Abstract
Tibia fracture followed by limb immobilization in rats evokes nociceptive and vascular changes resembling complex regional pain syndrome type I (CRPS I). Previously we observed that substance P (SP) and interleukin-1β (IL-1β) signaling contribute to chronic regional nociceptive sensitization in this model. It is known that inflammasome multiprotein complexes containing caspase-1 and NALP1 are involved in the activation of the IL-1β family of pro-nociceptive cytokines expressed in skin and other tissues. Therefore, we hypothesized that SP activated inflammasomes might contribute to mechanical allodynia after fracture. Using this model we observed that: 1) inflammasome components and products NALP1, caspase-1, IL-1β and IL-18 were present in low levels in normal skin, but expression of all these was strongly up-regulated after fracture, 2) NALP1, caspase-1 and IL-1β were co-expressed in keratinocytes, and the number of NALP1, caspase-1, and IL-1β positive cells dramatically increased at 4 weeks post-fracture, 3) LY303870, an NK1 receptor antagonist, effectively blocked fracture induced up-regulation of activated inflammasome components and cytokines, 4) IL-1β and IL-18 intraplantar injection induced mechanical allodynia in normal rats, and 5) both a selective caspase-1 inhibitor and an IL-1 receptor antagonist attenuated fracture induced hind paw mechanical allodynia. Collectively, these data suggest that NALP1 containing inflammasomes activated by NK1 receptors are expressed in keratinocytes and contribute to post-traumatic regional nociceptive sensitization. These findings highlight the possible importance of neuro-cutaneous signaling and innate immunity mechanisms in the development of CRPS.
1. Introduction
Complex regional pain syndrome (CRPS) is a frequent consequence of distal tibia [43] and radius fractures[1]. Recently we described a distal tibia fracture model in rats that exhibits chronic unilateral hindlimb warmth, edema, facilitated protein extravasation, allodynia, unweighting, and periarticular osteoporosis, a constellation of nociceptive, vascular, and bone changes closely resembling CRPS type (CPRS I) [13]. Using this model we observed elevated levels of interleukin 1β (IL-1β) and other cytokines in the hindpaw skin of the fractured limb [39; 40; 53]. Consistent with the animal model, patients with CRPS also exhibit increased inflammatory cytokine levels in the affected limb[12; 16; 17; 33]. Importantly, the continuous administration of the IL-1 receptor antagonist (IL-1ra) anakinra reduced fracture-induced nociceptive sensitization in the rat fracture model[26]. In situ hybridization and immunostaining demonstrated that epidermal keratinocytes were the main source of IL-1β[26]. The mechanisms underlying the post-traumatic up-regulation of cutaneous cytokines remain unclear, however.
Inflammasomes are multiprotein complexes responsible for the activation of caspase-1, a protease required for the processing and activation of pro-inflammatory cytokines IL-1β, IL-18, and IL-33[7; 27; 29; 30; 36]. The first of these, IL-1β, is a particularly well studied human and animal algogen. To date, three different inflammasomes have been described, and they are defined by the NOD-like receptor (NLR) protein they contain: the NALP1(Nacht, leucine-rich repeat and pyrin domain containing protein) inflammasome, the NALP3 inflammasome, and the IPAF inflammasome. The NALP1 inflammasome, which was the first molecular platform to be identified, is relatively widely expressed and is composed of NALP1, an adaptor known as apoptosis-associated speck-like protein containing card (ASC), and caspase-1[8; 11]. The expression and role of inflammasomes and inflammatory caspases has been studied in both human and rodent skin, and the findings indicate possible key roles for these regulators in the pathogenesis of inflammatory skin diseases including contact hypersensitivity[50], psoriasis[20], vitiligo[19], and sunburn-associated skin inflammation[9]. Diverse ligands and stimuli, such as microbe components (RNA and muramyl dipeptide (MDP)), endogenous danger signals released from dying cells (gout-associated uric acid crystals)[31] and ATP[28], and low intracellular K+ [36] concentrations trigger activation of the NALP3 inflammasome. Much less is known regarding the mechanism of activation of the NALP1 inflammasome, though MDP and ATP may stimulate assembly of this protein complex [9; 36].
Based on our recent findings that: 1) administration a selective NK1 receptor antagonist (LY303870) partially reversed nociceptive and vascular changes observed after tibia fracture[13], 2) that fracture chronically increased the expression of NK1 receptors in keratinocytes in the injured limb and the expression of SP in the ipsilateral sciatic nerve[52], and 3) that SP can stimulate keratinocytes to produce IL-1β in vitro and in vivo[6; 42], we hypothesized that SP might regulate NALP1 inflammasome activity in this CRPS model. The demonstration of such a pathway would be entirely novel and would support the hypothesis that neuro-cutaneous signaling is important in CRPS I.
2. Materials and methods
These experiments were approved by our Institutional Animal Care and Use Committee and followed the animal subjects guidelines of the IASP[58]. Adult (9-month-old) male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) were used in all experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding and were fed and watered ad libitum. During the experimental period the animals were fed Lab Diet 5012 (PMI Nutrition Institute, Richmond, IN), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g of vitamin D3, and were kept under standard conditions with a 12-h light dark cycle.
2.1 Surgery
Tibia fracture was performed under 2-4% isoflurane to maintain surgical anesthesia as we have previously described [13]. The right hindlimb was wrapped in stockinet (2.5 cm wide) and the distal tibia was fractured using pliers with an adjustable stop (Visegrip, Petersen Manufacturing, Dewitt, NE) that had been modified with a 3-point jaw. The hindlimb wrapped in casting tape (Delta-Lite, Johnson & Johnson, Raynham, MA) so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. The cast over the paw was applied only to the plantar surface; a window was left open over the dorsum of the paw and ankle to prevent constriction when post-fracture edema developed. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh. The rats were given subcutaneous saline and buprenorphine immediately after procedure (0.03 mg/kg) and on the next day after fracture for post-operative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. All rats used in this study had union at the fracture site after 4 weeks of casting.
