Abstract
Kidneys are important regulators of extracellular fluid volume homeostasis. Extracellular fluid volume is a key regulatory component of long-term blood pressure control influenced by controlling tubular sodium transport. In recent decades, renal P2 purinoceptors (P2 receptors) have come to the forefront as a mechanism for regulating extracellular fluid volume. P2 receptors are broadly distributed in renal tubular and vascular elements where they confer segmental control of renal vascular resistance, autoregulation, and tubular reabsorption. Activation or impairment of renal P2 purinoceptors is implicated in the regulating blood pressure or causing renal pathologies including hypertension. In this brief review, we discuss the role of renal vascular and tubular P2 purinoceptors in the regulation of renal hemodynamics, maintenance of extracellular fluid volume, regulation of sodium reabsorption and the control of blood pressure.
Keywords: P2 purinoceptors, ATP, blood pressure regulation, pressure natriuresis/sodium reabsorption
Introduction
Normal healthy kidney function is an important element in the regulation of blood pressure. High blood pressure affects approximately 67 million American adults, or nearly 1 out of every 3 [1]. The risk for cardiovascular and renal disease doubles with every 20mmHg increase in systolic blood pressure [2]. With treatment of high blood pressure costing the nation approximately 48 billion dollars a year, regulation of blood pressure is critical from a clinical, economic and societal stand point [3].
Blood pressure regulation is a complex and integrated mechanism. In 1961, Guyton developed a computational model predicting how the kidney is the centerpoint of long-term blood pressure control. He stated that, “Long-term regulation of mean arterial pressure is controlled principally by the kidneys” [4]. In 1969, Guyton et al. went on to show the important mechanistic linkage between long-term blood pressure control and sodium excretion, which includes the “pressure natriuresis relationship” [5]. Guyton’s model described this relationship between renal perfusion pressure, sodium excretion and blood pressure as an important mechanism for regulating circulatory volume [6]. The kidneys regulate circulatory volume by controlling sodium and water balance, thus maintaining extracellular fluid volume (ECFV) homeostasis. Simply put, an increase in sodium and water consumption leads to an increase in ECFV, which in turn increases blood volume. This increases venous return to the heart, inducing atrial stretch and increases cardiac output, thus increasing systemic blood pressure [6]. When arterial pressure increases, the nephron reduces sodium and water reabsorption thus increasing sodium and water excretion, to return ECFV and blood pressure to normal; the so called “pressure natriuresis” phenomenon. Conversely, with a decrease in blood pressure, the nephron increases sodium and water reabsorption, to increase ECFV and thereby increase blood pressure.
In the last two decades, the importance of renal P2 purinoceptors (P2 receptors) has come to light by the realization that multiple P2 receptor subtypes are expressed along renal vascular and tubular structures. The importance of the renal P2 purinoceptor system may be of similar to that of the renin-angiotensin-aldosterone system or sympathetic nerve activity for the regulation of blood pressure and maintenance of cardiovascular health. P2 receptors are broadly distributed throughout the kidney where they control renal vascular resistance, autoregulation, and regulation of tubular reabsorption. In this brief review we will discuss recent findings on the roles of P2 receptors in regulating renal hemodynamics, maintenance of ECFV, sodium reabsorption and blood pressure control.
Renal P2 Receptor Expression
P2 receptors are activated by ATP and can be divided into two distinct receptor families designated P2X and P2Y. Currently there are seven P2X receptor subtypes (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7) which function as ligand gated channels. Among many other roles, P2X receptors are important regulators of renal blood flow, vascular and endothelial function, tubular function, and inflammatory responses [7,8]. P2Y receptors are G-protein coupled receptors divided into approximately eight distinct subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14). Table 1 illustrates the distribution of P2X and P2Y receptors in renal vascular and tubular structures. Immunohistochemistry and western blot analyses indicate that P2X1,2,4,7 and P2Y1,2,4 receptors are found on arcuate, and interlobular arteries, as well as afferent arterioles [9–12•]. RT-PCR analysis and immunhistochemical staining reveals that P2Y1,2,4,6 receptors are found in isolated rat glomeruli [11,13]. Menzies et al. reported that P2X7 receptors are found in glomeruli [12•]. P2X4,5,6 and P2Y1,2,4,6 are located in the proximal tubule [8,11,14–16]. P2X and P2Y receptors have also been identified in the loop of Henle [8,11,14–18]. Messenger RNA and immunohistochemistry techniques reveal that P2X4,6 and P2Y1,2,4 receptors are expressed in the descending and ascending thin loops of Henle [8,11,14–21••]. Expression of P2X4,6 and P2Y1,2,4 receptors continues at low levels in the rat thick ascending limb [8,11,15,17•]. Marques et al. reported that P2X1,4,5 and P2Y2,6 receptors are expressed in the mouse thick ascending limb [22•]. P2X4,5,6 receptors are located on the basolateral membrane of the distal tubule [11,21••]. P2X1,2,4,5,6 are found in both the apical and basolateral membranes of the collecting duct epithelium [23]. Interestingly, there is only evidence for P2X4 receptor expression in human collecting duct cells [24]. P2Y1,2,4,6 receptors are also expressed in rat collecting duct as determined by immunostaining, protein expression and mRNA expression [16,23]. There is little information P2X3 and P2Y11–14 receptor expression in renal structures. Diverse P2 receptor expression in each nephron segment suggests that P2 receptors participate importantly in regulating solute and water reabsorption, which is essential for regulating ECFV and composition. Extracellular fluid balance is an important control point for regulating blood pressure and cardiovascular function.
