41.8%) | 40 (42.6%) | |
| Other | 70 (4.8%) | 1 (1.1%) | |
| Contrast volume (mL) | 110 (78) | 129 (89) | 0.027 |
| Contrast volume to creatinine clearance ratio | 2.4 (1.9) | 2.73 (2.3) | 0.069 |
| Contrast volume (mL)/CrCl | |||
| <3 mL/CrCl | 1070 (74.2%) | 67 (71.3%) | 0.75 |
| 3–3.9 mL/CrCl | 167 (11.6%) | 11 (11.7%) | |
| ≥4 mL/CrCl | 205 (14.2%) | 16 (17.0%) | |
| Fluoroscopy time (min) | 1429 (1098) | 1391 (779) | 0.74 |
| Periprocedural/Postprocedural bleeding | 113 (7.8%) | 10 (10.6%) | 0.33 |
| Length of in-hospital stay (days) | 6.7 (8.8) | 14.0 (28.9) | <0.001 |
Data are presented as mean±SD or n (%) as appropriate.
CABG, coronary artery bypass graft; CKD, chronic kidney disease; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional classification; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation; VARC-2, Valve Academic Research Consortium-2.
Figure 2.
(A) Creatinine changes: changes in creatinine from baseline to discharge in all patients. (B) Creatinine changes: distribution of the baseline creatinine level among the persistent acute kidney injury patients. (C) Creatinine changes: renal function (estimated glomerular filtration rate) at baseline and discharge.
Patients who developed pAKI were more often male (71.3%) despite a similar number of men and women being treated with TAVI (760 women, 780 men) (table 1). Baseline creatinine level was higher among those with pAKI, whereas eGFR was not significantly different. COPD was more frequently present in patients with pAKI. Other comorbidities, such as diabetes, prior hypertension and prior cardiac surgery/percutaneous coronary intervention, were equally common. Preprocedural echocardiography differed only by higher annulus diameter in patients with pAKI.
On TAVI procedure, patients who had pAKI received larger contrast volumes, whereas the contrast volume to CrCl ratio was borderline different. Hospitalisation was prolonged for patients with pAKI.
Predictors of pAKI
There were several univariate predictors of pAKI (table 2). However, after multivariable adjustment only male gender, lower baseline eGFR and apical vascular access site remained significantly associated with pAKI. Larger contrast volume and known COPD were borderline significantly associated with pAKI.
Table 2.
Predictors of persistent acute kidney injury
| Univariate OR (95% CI) | p Value | Multivariable OR* (95% CI) | p Value | |
| Age/10-year increase | 0.77 (0.59 to 1.00) | 0.052 | 0.85 (0.63 to 1.16) | 0.312 |
| eGFR/10 mL/min/1.73 m2 increase | 0.99 (0.98 to 1.00) | 0.179 | 0.88 (0.79 to 0.98) | 0.019 |
| Contrast volume per 10 mL increase | 1.03 (1.00 to 1.05) | 0.028 | 1.02 (1.00 to 1.05) | 0.052 |
| Male | 2.55 (1.61 to 4.04) | <0.001 | 2.68 (1.63 to 4.38) | <0.001 |
| Known diabetes | 1.30 (0.81 to 2.09) | 0.280 | 1.09 (0.66 to 1.80) | 0.735 |
| LVEF | ||||
| >50% | 1.0 (ref) | 1.0 (ref) | ||
| 40%–49% | 1.25 (0.72 to 2.19) | 0.418 | 1.02 (0.57 to 1.81) | 0.955 |
| 30%–39% | 1.59 (0.91 to 2.78) | 0.104 | 1.18 (0.66 to 2.12) | 0.580 |
| <30% | 1.01 (0.45 to 2.28) | 0.964 | 0.74 (0.32 to 1.72) | 0.489 |
| Logistic EuroSCORE I (n=1332) | 1.88 (0.42 to 8.51) | 0.407 | – | – |
| Prosthesis make | Ref (CoreValve) | – | – | |
| Edwards | 0.96 (0.63 to 1.47) | 0.856 | – | – |
| Other | 0.21 (0.03 to 1.53) | 0.119 | – | |
| COPD | 1.84 (1.17 to 2.91) | 0.009 | 1.59 (0.99 to 2.59) | 0.057 |
| Bleeding complication | 1.40 (0.51 to 2.78) | 0.978 | 1.64 (0.81 to 3.33) | 0.169 |
| Access site | 1.0 (ref: transfemoral) | 1.0 (ref: transfemoral) | ||
| Apical | 2.30 (1.47 to 3.79) | <0.001 | 2.23 (1.35 to 3.69) | 0.002 |
| Subclavian | 1.31 (0.31 to 5.63) | 0.713 | 1.34 (0.30 to 5.94) | 0.697 |
| Direct aortic | 3.17 (0.91 to 11.1) | 0.070 | 3.54 (0.95 to 13.3) | 0.060 |
| Prior CABG/PCI | 1.26 (0.83 to 1.92) | 0.278 | 0.90 (0.57 to 1.42) | 0.658 |
The following interaction terms were tested: gender and contrast volume to creatinine clearance ratio: p value 0.321; gender and age: p value 0.770; gender and diabetes: p value 0.995; gender and access site: p value 0.465.