2.2 Homogenization procedure and enzyme immunoassay for IL-1β, IL-18, and IL-33
Rat hindpaw dorsal skin was collected at 4 weeks after fracture and frozen immediately on dry ice. All tissues were minced into fine pieces in ice-cold T-PER tissue protein extraction reagent (PIERCE, Rockford, IL), pH 7.6, containing freshly added a cocktail of protease inhibitors: aprotinin (2 μg/ml), leupeptin (5 μg/ml), pepstatin (0.7 μg/ml), and PMSF (100 μg/ml) (Boehringer Mannheim, Germany) followed by homogenization using a rotor/stator homogenizer. Homogenates were centrifuged for 20 min at 14,000g, +4 °C. Supernatants were transferred to fresh precooled Eppendorf tubes. Triton X-100 (Boehringer Mannheim, Germany) was added at a final concentration 0.01%. The samples were centrifuged again for 5 min at 14,000g at 4 °C. The supernatants were aliquoted, stored at –80 °C and assayed in duplicate (after dilution in the standard buffer supplied) for IL-1β (R&D Systems, Minneapolis, MN), IL-18 (Biosource International, Camarillo, CA), IL-33 ELISA kit (R&D Systems), and NGF (Promega, Madison, WI) according to the manufacturer’s instructions using the standards provided. The lower limits of detection of the levels IL-1β, IL-18, IL-33, and NGF were 5 pg/mL, 4 pg/mL, 2.8 pg/mL, and 3.9 pg/mL respectively. Positive and negative controls were included in each assay. The optical density of the reaction product was read on a microplate reader at 450 nm, and values were normalized per gram of tissue assayed. Protein content was determined by the bicinchoninic acid protein assay reagent (Pierce, KMF Laborchemie, St. Augustin, Germany).
2.3 Western blotting procedure
Rat hindpaw dorsal skin was homogenized in 56.8 mol/l Tris buffer (pH 6.8) with freshly added protease inhibitors, 1.8% (v/v) β-mercaptoethanol, 0.1% glycerol. The homogenate was centrifuged at 13,000 × g for 15 min at 4 °C. The supernatant was used for western blot analysis. Total protein concentration of the homogenate was measured using a Bio-Rad DC protein assay reagent (Bio-Rad, Hercules, CA). Equal amounts of protein (50 μg) were subjected to SDS-PAGE (10% Tris-HCl acrylamide gel) and electrotransferred onto a polyvinylidene difluorided membrane. The blots were blocked overnight with 5 % non-fat dry milk in Tris-buffered saline with 0.5% Tween-20 (TBST), incubated with primary antibody against specific proteins 1 hr on a rocking platform. We used the following primary antibodies: rabbit anti-NALP1 (Cell Signaling Technology, Danvers, MA), rabbit ant-NALP1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-caspase-1 (BioVision Research, Mountain View, CA), and β-actin (Santa Cruz Biotechnology). After washing in TBST three times, the blot was incubated with anti-rabbit or anti-goat HRP-conjugated secondary antibody for 1 h at room temperature, and washed again, then incubated in ECL plus chemiluminescence reagents (Amersham, Piscataway, NJ) and visualized by PhosphoImager (Typhoon, GE Healthcare) and the band intensity was analyzed using ImageQuant 5.2 software (Molecular Dynamics, Piscataway, NJ). The specific protein/β-actin band intensity ratio represents the change of the specific protein after fracture. To detect β-actin expression, we stripped the primary antibody from the polyvinylidene difluorided membrane and re-probed the same blots with a second antibody using the same procedure used for the first antibody.
2.4 Tissue processing and immunofluorescence confocal microscopy
At four weeks post-fracture animals were euthanized and perfused with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS), pH 7.4, via the ascending aorta; the dorsal hindpaw skin including sub-dermal layers was immediately removed and post-fixed in 4% PFA for 2 hours, then the tissues were treated with 30% sucrose in PBS at 4°C before embedding in OCT. Following embedding, 8-μm thick slices were made using a cryostat, mounted onto Superfrost microscope slides (Fisher Scientific), and stored at -70°C.
Frozen sections were permeabilized and blocked with PBS containing 10% donkey serum and 0.3% Triton X-100, followed by exposure to the primary antibodies overnight at 4°C in PBS containing 2% serum. Upon detection of the first antigen, primary antibody from a different species against the second antigen was applied to the sections and visualized using an alternative fluorophore-conjugated secondary antibody. Sections were then rinsed in PBS and incubated with fluorophore-conjugated secondary antibodies against the immunoglobulin of the species from which the primary antibody was generated. After three washes, the sections were mounted with anti-fade mounting medium (Invitrogen). Images were obtained using confocal microscopy (Zeiss LSM/510 Upright 2 photon; Carl Zeiss) and stored on digital media. With regard to primary antibodies, rabbit anti rat NALP1, 1:100 (Cell Signaling Technology), rabbit anti rat caspase-1, 1:200 (Thermo Fisher Scientific), goat anti-rat IL-1β, 1:100 (R&D Systems), and monoclonal mouse anti-rat keratin, Pan Ab-1, 1:50 (clone AE1/AE3) (Thermo Fisher Scientific) were used. Double and triple labeling immunofluorescence was performed with donkey anti-mouse IgG (1:300) conjugated with Dylight 549, donkey anti-rabbit IgG (1:500) conjugated with Dylight 488 secondary antibodies, donkey anti-goat IgG (1:500) conjugated with fluorescein (FITC) secondary antibodies (Jackson ImmunoResearch Laboratories), incubated with respective primary antibodies. Control experiments included incubation of slices in primary and secondary antibody-free solutions both of which led to low intensity non-specific staining patterns in preliminary experiments.
2.5 Drug treatments
To test the hypothesis that SP signaling might regulate NALP1 inflammasome activity in the CRPS model, fracture rats were treated with either an NK-1 receptor antagonist LY303870 (Eli Lilly Co) at a dose of 20 mg/kg/day i.p. (n=7) or saline (n=8) for 8 days before euthanization. The dose was chosen on the basis of our previous studies [14].
To test the hypothesis that NALP1 inflammasome signaling mediates the vascular and nociceptive changes observed after tibia fracture in rats, a specific caspase-1 inhibitor (ac-YVAD-cmk (Calbiochem, Gibbstown, NJ) was applied locally by intraplantar injection at 4 weeks post-fracture. The inhibitor was diluted in DMSO according to manufacturer’s instruction then further diluted in sterile 0.45% NaCl immediately before the injection at a dose of 400μg/100μl (containing 8% DMSO, n=9), chosen on the basis of our preliminary studies. Controls were another set of fracture rats injected with vehicle (n=8).
To test the hypothesis that IL-1β mediates the nociceptive changes observed after tibia fracture in rats, anakinra IL-1 receptor antagonist (r-metHuIL-1ra, Kineret, Amgen) was injected once i.p. at a dose of 100 mg/kg (n=12) based on previous studies using IL-1ra in rodents [56]. In the control group, fracture rats (n=8) were injected equal amount of vehicle.
Mechanical nociceptive responses to intraplantar injection of IL-1β (Sigma) were evaluated in control rats. The cytokine was diluted in sterile PBS and injected at three different doses (10pg/50μl, 100pg/50μl, and 1000pg/50μl, n=6 per injection cohort), chosen on the basis of previous studies demonstrating hyperalgesic effects [26]. A similar study was performed using rat recombinant IL-18 (Sigma), which was diluted in sterile PBS and injected in three different doses (1ng/50μl, 10ng/50μl, and 50ng/50μl, n=6 per cohort) chosen on the basis of preliminary studies.