Table 1.
Distribution of P2 receptors in the renal vascular and tubular structures.
| P2 Receptor | Segment |
|---|---|
| P2X1 | vSMC[9,10,11,12], mTAL[22], CD[23] |
| P2X2 | vSMC[10,11] CD[23] |
| P2X4 | vSMC[11,12], PT[8,11], tDL[8,17], tAL[8], mTAL[8,17,21], DT[8,11], OMCD[23,24] |
| P2X5 | PT[11], mTAL[22], DT[21], CCD[11,23], OMCD[11,23], IMCD[11,23] |
| P2X6 | PT[8,11], tDL[8,11,17], tAL[8,11], mTAL[8,11,17], DT[8,11], CCD[23], OMCD[23], IMCD[23] |
| P2X7 | vSMC[12], Glom[11,12] |
| P2Y1 | vSMC[10,11], Glom[11,13], PT[11,16], tDL[21], mTAL[21], OMCD[16] |
| P2Y2 | vSMC[10], Glom[11,13], PT[11,14,16], tDL[14,16], tAL[14,16], mTAL[14,22], cTAL[14], CD[14], CCD[14], IMCD[14,16], OMCD[14,16] |
| P2Y4 | vSMC[9], Glom[11,13], PT[11,16], tAL[16], mTAL[21], CCD[23], OMCD[23], IMCD[23] |
| P2Y6 | Glom[11,13], PT[15], mTAL[15,22], cTAL[15], CCD[15,23], OMCD[15,23], IMCD[23] |
CCD, cortical collecting duct; CD, collecting duct; cTAL, cortical thick ascending limb; DT, distal tubule; Glom, Glomerulus; IMCD, inner medullary collecting duct; mTAL, medullary thick ascending limb, OMCD, outer medullary collecting duct, PT, proximal tubule, tAL, thin ascending limb; tDL, thin descending limb; vSMC, vascular smooth muscle cell. There is little to no expression reported for P2X3 and P2Y11–14 receptor expression in the kidney.
Ecto-adenosinetriphophatases (Ecto-ATPase) are enzymes that are activated by either Ca2+ or Mg2+ and are able to hydrolyze nucleoside triphosphates (ATP, UTP) and nucleoside diphosphates (ADP, UDP) [25]. Sabolic et al. have shown that ecto-ATPases are found all along renal tubules, arterioles and peritubular capillaries [25]. These ecto-ATPases can metabolize ATP and other nucleotides and nucleosides that are released into tubular fluid or renal interstitial spaces and, in essence, provide a physiological “brake” to regulate endogenous P2 receptor-dependent signaling mechanisms. Consequently, the synergy between the tissue specific ATP-release and ATP catabolism yield a closely regulated method for controlling P2 receptor dependent mechanisms. Accordingly, this could represent a highly sensitive way of regulating renal tubular transport and renal hemodynamics, and thus contribute significantly to the control of extracellular fluid volume and blood pressure regulation.