*The multivariable model was adjusted for age, sex, LVEF, baseline eGFR, contrast volume, COPD, diabetes, known hypertension, prior coronary revascularisation, bleeding complication and procedural access site.
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Association between pAKI and mortality
By 30 days after discharge, 1.9% (29/1540) of the patients had died. More patients with pAKI had died compared with those without pAKI (7.5% vs 1.5%, p<0.001). On a multivariable logistic regression analysis including pAKI, age and gender, pAKI was found to independently predict 30-day mortality (HR 5.03, 95% CI (2.05 to 12.3), p<0.001). In comparison, age per 10-year increase (HR 1.71, 95% CI (0.90 to 3.22), p=0.099) and male gender (HR 1.77, 95% (CI 0.80 to 3.91), p=0.156) were not associated with 30-day mortality.
At 1 year 10.4% (161/1540) of the patients had died, with a larger proportion dying in the pAKI group (29.8% vs 9.2%, p<0.001). In the long-term with a median follow-up of 1.8 years (IQR 0.7–3.0), 27.0% (416/1540 patients) had died, with more patients dying in the pAKI group (50.0% vs 25.5%, p<0.001) (figure 3). The presence of pAKI predicted a doubled risk of death even after adjustment of other covariates, both at 1 year and in the long term (table 3).
Figure 3.
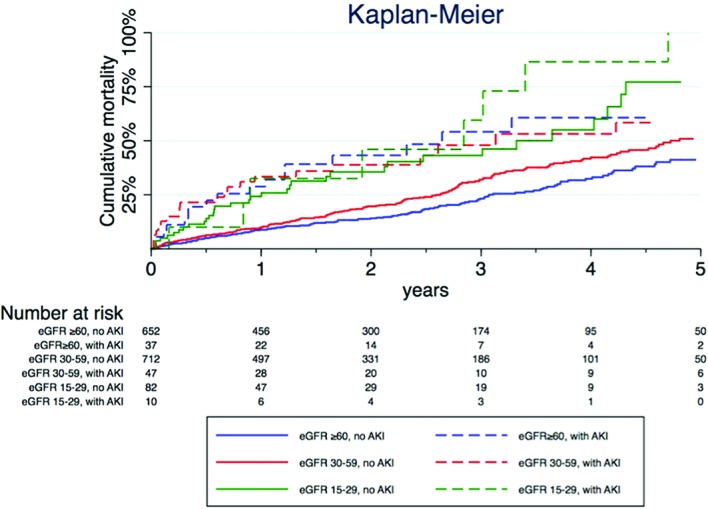
Kaplan-Meier curve.
Table 3.