2.6. Hindpaw nociception
To measure mechanical allodynia in the rats an up–down von Frey testing paradigm was used as we have previously described[13; 21]. Rats were placed in a clear plastic cylinder (20 cm in diameter) with a wire mesh bottom and allowed to acclimate for 15 min. The paw was tested with a series of 8 von Frey hairs ranging in stiffness from 0.41 to 15.14 g. The von Frey hair was applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads. The fiber was pushed until it slightly bowed and then it was jiggled in that position for 6s. Stimuli were presented at an interval of several seconds. Hindpaw withdrawal from the fiber was considered a positive response. The initial fiber presentation was 2.1 g and the fibers were presented according to the up–down method of Dixon to generate six responses in the immediate vicinity of the 50% threshold. Stimuli were presented at intervals of several seconds.
2.7. Hindpaw volume
A laser sensor technique was used to determine the dorsal-ventral thickness of the hindpaw, as we have previously described [13; 14; 21]. Before baseline testing the bilateral hindpaws were tattooed with a 2–3 mm spot on the dorsal skin over the midpoint of the third metatarsal. For laser measurements each rat was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. The paw was gently held flat on the table with a small metal rod applied to the top of the ankle joint. Using optical triangulation, a laser with a distance measuring sensor was used to determine the distance to the table top and to the top of the hindpaw at the tattoo site and the difference was used to calculate the dorsal–ventral paw thickness. The measurement sensor device used in these experiments (4381 Precicura, Limab) has a measurement range of 200 mm with a 0.01 mm resolution.
2.8. Hindpaw temperature
The room temperature was maintained at 23°C and humidity ranged between 25% and 45%. The temperature of the hindpaw was measured using a fine wire thermocouple (Omega) applied to the paw skin, as previously described[13; 14; 21]. The investigator held the thermistor wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw; the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature.
2.9 Statistical analysis
Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by post hoc Newman-Keuls multiple comparison testing to compare fracture control and caspase-1 inhibitor and IL1-ra treated fracture rats. Behavioral data collected over time after intraplantar injections of IL-1β / IL-18 were analyzed by a repeated measures ANOVA on data for each test time point, comparing various treatment groups (IL-1β/IL-18 doses). A Bonferroni test was used to determine the source of differences between IL-1β/IL-18 doses. For simple comparisons of two means, two-tailed t-testing was performed. All data are presented as the mean ± SE of the mean, and differences are considered significant at a p value less than 0.05 (Prism 4, GraphPad Software).
3. Results
3.1. Tibia fracture chronically increased NALP1 inflammasome levels in hindpaw skin
We previously observed an increase in IL-1β mRNA and protein expression levels in ipsilateral hindpaw skin 4 weeks post-fracture in the tibia fracture CRPS model[39; 40; 53], and that this cytokine serves to support nociceptive sensitization[26]. Since IL-1 β is synthesized in a biologically inactive pro-form, the maturation of IL-1β requires processing by inflammasome associated caspase-1. Therefore we postulated that the NALP1 inflammasome may be activated by fracture.
To test this hypothesis, hindpaw skin from fracture and intact rats were homogenized and subjected to western blot analysis. Figure 1 demonstrates that 4 weeks after distal tibia fracture mature NALP1 and caspase-1 are strongly up-regulated. In these studies two NALP1 isoforms with apparent molecular sizes of 165kDa and 70 kDa were detectable, but expressed in very low levels in control hindpaw skin (Fig. 1A). NALP1 is known to be expressed in these long and short forms both of which have biological activity [22]. After fracture the 165kDa isoform protein levels decreased significantly, but the 70-kDa isoform increased 5-fold in the skin of the fractured limb (Fig 1A, B).
Figure 1. Changes in hind paw skin inflammasome component protein levels after fracture.
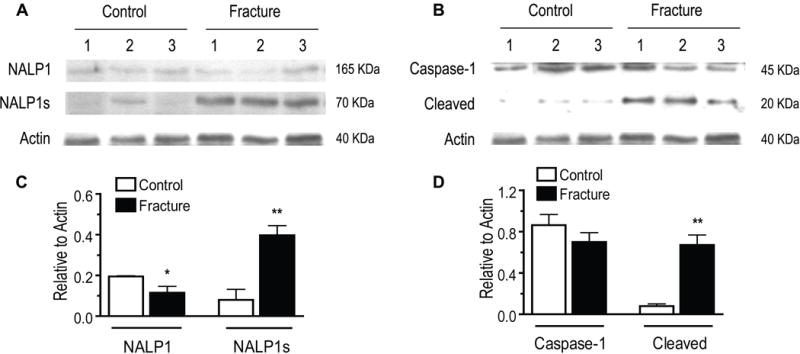
At 4 weeks post-fracture there was a reduction in full-length NALP1 (165kDa) protein levels in hindpaw skin, as measured by western blot. Conversely, the short form of NALP1 (NALPs, 70 kDa) and active cleaved fragments caspase-1 (20 kDa) were up-regulated 5-fold and 8-fold, respectively, after fracture. Panels A and C show western band appearances and scanned densities for full length NALP1 and short form (NALP1s) for samples from control and fracture groups (n=4/group). Panels B and D provide band appearances and quantification for full length and cleaved caspase-1 in the same samples (n=4/group). *p<0.05, **p<0.01 for fracture vs control values.
Caspase-1 is expressed as a proenzyme of 45 kDa, which binds to NALP1 and is activated to form large (20kDa, p20) and small (10kDa, p10) subunits [11; 45]. Although we found that the procaspase-1 levels in fracture skin did not significantly differ from control, the levels of the activated p20 fragment were 8-fold higher in ipsilateral hindpaw skin at 4 weeks post-fracture (Fig. 1C, D).
3.2. Keratinocytes express NALP1 inflammasome proteins after fracture
Since the keratinocyte is a major cellular source of IL-1β in hindpaw skin 4 weeks post-fracture[26], we determined if these cells specifically up-regulate NALP1 and caspase-1 expression. First, keratinocytes in the epidermis were stained using Pan-Ab1 antigen, a keratin marker, and co-stained with an antibody against rat NALP1. Only weak NALP1 imunoreactivity was detected in a few keratinocytes in control hindpaw skin (Fig. 2A), while strong staining for NALP1 was seen in many keratinocytes across multiple layers of the epidermis 4 weeks after fracture. After fracture most of the NALP1 labeled cells were distributed in the deeper epidermal layers (Fig. 2), which co-labeled for keratin including the stratum basal layer where new keratinocytes are generated. Fig. 2B illustrates the 7-fold increase in NALP1 positive cells in ipsilateral hindpaw skin after fracture compared with controls. Similar to NALP1 expression, caspase-1 protein was also expressed at very low basal levels in the kertinocytes in normal skin (Fig. 3 upper panels), whereas at 4 weeks post-fracture there was a dramatic increase in caspase-1 immunoreactivity in the epidermal keratinocytes (Fig. 3 middle panel).