P2 Receptor Function in the Renal Vasculature
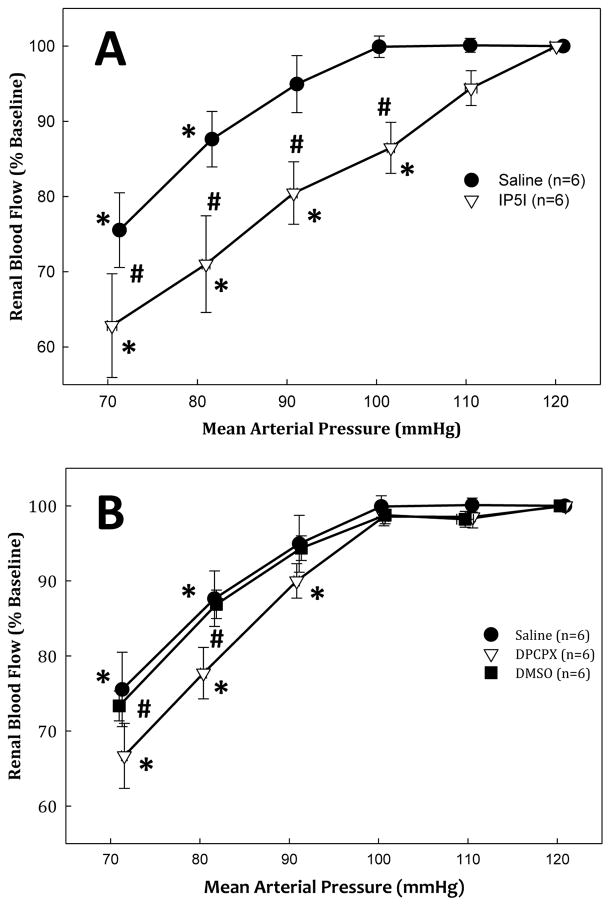
Several studies have shown that P2X1 receptors are found on the afferent arteriole, while one study proposed that P2Y1 receptors are on both the afferent and efferent arterioles [11,20,26,27•]. P2X1 receptor activation regulates renal vascular resistance and confers autoregulatory control of renal blood flow, glomerular capillary pressure and thus glomerular filtration rate [28]. Using the juxtamedullary nephron preparation, Inscho et al. reported that P2X1 receptor blockade with NF279, blocked the pressure-mediated vasoconstriction when renal perfusion pressure was increased, consistent with an impaired autoregulatory response [29]. They also reported that afferent arteriole autoregulatory behavior was significantly blunted in P2X1 receptor knockout mice (P2X1−/−) compared with wild type controls [29]. Osmond et al. reported that whole kidney autoregulation of renal blood flow was impaired in rats treated with the P2X1 receptor antagonist, IP5I (Figure 1: Panel A) [28]. Whole kidney autoregulation of renal blood flow was unaltered during treatment with an A1 receptor antagonist, DPCPX (Figure 1: Panel B) [28]. These studies suggest that P2X1 receptors are important for afferent arteriole and whole kidney autoregulation. Recently, Schnermann et al. investigated the role of P2X receptors in mediating preglomerular resistance adjustments invoked by tubuloglomerular feedback signaling [30]. They reported that mouse tubuloglomerular feedback responses remained essentially normal during P2 receptor blockade suggesting that adenosine and P1 receptors may contribute to tubuloglomerular feedback induced vasoconstriction in vivo [30]. These studies suggest that there may be important differences in the roles of P1 and P2 receptors in regulating renal microvascular function. Menzies et al. reported that “selective” blockade of P2X7 receptors with Brilliant Blue G, reduced blood pressure and decreased renal vascular resistance, thus suggesting that P2X7 receptor activation may increase renal vascular resistance [12•,31••]. Consequently, impaired vascular P2 receptor activation may blunt renal autoregulatory control, permitting pathological increases in glomerular capillary pressure, leading to glomerular injury.
Figure 1.
Percent change of baseline renal blood flow (RBF) in response to decreasing renal perfusion during P2X1 receptor antagonism. Percent change from baseline RBF in response to decrements in renal perfusion pressure with (open triangles) and without (closed circles) P2X1 receptor antagonism by IP5I. Panel B: percent change from baseline RBF in response to decrements in renal perfusion pressure with A1 receptor inhibition by DPCPX (open triangles) and during DMSO vehicle infusion (closed squares) and saline experiments (close circles). * P <0.05 vs. baseline. #P < 0.05 between saline vehicle. Figure modified from [27].