Cox regression analysis of 1-year and long-term mortality
| Univariate HR (95% CI) | p Value | 1-Year mortality Multivariable HR (95% CI) |
p Value | Long-term mortality Multivariable HR (95% CI) |
p Value | |
| Age (per 1-year increase) | 1.12 (0.97 to 1.29) | 0.132 | 1.01 (0.98 to 1.03) | 0.497 | 1.19 (1.01 to 1.41) | 0.038 |
| Persistent acute kidney injury | 2.12 (1.57 to 2.88) | <0.001 | 2.74 (1.80 to 4.16) | <0.001 | 2.04 (1.49 to 2.81) | <0.001 |
| eGFR (per 1 mL/min/1.73 m2 increase) | 0.93 (0.88 to 0.97) | 0.002 | 0.99 (0.99 to 1.00) | 0.102 | 0.94 (0.90 to 0.99) | 0.031 |
| Male | 1.42 (1.17 to 1.73) | <0.001 | 1.54 (1.09 to 2.18) | 0.015 | 1.50 (1.22 to 1.86) | <0.001 |
| NYHA I | Ref | – | – | – | – | – |
| NYHA II | 1.17 (0.16 to 8.68) | 0.878 | – | – | – | – |
| NYHA III | 1.30 (0.18 to 9.23) | 0.796 | – | – | – | – |
| NYHA IV | 1.93 (0.27 to 13.8) | 0.514 | – | – | – | – |
| LVEF >50% | Ref | Ref 1.0 | ||||
| LVEF 40%–49% | 1.47 (1.15 to 1.87) | 0.002 | 1.69 (1.13 to 2.53) | 0.011 | 1.36 (1.06 to 1.75) | 0.018 |
| LVEF 30%–39% | 1.43 (1.10 to 1.88) | 0.009 | 1.52 (0.97 to 2.38) | 0.070 | 1.33 (1.00 to 1.76) | 0.049 |
| LVEF <30% | 1.54 (1.09 to 2.19) | 0.014 | 1.99 (1.22 to 3.20) | 0.005 | 1.36 (0.96 to 1.94) | 0.088 |
| Prior stroke | 1.26 (0.98 to 1.62) | 0.073 | 1.14 (0.75 to 1.72) | 0.482 | 1.14 (0.87 to 1.48) | 0.341 |
| Known diabetes | 1.36 (1.09 to 1.69) | 0.007 | 1.47 (1.01 to 2.11) | 0.040 | 1.46 (1.15 to 1.85) | 0.002 |
| Known hypertension | 0.82 (0.67 to 1.01) | 0.069 | 0.71 (0.50 to 1.00) | 0.052 | 0.76 (0.61 to 0.95) | 0.014 |
| Prior cardiac surgery/prior PCI | 0.59 (0.42 to 0.82) | 0.002 | 0.66 (0.53 to 0.82) | <0.001 | ||
| Prior cardiac surgery | 0.70 (0.57 to 0.87) | 0.001 | – | – | – | – |
| Prior PCI | 0.94 (0.76 to 1.16) | 0.578 | – | – | – | – |
| Prior COPD | 1.28 (1.02 to 1.61) | 0.034 | 1.04 (0.71 to 1.52) | 0.842 | 1.18 (0.93 to 1.50) | 0.175 |
| Peripheral arterial disease | 1.22 (0.97 to 1.51) | 0.084 | 1.48 (1.02 to 2.16) | 0.041 | 1.24 (0.97 to 1.58) | 0.082 |
| Atrial fibrillation | 1.33 (1.09 to 1.62) | 0.004 | 1.39 (1.01 to 1.91) | 0.044 | 1.28 (1.05 to 1.57) | 0.016 |
| Logistic EuroSCORE I (n=1332) | 2.48 (1.21 to 5.07) | 0.013 | – | – | – | – |
| Prosthesis make | Ref (CoreValve) | Ref (CoreValve) | Ref (CoreValve) | |||
| Edwards | 0.92 (0.76 to 1.12) | 0.418 | 0.83 (0.60 to 1.15) | 0.276 | 0.90 (0.73 to 1.11) | 0.315 |
| Other | 0.54 (0.20 to 1.48) | 0.236 | 0.60 (0.22 to 1.65) | 0.322 | 0.59 (0.22 to 1.61) | 0.305 |
COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Sensitivity analyses: gender and two alternative definitions of pAKI
When using the VARC-2 definition, pAKI was more frequent among men (see online supplementary table 1). However, changing the pAKI definition to a 25% change in creatinine from baseline to discharge, male gender was more equally distributed among those developing pAKI (see online supplementary table 2). Predictors of a 25% change in creatinine were only access site (apical) and presence of COPD, whereas male gender and contrast volume were not (see online supplementary table 3). However, both male gender and this alternative pAKI definition were predictive of 1-year mortality (see online supplementary table 4).
openhrt-2016-000554supp001.pdf (136KB, pdf)
When pAKI was based on at least fulfilling the ‘R’ or higher of the RIFLE definition of kidney injury, more men had pAKI. Predictive of this AKI definition was male gender, apical access site and bleeding complications (see online supplementary table 3). In the Cox regression model, male gender as well as this alternative AKI definition remained predictive of 1-year mortality (see online supplementary table 4).