Figure 2. Co-immunostaining for NALP1 protein (green) and keratin (red, a keratinocyte marker) in the hindpaw skin 4 weeks post-fracture with and without LY303870 treatment.
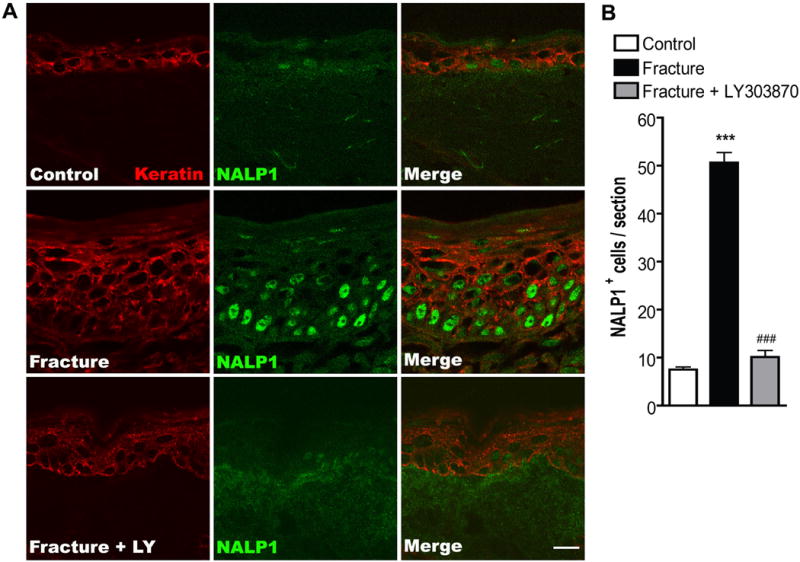
(A) Top panels are images from a normal control rat, middle panels are from fracture rat, and lower panels are from a fracture rat treated with a Substance P NK1 receptor antagonist (LY303870). Fracture increased keratinocyte proliferation and expression of NALP1 protein and LY303870 treatment prevented this increase. Scale bar = 10 μm. (B) There was a 7-fold increase in NALP1 positive cells in the epidermis at 4 weeks post-fracture and this increase was blocked in fracture rats treated with LY303870. ***P<0.001 for fracture (n=8) vs control (n=8) values, ###P<0.001 for fracture (n=8) vs fracture + LY303870 (n=7).
Figure 3. Representative fluorescence photomicrographs of caspase-1 protein (green) and keratin (red) in the dorsal hindpaw skin at 4 weeks post-fracture.
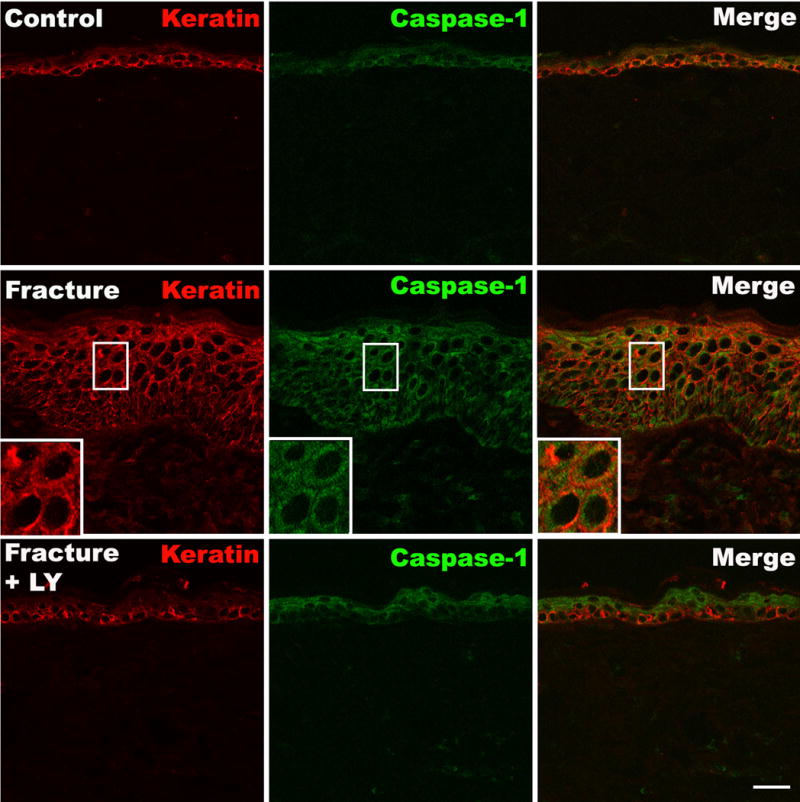
Upper panels show minimal caspase-1 immunostaining in normal epidermal keratinocytes. Middle panels show a dramatic increase in caspase-1 immunostaining in keratinocytes after fracture. Lower panels show that LY303870 administration blocked the fracture induced increase in caspase-1 expression. Scale bar=20μm.
3.3. NALP1, IL-1β, and caspase-1 were co-induced in keratinocytes 4 weeks after fracture
Skin sections were incubated with an antibody directed against the rat keratin Pan-Ab1 antigen and co-stained with antibodies against NALP1, IL-1β, or caspase-1. We observed that some control skin epidermal keratinocytes co-expressed low levels of NALP1 and IL-1β. In contrast, at 4 weeks post-fracture robust NALP1 and IL-1β co-staining was observed in keratinocytes throughout the epidermis (Fig. 4). Extracellular IL-1β imunoreactivity was also detected, suggesting that IL-1β was being released by keratinocytes. Similarly, caspase-1, IL-1β, and keratin triple labeling revealed that epidermal keratinocytes in control animals co-expressed low levels of caspase-1 and IL-1β, while after fracture high levels of caspase-1 and IL-1β immunoreactivity were co-localized in keratinocytes across the epidermis (Fig. 5).
Figure 4. Fluorescence photomicrographs of keratin (red), IL-1β (green) and NALP1 (blue) immunostaining in hindpaw skin at 4 weeks post-fracture.
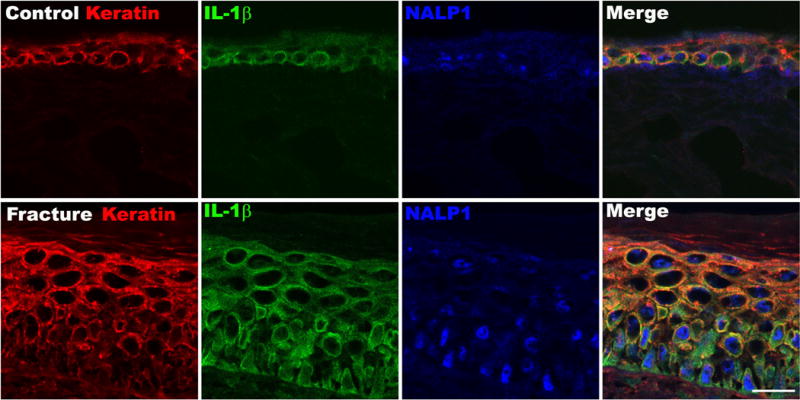
Top panels are from a representative normal control rat, bottom panels are from a fracture rat. Triple labeling demonstrates an increased co-expression of the NALP1 inflammasome component and the pro-inflammatory cytokine IL-1β in keratinocytes after fracture. Scale bar=25μm.