Activation of P2Y receptors in the renal vasculature can produce either vasoconstriction or vasodilation [32]. Studies using the juxtamedullary nephron preparation demonstrated that stimulation of either P2Y1 (2 methlythio-ATP) or P2Y2,4 receptors (UTP) vasoconstricts afferent arterioles [33]. Using isolated perfused kidneys, Eltze et al. showed that P2Y receptor agonists (ATP, ADP, UTP, ATPγS) induced vasodilation at low concentrations, and vasoconstriction at high concentrations [34]. This vasodilation elicited by “selective” P2Y receptor agonists seems to involve endothelial factors including nitric oxide (NO) or endothelium-derived relaxing factor, and is independent of prostaglandins [34]. Evidence for P2Y2 receptor dependent vasodilation has emerged from studies using P2Y2 receptor knockout mice (P2Y2−/−), which exhibited increased renal vascular resistance compared to wild-type controls [35]. This suggests that P2Y2 receptors exert basal vasodilatory influence on the renal vasculature. Furthermore, Rieg et al. showed that P2Y2 receptor activation decreases blood pressure in wild type mice and deletion of P2Y2 receptors (P2Y2−/−) increases systemic blood pressure [36]. These data show that P2Y receptors exert a vasodilatory influence that reduces systemic blood pressure. Therefore, P2X7 and P2Y2 receptors may provide novel therapeutic targets for regulating systemic blood pressure. Activation or impairment of renal vascular P2 receptors appear to play and important role in regulating renal vascular resistance, glomerular capillary pressure, and whole kidney autoregulation, therefore contributing to the regulation of renal function and systemic blood pressure.
P2 Receptor Activation in Renal Tubular Segments
Sodium balance and regulation of ECFV is controlled by regulating sodium transport along the nephron. An increase in systemic blood pressure increases renal perfusion pressure to the kidney. While autoregulation is efficient, it is not perfect, leading to a subtle increase in glomerular capillary pressure and a small increase in glomerular filtration rate which can increase tubular fluid flow rate, stimulate ATP release from tubular epithelium where it can activate P2 receptors and reduce epithelial sodium transport. Therefore, regulation of ECFV occurs in part by P2-receptor-mediated regulation of salt and water reabsorption by the nephron [37,38••].
Proximal Tubule
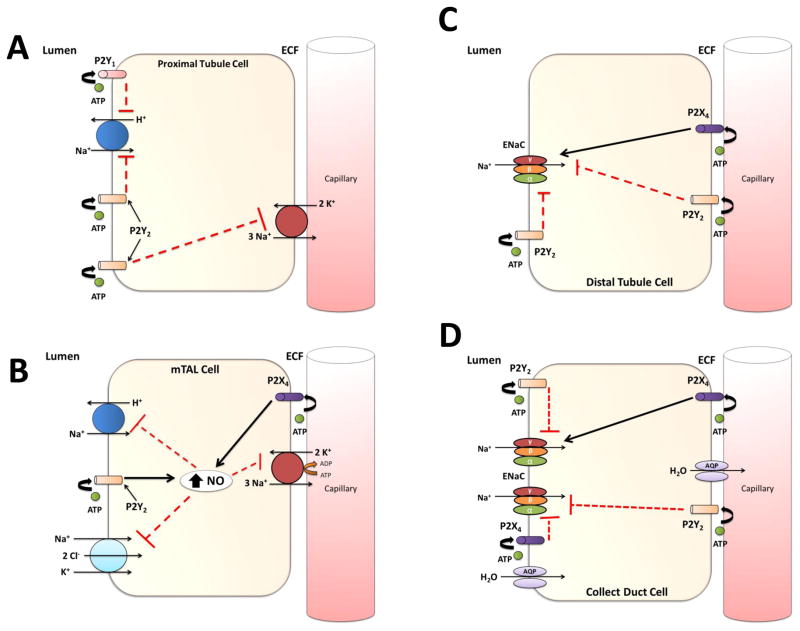
Activation of P2 receptors in renal tubules can inhibit renal sodium transport. P2Y receptors seem to predominate in the proximal tubule. Activation of P2Y2 receptors inhibits sodium and bicarbonate reabsorption by decreasing the activity of the Na+/H+ exchanger and Na+/K+ ATPase activity [39,40]. Microperfusion of P2Y1 receptor agonists in the proximal tubule lumen inhibit the Na+/H+ exchanger 3 in vivo and reduces Na+ reabsorption [41]. Increases in renal perfusion pressure cause a subtle increase in glomerular filtration rate thus increasing the filtered load received by the proximal tubule. This stimulates increased shear stress in the proximal tubule leading to local release of ATP and in turn activates proximal tubule P2Y receptors through autocrine/paracrine mechanisms. Activation of P2Y receptors reduces proximal tubule sodium reabsorption and enhances solute and water delivery to the distal nephron and contributes to the pressure natriuresis response to reduce ECFV and thus blood pressure (Figure 2: Panel A).
Figure 2.