Discussion
In this large nationwide registry-based study, consisting of 1540 unselected consecutive patients, pAKI that was present at discharge from hospital was present in 6.1% of the patients. The factors associated with the presence of pAKI were baseline renal function, male sex and access site, whereas contrast volume was borderline significant. With the increasing use of TAVI, identifying patients at risk and preventing pAKI are an important aim, because pAKI is associated with both lengthened in-hospital stay and increased mortality. Even though the majority of patients fulfilled the VARC2 criteria7 by only having at least a 26 mmol/L increase in their baseline creatinine to discharge, this doubled the risk of both short-term and long-term mortality in all renal function categories.
Several factors were associated with the development of pAKI. Baseline renal function predicted pAKI, whereas contrast volume use was a borderline predictor of pAKI. Contrast is well known to be directly nephrotoxic, and precautions are usually undertaken to reduce this risk. A prior study has evaluated the dose of contrast given in relation to renal function and found this ratio predictive of AKI.1 Contrast-induced nephropathy may occur more often in vulnerable patients with underlying atherosclerotic disease.12 This study also confirms that patients undergoing TAVI through an apical access site compared with a transfemoral route are at increased risk of developing persistent AKI. These patients usually represent a group with more advanced atherosclerotic disease and worse outcome, as shown by others.13
A surprising finding in this study was that male gender predicted pAKI. There were significantly more male than female patients who developed pAKI. This occurred despite TAVI being performed in an equal number of men and women. In general, men who had TAVI had the same logistic EuroSCORE as women, were on average a few years younger than women, but appeared to have a higher burden of atherosclerotic disease, reflected by the higher number of prior percutaneous coronary interventions and prior stroke. Despite women having more reported periprocedural bleeding complications than men, which are factors that could have made them more prone to having AKI, male gender remained associated with having pAKI.
A possible explanation for men being more likely to have pAKI could lie in the pAKI definition. Men have higher baseline creatinine values compared with women, and therefore a ≥26 mmol/L increase in creatinine would more often occur in men, being a less percentage increase than in women. In a sensitivity analysis, when pAKI was instead defined as a 25 percentage point increase rather than an absolute increase in creatinine, an equal number of men and women would have had pAKI. In this alternative definition of pAKI, only apical access and COPD were predictive of pAKI. However, for long-term outcome, both pAKI and male gender were predictive. Therefore, as seen in other studies as well, the increased risk for men undergoing TAVI to have pAKI compared with women is probably only partially mediated by AKI itself, and other factors specific to men are probably present that influence long-term outcome.14
Mortality was doubled in patients who had pAKI in this study. Renal function is predictive of adverse outcome,15 but adding pAKI independently worsened the outcome. Recent studies showed that kidney function before TAVI, and especially the occurrence of AKI, significantly influences the outcome in patients after TAVI.16–18 The reasons for the association of chronic kidney disease with adverse outcomes after invasive and surgical procedures are not fully understood.
A limitation of this study is that we used a modified version of the VARC2-AKI definition7. Only creatinine on discharge was available, and therefore a peak creatinine between the first 72 hours and 7 days post-TAVI could not be detected. Therefore, most of the patients probably have recovered renal function after AKI by the time of discharge (median 6 days (IQR 4–7)), and therefore we found a low incidence of pAKI. However, the patients identified by this extended definition of creatinine elevation has for other patients been shown to be associated with worse prognosis.16 In this study, we also did not include patients with an eGFR <15 mL/min/1.73 m2, as these patients are a very vulnerable group with very high risk of developing both AKI and dialysis. Furthermore, in the models of predictors for long-term outcome, residual confounding could remain. Unfortunately, such frailty was not collected and could not be adjusted for.
Conclusion
The clinical implications are that AKI after TAVI is associated with a doubled mortality. Therefore, efforts should be made to avoid AKI. Special attention should be given to vulnerable groups undergoing TAVI at risk of AKI, such as male patients, patients with already low renal function on admission and patients requiring transapical procedures.