Figure 5. Fluorescence photomicrographs of keratin (red), caspase-1 (green) and IL-1β (blue) immunostaining in the hindpaw plantar skin at 4 weeks post-fracture.
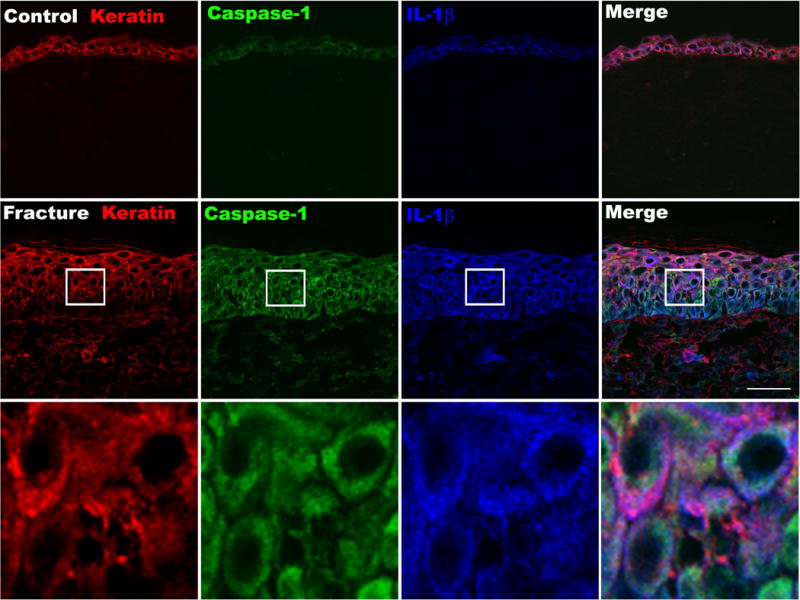
Top panels are from a normal control rat, middle panels are from a fracture rat, and the lower panels are the enlargements of the box regions. Triple labeling demonstrates the increased co-expression of the caspase-1 inflammasome component and IL-1β in the epidermis after fracture. Scale bar=35μm.
3.4. Tibia fracture increased expression of IL-1β, IL-18, and NGF in hindpaw skin
IL-1β, IL-18, and IL-33 are all synthesized as biologically inactive precursor molecules inside cells, and active inflammasome-associated caspase-1 is capable of cleaving them into their biologically active mature forms. Using ELISA analysis we measured significant increases in IL-1β and IL-18 in ipsilateral hindpaw skin at 4 weeks after fracture; IL-1β and IL-18 were increased 3-fold and 2-fold respectively in the fracture group compared to controls (Fig. 6A and B). Although a similar upward trend was observed for IL-33 levels after fracture, but this failed to reach significance (Fig. 6C).
Figure 6. Skin IL-1 family cytokine levels after fracture.
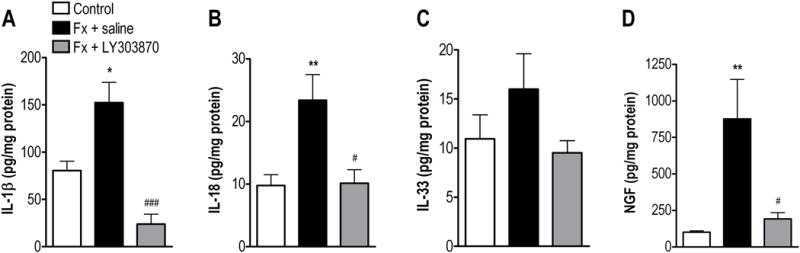
IL-1β (A), IL-18 (B), IL-33 (C) and NGF (D) protein levels all increased in hindpaw skin at 4 weeks post-fracture but the increase in IL-33 did not reach significance. Systemic treatment with the SP NK1 receptor antagonist LY303870 for 8 days blocked the increased hindpaw skin expression of IL-1β, IL-18, and NGF after fracture. *p<0.05, **p<0.01 for fracture (n=8) vs control values (n=8). ###P<0.001, #p<0.05 for fracture (n=8) vs fracture+LY303870 (n=7).
As IL-1β can act as an intermediate inflammatory mediator serving to up-regulate NGF expression [26; 38; 41; 46] and NGF contributes to the nociceptive sensitization in the fracture model [40], hindpaw skin levels of this neurotrophin were also assessed by ELISA. Figure 6D illustrates an 8-fold increase in hindpaw NGF levels at 4 weeks after fracture.
3.5. SP signaling mediates the post-fracture increase in NALP1 inflammasome proteins
To test whether peripheral SP signaling via NK1 receptor activation mediates the post-fracture increase in NALP1 inflammasome protein levels in the CRPS model, fracture animals were chronically treated with the selective NK1 receptor antagonist LY303870 for 8 days. Fig.2A (lower panel) shows representative confocal immunofluorescence microscopy results for NALP1 in keratinocytes in dorsal hind paw skin from LY303870 treated fracture animals, and Fig. 2B demonstrates quantification of NALP1-positive cells in the hindpaw epidermis. These results demonstrate that LY303870 effectively blocked fracture-induced NALP1 protein expression. LY303870 also markedly reduced fracture induced increases in caspase-1 protein expression (Fig. 3 lower panels). Finally, LY303870 treatment completely reversed the fracture-induced increases in IL-1β, IL-18, and NGF levels as measured by ELISA in hindpaw skin homogenates (Fig. 6).
3.6. Intraplantar IL-1β and IL-18 dose-dependently induced mechanical allodynia in intact rats
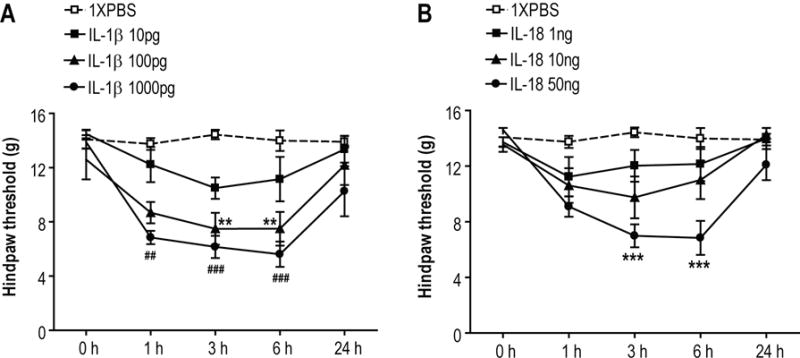
The nociceptive and vascular effects of IL-1β and IL-18 injections were examined in normal intact rats. Intraplantar IL-1β injection dose-dependently induced prolonged mechanical allodynia lasting between 1 and 6 h after drug administration at doses between 100 and 1000 pg (Fig.7A). IL-18 injection also dose-dependently induced prolonged mechanical allodynia lasting between 3 and 6 h after drug administration, although the apparent potency of this cytokine was approximately 500-fold lower (Fig.7B).