A summary of P2 receptor regulation of sodium transport throughout major segments of the nephron. Panel A (Proximal Tubule): Activation of apical P2Y1 and P2Y2 receptors by luminal ATP inhibits the activity of the Na+/H+ exchanger. P2Y2 receptor activation can also inhibit Na+/K+ ATPase in the basolateral membrane. Panel B (Medullary thick ascending limb): Basolateral P2X4 and apical P2Y2 receptor stimulation by ATP causes an increase in intracellular nitric oxide, that reduces sodium reabsorption through mechanisms of inhibiting the Na+/H+ exchanger, N+/K+/2Cl− co-transporter, and the Na+/K+ ATPase. Panel C (Distal Tubule): P2 receptors in the distal tubule cause both an inhibition and stimulation of epithelial sodium channel (ENaC) activity. Activation of basolateral and apical P2Y2 receptors reduces sodium transport through inhibition of ENaC activity. P2X4 receptor stimulation by ATP causes an increase in sodium reabsorption through mechanisms associated with ENaC channel activity. Panel D (Collecting Duct): P2 receptor activation by ATP causes a reduction or stimulation in sodium reabsorption in a collecting duct cell. Apical and basolateral P2Y2, and basolateral P2X4 receptor, at high levels of ATP, activity inhibits ENaC activity, reducing sodium transport. Low levels of ATP may stimulate ENaC activity through mechanisms linked to P2X4.
Loop of Henle
Increasing tubular fluid flow rate through the loop of Henle stimulates ATP release that activates P2 receptors in an autocrine/paracrine manner. Garvin et al. demonstrated that ATP and UTP increase intracellular nitric oxide (NO) production in cell suspensions of rat medullary thick ascending limb (mTAL) [42]. This ATP-mediated increase in NO production inhibits Na+/K+ ATPase and Na+/H+ exchanger activity in mTAL. Marques et al. reported that stimulation of P2X4 receptors increased intracellular NO, inhibiting the apical Na+/K+/2CL− (NKCC2) co-transporter [22•]. In a study using P2Y2−/− mice, Vallon’s group showed there is an increase NKCC2 co-transporter protein, suggesting an inhibitory role of P2Y2 receptors on sodium reabsorption and NKCC2 transporter expression in mouse mTAL [14,35]. These data suggest that increases in tubular fluid flow rate releases ATP that activates both basolateral and apical P2 receptors in the loop of Henle increasing intracellular NO production and reducing sodium reabsorption. The increase in glomerular filtration rate leads in an increase in tubular fluid flow rate from the proximal tubule that facilitates release of ATP from the tubular epithelium and the activation of P2 receptors in the loop of Henle. P2 receptor activation in this segment reduces sodium transport by the inhibition of Na+/K+ ATPase, Na+/H+ exchanger, and NKCC2 co-transporter, potentiating the pressure natriuresis response and decreasing blood pressure (Figure 2: Panel B).
Distal Tubule
Functional aspects of P2 receptors in the distal tubule have been limited to studies using cultured cell lines. A study by Zhang et al. has shown that addition of 2-methylthio-ATP, basolaterally to A6 (Xenopus kidney) cells stimulates epithelial sodium channel (ENaC) activity purportedly by P2X4 receptor activation, [43]. Extracellular ATP inhibited sodium and calcium absorption and increased intracellular Ca2+ concentration in cultured rabbit connecting tubule cells, likely by P2Y2 receptor activation [44]. These findings provide suggestion that P2 receptors in the distal tubule might regulate sodium transport via modulating ENaC activity in this segment (Figure 2: Panel C).
Collecting Duct
The rate limiting step in sodium reabsorption in the collecting duct is the apical expression of ENaC. ATP was first shown to inhibit ENaC in primary and immortalized mouse cell culture [45–47]. Work from Sipos et al. suggests that ATP release from collecting duct epithelium into the tubular fluid inhibits ENaC activity in the collecting duct, contributing to the pressure natriuretic response [48]. Patch clamp studies in isolated split open cortical collecting ducts of P2Y2−/− and WT mice showed that ATP reduces the open probability of ENaC via P2Y2 receptor activation [49]. Pochynyuk et al. also reported that P2Y2−/− mice developed salt-sensitive hypertension due to the lack of inhibition of ENaC [49]. Wildmann et al. reported that high extracellular sodium concentration levels (145 mM) evoked a P2X4 receptor mediated inhibition of ENaC in rat collecting duct, while low extracellular sodium concentration level (50 mM) increased ENaC activity [23]. P2X4−/− mice on a low Na+ diet had a lower mean arterial pressure compared to their WT littermates [50••]. Taken together, these data suggest that an increase in tubular fluid flow rate releases ATP into the tubular lumen, activating P2X and P2Y receptors in the collecting duct. Activation of P2 receptors inhibits or in some studies stimulates ENaC activity and sodium reabsorption, thus modulating urinary sodium excretion and regulating systemic blood pressure (Figure 2: Panel D).