Footnotes
Contributors: KS, AR, TJ and GV had the conception and planned the study. AR conducted the SWEDHEART-registry in order to provide us with the file with the TAVI-registry and it was his responsibility to apply the study protocol to the Ethics Board in Stockholm, Sweden. ME as a nephrologist supported us with important knowledge about the definition of the acute kidney injury and the definition of persistent kidney injury and their clinical implications. KS (as the main supervisor) and me had the responsibility to perform the statistical analysis, to design the tables and the figures and to write the article. All the co-authors (ME, AR and TG) have constributed with their comments to improve the statistical analysis, the knowledge and the quality of the manuscript by revising our results critically for important intellectual content. All the contributors (GV, ME, AR, TG, KZ) have made the final approval of the version to be published by reading and accepting all the Changes that were performed during this process.
Funding: KS and ME were supported by the Stockholm County Council (clinical postdoctoral appointment).
Competing interests: Dr TJ reports consulting and lecture fees from Astra Zeneca and Aspen. Dr KS reports lecture fees from Astra Zeneca, Aspen and St Jude. Dr ME reports payment for lectures for Amgen. Dr AR reports consulting fees from Medtronic and Symetis, and educational grant from Medtronic.
Patient consent: Obtained.
Ethics approval: This study protocol was approved by the Ethics Board in Stockholm, Sweden.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Yamamoto M, Hayashida K, Mouillet G, et al. . Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv 2013;6:479–86. 10.1016/j.jcin.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 2. Nuis RJ, Rodés-Cabau J, Sinning JM, et al. . Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2012;5:680–8. 10.1161/CIRCINTERVENTIONS.112.971291 [DOI] [PubMed] [Google Scholar]
- 3. Seiffert M, Sinning JM, Meyer A, et al. . Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631–40. 10.1007/s00392-014-0692-4 [DOI] [PubMed] [Google Scholar]
- 4. Arsalan M, Squiers JJ, Farkas R, et al. . Prognostic Usefulness of acute kidney Injury after Transcatheter aortic valve replacement. Am J Cardiol 2016;117:1327–31. 10.1016/j.amjcard.2016.01.037 [DOI] [PubMed] [Google Scholar]
- 5. Barbash IM, Ben-Dor I, Dvir D, et al. . Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J 2012;163:1031–6. 10.1016/j.ahj.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 6. Ohno Y, Maekawa Y, Miyata H, et al. . Impact of periprocedural bleeding on incidence of contrast-induced acute kidney injury in patients treated with percutaneous coronary intervention. J Am Coll Cardiol 2013;62:1260–6. 10.1016/j.jacc.2013.03.086 [DOI] [PubMed] [Google Scholar]
- 7. Kappetein AP, Head SJ, Généreux P, et al. . Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438–54. 10.1016/j.jacc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Bagur R, Webb JG, Nietlispach F, et al. . Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865–74. 10.1093/eurheartj/ehp552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Najjar M, Salna M, George I. Acute kidney injury after aortic valve replacement: incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther 2015;13:301–16. 10.1586/14779072.2015.1002467 [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 12. Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart 2016;102:638–48. 10.1136/heartjnl-2014-306962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thourani VH, Jensen HA, Babaliaros V, et al. . Transapical and Transaortic Transcatheter Aortic Valve Replacement in the United States. Ann Thorac Surg 2015;100:1718–27. 10.1016/j.athoracsur.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 14. Kodali S, Williams MR, Doshi D, et al. . Sex-Specific differences at presentation and outcomes among patients undergoing transcatheter aortic valve replacement: a Cohort Study. Ann Intern Med 2016;164:377–84. 10.7326/M15-0121 [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto M, Hayashida K, Mouillet G, et al. . Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol 2013;62:869–77. 10.1016/j.jacc.2013.04.057 [DOI] [PubMed] [Google Scholar]
- 16. Brown JR, Kramer RS, Coca SG, et al. . Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 2010;90:1142–8. 10.1016/j.athoracsur.2010.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duncan A, Ludman P, Banya W, et al. . Long-Term Outcomes after Transcatheter aortic valve replacement in High-Risk Patients with severe aortic Stenosis. JACC Cardiovasc Interv 2015;8:645–53. 10.1016/j.jcin.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 18. Giordana F, D'Ascenzo F, Nijhoff F, et al. . Meta-analysis of predictors of all-cause mortality after transcatheter aortic valve implantation. Am J Cardiol 2014;114:1447–55. 10.1016/j.amjcard.2014.07.081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2016-000554supp001.pdf (136KB, pdf)