Figure 7. Cytokine induced allodynia in rat hind paws.
Intraplantar injection of IL-1β (n = 6 per injection cohort) and IL-18 (n = 6 per injection cohort) in normal control rats. (A) IL-1 (100 and 1000 pg) reduced von Frey mechanical nociceptive thresholds at 1, 3, and 6 h after injection. (B) Only the highest dose of IL-18 (50ng) reduced von Frey thresholds at 3 and 6 h. **p<0.01, ***p<0.001 for drug vs vehicle threshold values
3.7. Caspase-1 inhibition and IL-1ra attenuated fracture induced nociceptive behavior
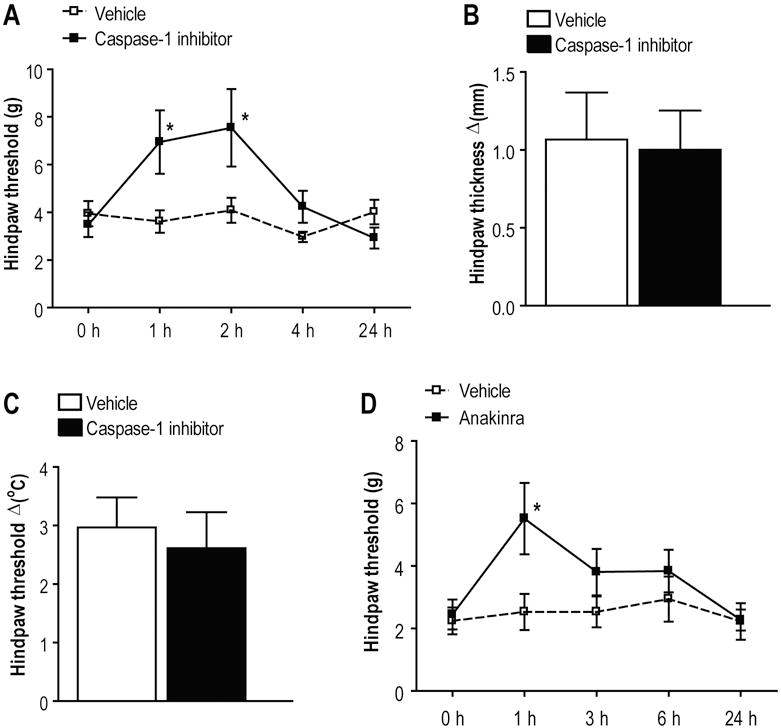
Based on these data we postulate that tibia fracture in rats evokes NALP1 inflammasome activation leading to excessive expression of the pro-nociceptive cytokines IL-1β and IL-18 that contribute to the nociceptive sensitization observed in the fracture model. To test this hypothesis a specific caspase-1 inhibitor (ac-YVAD-cmk) was administered locally by intraplantar injection to fracture rats at 4 weeks post-injury. Figure 8A illustrates that caspase-1 inhibitor treatment attenuated fracture induced local mechanical allodynia at 1 and 2 h after injection. There was no ac-YVAD-cmk treatment effect on hindpaw edema or warmth in the fracture rats after these local injections (Figs. 8B, C). Consistent with the caspase-1 inhibitor findings, administration of an IL-1β receptor antagonist (IL-1ra) also reduced mechanical allodynia transiently after injection (Figure 8D). Neither caspase-1 inhibitor application nor IL-1ra treatment had any effect on the nociceptive thresholds of control animals or the hindpaws of limbs contralateral to fracture (data not shown).
Figure 8. Pharmacological inhibition of IL-1β production and signaling.
(A,B,C) Intraplantar administration of a caspase-1 inhibitor (ac-YVAD-cmk) reduced post-fracture von Frey mechanical allodynia, but had no effect on hindpaw warmth and edema (D) Intraperitoneal administration of a IL-1β receptor antagonist (IL-1ra, anakinra) also reduced hindpaw mechanical allodynia in fracture rats. *p<0.05 for drug (n=9) vs vehicle (n=8) threshold values
4. Discussion
A growing body of clinical and preclinical evidence points to peripherally generated cytokines as supporting CRPS I, particularly during the acute phases of the syndrome. Moreover, there is compelling evidence indicating that the IL-1 family of cytokines, especially IL-1β, is involved in the modulation of nociceptive information[38; 47; 48]. IL-1β can act both directly on neurons or indirectly as an intermediate inflammatory mediator serving to up-regulate NGF and other cytokines [3; 26; 38; 41; 46]. Recently, we described a tibia fracture model in rats that exhibits chronic unilateral hindlimb allodynia, unweighting, warmth, edema, and periarticular osteoporosis, a symptom complex closely resembling CRPS [13]. We first observed that SP signaling through NK1 receptors contributes to the development of post-traumatic inflammation and pain in this model [13; 52]. Later it was shown that elevated levels of IL-1β and other inflammatory mediators are present in the hindpaw skin including epidermal keratinocytes of the fractured limb [39; 40; 53], and that IL-1β signaling contributes to nociceptive sensitization after tibia fracture either directly or by stimulating NGF expression[26]. We therefore pursued the hypothesis that post-traumatic facilitation of neuro-cutaneous signaling activates inflammasomes in keratinocytes, thus inducing IL-1β production and nociceptive sensitization after tibia fracture.
Inflammasomes are intracellular multi-protein complexes which are activated under a variety of circumstances. Our emerging understanding of inflammasome function is that they are important components of the innate immune system[5; 18; 29; 30; 35; 36]. In this study we investigated possible mechanisms of NALP1 inflammasome activation leading to elevated IL-1β family cytokine production in skin and their role in chronic nociceptive sensitization after fracture. The data presented here provide evidence for the first time that rat keratinocytes contain key components of the NALP1 inflammasome, and dysregulated activation of this inflammasome elicited by fracture may result in aberrant release of IL-1β, IL-18 and, through an indirect mechanism, NGF. These substances subsequently support nociceptive sensitization characteristic of the rat fracture CRPS I model.
Assembly of the inflammasome depends on NOD-like receptor family members such as NALPs. The NALPs serve as sensors and scaffolding proteins that activate caspase-1, thus promoting IL-1β maturation[4; 5; 18; 27]. In this study, we were able to show that only a few keratinocytes in normal rat skin express NALP1. Protein levels of the 70 kDa short form of NALP1 were increased dramatically in the hindpaw skin after fracture (Figs. 1A, C) and the number of keratinocytes expressing strong NALP1 immunoreactivity increased as well (Fig. 2). Moreover, our immunohistochemical data show that NALP1, caspase-1 and the key active product of inflammasome activity IL-1β are co-expressed in keratinocytes after fracture (Fig. 4).