Conclusion
P2 receptors are highly expressed in renal vascular, glomerular and tubular structures. Many studies have investigated the mechanisms by which P2 receptors contribute to the regulation of blood pressure control and overall kidney function. Activation of renal vascular P2 receptors contributes to the maintenance of a stable glomerular capillary pressure, and preservation of glomerular hemodynamics. P2 receptor activation in the nephron inhibits sodium reabsorption facilitating enhanced sodium excretion and contributes to the control of blood pressure. Certainly, the P2 receptor system represents an important renal tubular and vascular regulatory system that is tightly integrated into other neural, endocrine and cardiovascular mechanisms to regulate extracellular fluid volume and composition and thereby contribute to the regulation of body fluid homeostasis and blood pressure. Better understanding of the influence of P2 purinoceptors on renal vascular and tubular function will be important for clarifying their role in regulating renal hemodynamics and tubular transport mechanisms, and may lead to better therapeutic interventions for the treatment of hypertension.
Highlights.
Renal distribution of P2 purinoceptors in vascular, glomerular and tubular structures.
P2 receptors are important for renal autoregulation, and for regulation of renal and glomerular hemodynamics.
P2 receptors regulate tubular function, sodium reabsorption, extracellular fluid volume and blood pressure.
Acknowledgments
The authors would like to acknowledge grant support from National Institutes of Health (DK-44628, HL-098135, HL-095499) for EW Inscho and the American Heart Association pre-doctoral fellowship (14PRE20460061) for JP Van Beusecum.
Footnotes
Financial disclosure and conflict of interest statements: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Vital signs: Awareness and treatment of uncontrolled hypertension among adults--united states, 2003–2010. Morbidity and Mortality Weekly Report. 2012;61:703–709. [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Guyton AG. Physiologic regulation of arterial pressure. The American Journal of Cardiology. 1961;8(3):401–407. doi: 10.1016/0002-9149(61)90159-x. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, Coleman TG. Quantitative analysis of the pathophysiology of hypertension. 1969. Journal of the American Society of Nephrology. 1999;10(10):2248–2258. [PubMed] [Google Scholar]
- 6.Guyton AC, Coleman TG, Granger HJ. Circulation: Overall regulation. Annual Review of Physiology. 1972;34:13–46. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 7.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annual Review of Physiology. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 8.Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: The current state of play. News in Physiological Sciences. 2003;18:237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- 9.Inscho EW. P2 receptors in regulation of renal microvascular function. American Journal of Physiology-Renal Physiology. 2001;280(6):F927–944. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- 10.Knight GE, Oliver-Redgate R, Burnstock G. Unusual absence of endothelium-dependent or -independent vasodilatation to purines or pyrimidines in the rat renal artery. Kidney International. 2003;64(4):1389–1397. doi: 10.1046/j.1523-1755.2003.00233.x. [DOI] [PubMed] [Google Scholar]
- 11.Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: An immunohistological study. Cells, Tissues, Organs. 2003;175(2):105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- 12•.Menzies RI, Unwin RJ, Dash RK, Beard DA, Cowley AW, Jr, Carlson BE, Mullins JJ, Bailey MA. Effect of P2X4 and P2X7 receptor antagonism on the pressure diuresis relationship in rats. Frontiers in Physiology. 2013;4:305. doi: 10.3389/fphys.2013.00305. Interesting study comparing pressure diuresis relationships in Lewis and F344 rats. The results demonstrate both a physiological and possibly a pathophysiological role for P2X7 for renal vascular and/or systemic vascular function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ. P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiology. 2004;96(3):p79–p90. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- 14.Vallon V, Rieg T. Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system. American Journal of Physiology Renal Physiology. 2011;301(3):F463–475. doi: 10.1152/ajprenal.00236.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey MA, Imbert-Teboul M, Turner C, Srai K, Burnstock G, Unwin RJ. Evidence for basolateral P2Y6 receptors along the rat proximal tubule: Functional and molecular characterization. Journal of the American Society of Nephrology. 2001;12(8):1640–1647. doi: 10.1681/ASN.V1281640. [DOI] [PubMed] [Google Scholar]
- 16.Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney International. 2000;58(5):1893–1901. doi: 10.1111/j.1523-1755.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 17•.Wildman SS. Emerging key roles for P2X receptors in the kidney. Frontiers in Physiology. 2013;4:262. doi: 10.3389/fphys.2013.00262. A recent review summarizing the current and novel evidence for the involvement for P2X receptors in the regulation of renal vascular and tubular function in normal physiology and pathophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y(2) purinoceptor in rat renal inner medulla and lung. American Journal of Physiology-Renal Physiology. 2000;278(1):F43–51. doi: 10.1152/ajprenal.2000.278.1.F43. [DOI] [PubMed] [Google Scholar]
- 19.Bailey MA, Hillman KA, Unwin RJ. P2 receptors in the kidney. Journal of the Autonomic Nervous System. 2000;81(1–3):264–270. doi: 10.1016/s0165-1838(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 20.Chan CM, Unwin RJ, Burnstock G. Potential functional roles of extracellular ATP in kidney and urinary tract. Experimental Nephrology. 1998;6(3):200–207. doi: 10.1159/000020524. [DOI] [PubMed] [Google Scholar]
- 21••.Burnstock G, Evans LC, Bailey MA. Purinergic signalling in the kidney in health and disease. Purinergic Signalling. 2014;10(1):71–101. doi: 10.1007/s11302-013-9400-5. A complete and comprehensive review on historical and recent studies and the current views on the wide variety of events mediated by receptors for purines and pyrimidines in physiology and pathophysiological conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Marques RD, de Bruijn PI, Sorensen MV, Bleich M, Praetorius HA, Leipziger J. Basolateral P2X receptors mediate inhibition of NaCl transport in mouse medullary thick ascending limb (mTAL) American Journal of Physiology-Renal Physiology. 2012;302(4):F487–494. doi: 10.1152/ajprenal.00570.2011. An interesting article investigating the role P2X1, P2X4, and P2X5 receptors play in cation transport inhibition in mouse medullary thick ascending limb. This study provides pharmacological, molecular and knockout mouse evidence that P2X4 is a novel role for an ionotropic receptor causing transport inhibition in renal tubular epithelium in mouse medullary thick ascending limb. [DOI] [PubMed] [Google Scholar]
- 23.Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. Journal of the American Society of Nephrology. 2008;19(4):731–742. doi: 10.1681/ASN.2007040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chabardès-Garonne D, Méjean A, Aude J-C, Cheval L, Di Stefano A, Gaillard M-C, Imbert-Teboul M, Wittner M, Balian C, Anthouard V, Robert C, et al. A panoramic view of gene expression in the human kidney. Proceedings of the National Academy of Sciences. 2003;100(23):13710–13715. doi: 10.1073/pnas.2234604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabolic I, Culic O, Lin SH, Brown D. Localization of ecto-ATPase in rat kidney and isolated renal cortical membrane vesicles. The American Journal of Physiology. 1992;262(2 Pt 2):F217–228. doi: 10.1152/ajprenal.1992.262.2.F217. [DOI] [PubMed] [Google Scholar]
- 26.Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Experimental Biology and Medicine (Maywood, NJ) 2007;232(6):715–726. [PubMed] [Google Scholar]
- 27•.Vallon V, Stockand J, Rieg T. P2Y receptors and kidney function. Wiley Interdisciplinary Reviews-Membrane Transport and Signaling. 2012;1(6):731–742. doi: 10.1002/wmts.61. A recent review on the ATP/UTP/P2Y receptor system and how this system mediates renal vascular hemodynamics and tubular transport throughout the nephron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osmond DA, Inscho EW. P2X(1) receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. American Journal of Physiology-Renal Physiology. 2010;298(6):F1360–1368. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. The Journal of Clinical Investigation. 2003;112(12):1895–1905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnermann J. Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice. American Journal of Physiology-Renal Physiology. 2011;300(2):F339–344. doi: 10.1152/ajprenal.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Menzies RI, Unwin RJ, Bailey MA. Renal P2 receptors and hypertension. Acta Physiologica (Oxford, England) 2014 doi: 10.1111/apha.12412. A review on the role of purinergic signaling in the renal control of blood pressure. This review integrates the effects of P2 receptors on vascular and tubular function when challenged with increases in renal perfusion pressure, specifically P2X7, as a potential therapeutic target in the treatment of hypertension. [DOI] [PubMed] [Google Scholar]