It has been demonstrated that IL-1β and IL-18 are synthesized as inactive pro-forms in tissues, and their activation requires the cysteine protease caspase-1, a key component of the NALP1 inflammasome. After cleavage of their proforms by caspase-1, these cytokines become biologically active and are subsequently secreted from the cells in which they are produced [57]. Using western blots we detected little caspase-1 in control skin consistent with previous studies on unperturbed tissues [32; 37]. However fracture skin contained an 8-fold increase level of cleaved caspase-1 after fracture compared to control skin. Furthermore, after fracture caspase-1 was co-expressed with IL-1β in the epidermal keratinocytes of the hindpaw skin (Fig. 5). Additional pharmacological data demonstrated that both caspase-1 and IL-1 receptor are required for nociceptive sensitization in this CRPS-I model. Taken together our results demonstrate that both the sensing (NALP1) and enzymatic (caspase-1) portions of the NALP1 inflammasome are up-regulated after fracture, and that the mature IL-1β produced supports the allodynia measured in the rat fracture model of CRPS I.
One key question is how the keratinocyte’s inflammasomes become activated after fracture. While common causes of inflammation like infection and severe tissue trauma are not generally present in CRPS, facilitated neurogenic signaling and inflammation are frequently observed in CRPS patients[24; 34; 51]. After tibia fracture enhanced SP signaling contributes to the development of chronic nociceptive and vascular abnormalities in rats[13; 14]. We recently observed fracture enhanced SP expression in sciatic nerve and NK1 receptor expression in keratinocytes[52]. Our data present here using the selective NK1 receptor antagonist LY303870 suggest that SP signaling is required for NALP1, caspase-1, IL-1β, IL-18, and NGF up-regulation after fracture. It is true that the systemic administration of LY303870 does not allow us to conclude with certainty that local hindpaw NK1 receptors are the only population of NK1 receptors supporting peripheral inflammatory changes. However, our group has reported previously that intraplantar application of SP can induce IL-1β production and cause allodynia[42]. Others have reported that SP is able to up-regulate the production of IL-1β in murine and human keratinocytes in vitro[6; 49]. Therefore, it is possible that the enhanced SP signaling seen in human CRPS and our rat fracture model of CRPS serves to directly stimulate inflammasome activity in keratinocytes and ultimately cytokine production and pain. Our model for SP activation of inflammasome activity and subsequent cytokine production is provided as in figure 9.
Figure 9. Proposed model of SP-induced keratinocyte NALP1 inflammasome activation, cytokine production and cutaneous sensory neuron sensitization in a rat fracture model of CRPS.
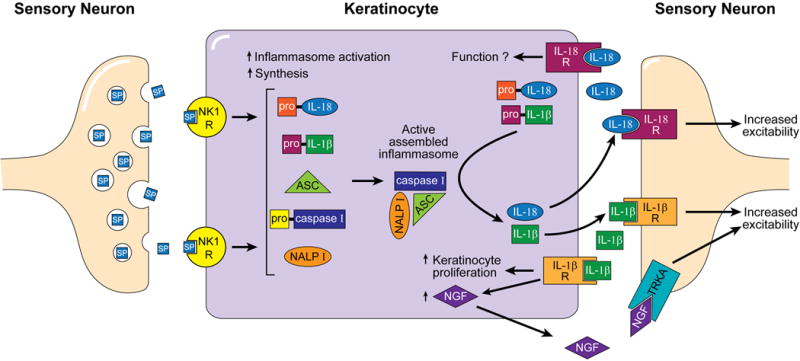
Activation of nociceptors after fracture leads to the increased neural expression SP and up-regulation of the SP NK1 receptor in cutaneous keratinocytes. This facilitated SP signal triggers NALP1 inflammasome activation and assembly in keratinocytes. Active assembled inflammasomes cleave pro-IL-1β and pro-IL-18 to form the pro-inflammatory cytokines IL-1β and IL-18. Active IL-1β and IL-18 are secreted from keratinocytes in which they are produced and can then activate their respective receptors that are expressed on the surface of keratinocytes. IL-1β receptor activation on keratinoctytes stimulates proliferation and NGF production and release. These pro-inflammatory mediators (IL-1β, IL-18, and NGF) also bind to their respective receptors (i.e., IL-1β, IL-18, TrkA receptors) expressed on cutaneous sensory neurons, resulting in nociceptive sensitization.
It should be noted that several studies have attempted to measure cytokine levels in the skin of patients affected by CRPS I. For example, using a suction blister technique, Heijmans-Antonissen et al.[15] measured levels of 25 mediators in blister fluid. In these experiments IL-1β was essentially undetectable though IL-6 and TNFα were abundant. These data were in keeping with other reports using similar methodology [16; 17]. Furthermore, while elevated levels of cytokines like IL-6 and TNFα have been identified in the skin of CRPS patients in a reproducible way, the absolute levels of these mediators correlate poorly with disease status [54; 55]. On the other hand, the typical duration of disease in the human studies was several months to years, certainly a more chronic situation than is modeled in the rats. While it is possible that fundamentally different mechanisms support sensitization in human CRPS compared to the rat model, we would suggest that an equally plausible situation is one involving a transition of supporting mediators as the disease becomes more chronic. Thus acute mechanisms (and acutely effective treatments) may be different from more chronic mechanisms and successful treatments.
Lastly, while SP/NK1 mediated activation of cutaneous inflammasomes may be a novel observation, there are other data suggesting that the central and peripheral nervous systems can control peripheral inflammation. For example, two recent reports provided evidence that glia and possibly neurons in the CNS control arthritis through signaling mechanisms involving p38, TNFα and Il-1β[2; 10]. Additional data provided by Sorkin et al. demonstrated that the activation of CNS adenosine receptors could reduce peripheral inflammation and neutrophil accumulation after carrageenan injection [44]. Our fracture CRPS model is essentially devoid of neutrophil accumulation in the sensitized skin, however[53]. Other studies have shown that the peripheral sympathetic nervous system supports arthritis in animal models as well as small capsaicin-sensitive afferent peripheral nerve fibers[25]. Adding to this body of evidence are the studies of Kyrkanides et al. who demonstrated that the over expression of the mu opioid receptor (MOR) on primary sensory neurons could block the full expression of arthritis in rat temperomandibular joints[23], presumably by reducing overall neuronal activity. In contrast to the aforementioned studies, however, our studies have identified a specific peripheral mechanism of inflammation not normally associated with CRPS to explain at least part of the nociceptive sensitization seen after tibial fracture.