- 32.Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signalling. 2009;5(4):447–460. doi: 10.1007/s11302-009-9147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inscho EW, Cook AK, Mui V, Miller J. Direct assessment of renal microvascular responses to P2-purinoceptor agonists. The American Journal of Physiology. 1998;274(4 Pt 2):F718–727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- 34.Eltze M, Ullrich B. Characterization of vascular P2 purinoceptors in the rat isolated perfused kidney. European Journal of Pharmacology. 1996;306(1–3):139–152. doi: 10.1016/0014-2999(96)00244-0. [DOI] [PubMed] [Google Scholar]
- 35.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB Journal. 2007;21(13):3717–3726. doi: 10.1096/fj.07-8807com. [DOI] [PubMed] [Google Scholar]
- 36.Rieg T, Gerasimova M, Boyer JL, Insel PA, Vallon V. P2Y(2) receptor activation decreases blood pressure and increases renal Na(+) excretion. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2011;301(2):R510–518. doi: 10.1152/ajpregu.00148.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: What we know and what we need to find out. Clinical and Experimental Pharmacology & Physiology. 2005;32(5–6):400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 38••.Guan Z, Fellner RC, Beusecum JV, Inscho EW. P2 receptors in renal autoregulation. Current vascular pharmacology. 2014;12:818–828. doi: 10.2174/15701611113116660152. A recent review on the current views of P2 receptors and their role in renal autoregulatory resistance adjustments. This review integrates recent ideas regarding impaired autoregulatory responses in pathological settings in hypertension and inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey MA, Shirley DG. Effects of extracellular nucleotides on renal tubular solute transport. Purinergic Signalling. 2009;5(4):473–480. doi: 10.1007/s11302-009-9149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin W, Hopfer U. Purinergic-mediated inhibition of Na+-K+-ATPase in proximal tubule cells: Elevated cytosolic Ca2+ is not required. The American Journal of Physiology. 1997;272(4 Pt 1):C1169–1177. doi: 10.1152/ajpcell.1997.272.4.C1169. [DOI] [PubMed] [Google Scholar]
- 41.Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. American Journal of Physiology-Renal Physiology. 2004;287(4):F789–796. doi: 10.1152/ajprenal.00033.2004. [DOI] [PubMed] [Google Scholar]
- 42.Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: Clinical implications. Annual Review of Physiology. 2011;73:359–376. doi: 10.1146/annurev-physiol-012110-142247. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Sanchez D, Gorelik J, Klenerman D, Lab M, Edwards C, Korchev Y. Basolateral P2X4-like receptors regulate the extracellular ATP-stimulated epithelial Na+ channel activity in renal epithelia. American Journal of Physiology-Renal Physiology. 2007;292(6):F1734–1740. doi: 10.1152/ajprenal.00382.2006. [DOI] [PubMed] [Google Scholar]
- 44.van Baal J, Hoenderop JG, Groenendijk M, van Os CH, Bindels RJ, Willems PH. Hormone-stimulated Ca2+ transport in rabbit kidney: Multiple sites of inhibition by exogenous atp. The American Journal of Physiology. 1999;277(6 Pt 2):F899–906. doi: 10.1152/ajprenal.1999.277.6.F899. [DOI] [PubMed] [Google Scholar]
- 45.Cuffe JE, Bielfeld-Ackermann A, Thomas J, Leipziger J, Korbmacher C. ATP stimulates Cl- secretion and reduces amiloride-sensitive Na+ absorption in M-1 mouse cortical collecting duct cells. The Journal of Physiology. 2000;524(Pt 1):77–90. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas J, Deetjen P, Ko WH, Jacobi C, Leipziger J. P2Y(2) receptor-mediated inhibition of amiloride-sensitive short circuit current in M-1 mouse cortical collecting duct cells. The Journal of Membrane Biology. 2001;183(2):115–124. doi: 10.1007/s00232-001-0059-4. [DOI] [PubMed] [Google Scholar]
- 47.Koster HP, Hartog A, van Os CH, Bindels RJ. Inhibition of Na+ and Ca2+ reabsorption by P2u purinoceptors requires PKC but not Ca2+ signaling. The American Journal of Physiology. 1996;270(1 Pt 2):F53–60. doi: 10.1152/ajprenal.1996.270.1.F53. [DOI] [PubMed] [Google Scholar]
- 48.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. Journal of the American Society of Nephrology. 2009;20(8):1724–1732. doi: 10.1681/ASN.2008101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. The Journal of Biological Chemistry. 2008;283(52):36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Craigie E, Birch RE, Unwin RJ, Wildman SS. The relationship between P2X4 and P2X7: A physiologically important interaction? Frontiers in Physiology. 2013;4:216. doi: 10.3389/fphys.2013.00216. A recent review analyzing the current in vitro and in vivo studies and the interactions between P2X4 and P2X7 and their potential impact of these receptors on the regulation of renal function. [DOI] [PMC free article] [PubMed] [Google Scholar]