Collectively, these data suggest that interleukin processing inflammasomes containing NALP1 and caspase-1 in keratinocytes are part of an important mechanism underlying chronic regional nociceptive sensitization after fracture. The release of SP from primary afferent nerve terminals acting through NK-1 receptors is part of the mechanism of activation. This inflammasome activity may not, however, support all manifestations of CRPS such as limb warmth and edema. Still, controlling inflammasomes and associated signaling pathways provide novel avenues for new therapeutic approaches for CRPS. Largely unresolved at this point is the mechanism whereby initial distant tissue injury leads to the activation of inflammasomes in skin not involved in the initial injury. Furthermore the events leading from keratinocyte NK1 receptor activation to stimulation of NALP1 and caspase-1 synthesis, maturation and assembly are unclear. Thus while the existence of a neuro-cutaneous mechanism supporting CRPS appears plausible, further investigation is required to fully map the signaling pathways that generate chronic post-traumatic inflammation and pain.
Acknowledgments
This work was funded by Department of Veteran Affairs, Veterans Health Administration, Rehabilitation Research and Development Service grant F4516I and NIH award GM079126. We would like to thank Amgen (Thousand Oaks, CA) for generously providing the IL-1ra used in these experiments. Also we thank Dr. Frances Davies for her critical reading of the manuscript.
ABREVIATIONS
- SP
Substance P
- NK1R
Substance P NK1 receptor
- IL-1β
interleukin-1β
- IL-1βR
interleukin-1β receptor
- IL-18
interleukin 18
- IL-18R
interleukin 18 receptor
- NALP
Nacht leucine-rich repeat and pyrin domain containing protein
- ASC
caspase-activating recruitment domain
- NGF
nerve growth factor
- TRKA
high affinity neurotropin receptor
Footnotes
The authors do not have financial or other relationships that might lead to conflict of interest.
References
- 1.Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after Colles’ fracture. J Bone Joint Surg Br. 1990;72(1):105–110. doi: 10.1302/0301-620X.72B1.2298766. [DOI] [PubMed] [Google Scholar]
- 2.Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3(9):e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283(38):25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carneiro LA, Magalhaes JG, Tattoli I, Philpott DJ, Travassos LH. Nod-like proteins in inflammation and disease. J Pathol. 2008;214(2):136–148. doi: 10.1002/path.2271. [DOI] [PubMed] [Google Scholar]
- 5.Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4(1):34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- 6.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40(4):251–263. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 8.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279(21):21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 9.Faustin B, Reed JC. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008;18(1):4–8. doi: 10.1016/j.tcb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino PM, Tallents RH, Miller JN, Brouxhon SM, O’Banion MK, Puzas JE, Kyrkanides S. Spinal interleukin-1beta in a mouse model of arthritis and joint pain. Arthritis Rheum. 2008;58(10):3100–3109. doi: 10.1002/art.23866. [DOI] [PubMed] [Google Scholar]
- 11.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1–2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121(1–2):158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Heijmans-Antonissen C, Wesseldijk F, Munnikes RJ, Huygen FJ, van der Meijden P, Hop WC, Hooijkaas H, Zijlstra FJ. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;2006(1):28398. doi: 10.1155/MI/2006/28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11(1):47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunol Lett. 2004;91(2–3):147–154. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Iversen L, Johansen C. Inflammasomes and inflammatory caspases in skin inflammation. Expert Rev Mol Diagn. 2008;8(6):697–705. doi: 10.1586/14737159.8.6.697. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 20.Johansen C, Moeller K, Kragballe K, Iversen L. The activity of caspase-1 is increased in lesional psoriatic epidermis. J Invest Dermatol. 2007;127(12):2857–2864. doi: 10.1038/sj.jid.5700922. [DOI] [PubMed] [Google Scholar]
- 21.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104(1–2):75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 22.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55(5):443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kyrkanides S, Fiorentino PM, Miller JN, Gan Y, Lai YC, Shaftel SS, Puzas JE, Piancino MG, O’Banion MK, Tallents RH. Amelioration of pain and histopathologic joint abnormalities in the Col1-IL-1beta(XAT) mouse model of arthritis by intraarticular induction of mu-opioid receptor into the temporomandibular joint. Arthritis Rheum. 2007;56(6):2038–2048. doi: 10.1002/art.22635. [DOI] [PubMed] [Google Scholar]
- 24.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183(1):197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 25.Levine JD, Dardick SJ, Roizen MF, Helms C, Basbaum AI. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li WW, Sabsovich I, Guo TZ, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144(3):303–313. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7(1):31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 28.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 29.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 30.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 31.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani H, Black R, Kupper TS. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta. Different strategies of IL-1 production and processing in monocytes and keratinocytes. J Clin Invest. 1991;87(3):1066–1071. doi: 10.1172/JCI115067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators Inflamm. 2005;2005(6):366–372. doi: 10.1155/MI.2005.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyen WJ, Arntz IE, Claessens RM, Van der Meer JW, Corstens FH, Goris RJ. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response? Pain. 1993;55(2):151–157. doi: 10.1016/0304-3959(93)90144-E. [DOI] [PubMed] [Google Scholar]
- 35.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21(1):10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19(6):615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Raymond AA, Mechin MC, Nachat R, Toulza E, Tazi-Ahnini R, Serre G, Simon M. Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. Br J Dermatol. 2007;156(3):420–427. doi: 10.1111/j.1365-2133.2006.07656.x. [DOI] [PubMed] [Google Scholar]
- 38.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60(1):57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137(3):507–519. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138(1):47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145(3):341–349. doi: 10.1016/j.pain.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75(3):450–452. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- 44.Sorkin LS, Moore J, Boyle DL, Yang L, Firestein GS. Regulation of peripheral inflammation by spinal adenosine: role of somatic afferent fibers. Exp Neurol. 2003;184(1):162–168. doi: 10.1016/s0014-4886(03)00102-x. [DOI] [PubMed] [Google Scholar]
- 45.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 46.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, Cunha FQ. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun. 2007;21(5):535–543. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Verri WA, Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A. 2008;105(7):2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viac J, Gueniche A, Doutremepuich JD, Reichert U, Claudy A, Schmitt D. Substance P and keratinocyte activation markers: an in vitro approach. Arch Dermatol Res. 1996;288(2):85–90. doi: 10.1007/BF02505049. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127(8):1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 51.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91(3):251–257. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 52.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144(3):278–286. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. Eur J Pain. 2009;13(3):253–262. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm. 2008;2008:469439. doi: 10.1155/2008/469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Tumor necrosis factor-alpha and interleukin-6 are not correlated with the characteristics of Complex Regional Pain Syndrome type 1 in 66 patients. Eur J Pain. 2008;12(6):716–721. doi: 10.1016/j.ejpain.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun. 2008;22(7):1072–1077. doi: 10.1016/j.bbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4(3):198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


