Abstract
We have previously shown that topical opioids including morphine and its congeners promote healing of full thickness ischemic wounds in rats. We examined the contribution of mu opioid receptor (MOPr)-mediated healing of full thickness ischemic wounds using MOPr and delta or kappa opioid receptor (DOPr or KOPr) knockout (KO) mice. Wound closure in the early (day 5) as well as later phases was delayed in topical morphine or PBS treated MOPr-KO mice compared to reciprocal treatments of wounds in wild-type (WT) mice. MOPr expression was significantly upregulated at 30 min in the wound margins and colocalized with wound margins and vasculature in the epidermal and dermal layers of the skin. We next examined whether neuropeptide expression was involved in the mechanism of MOPr-mediated wound closure. Substance P (SP) and calcitonin gene related peptide (CGRP) immunoreactivity (ir) was significantly increased in the skin of MOPr-KO mice as compared to WT mice. Neuropeptide-ir was increased significantly in PBS-treated wounds of MOPr and WT mice, but morphine treatment reduced neuropeptide immunoreactivity in both as compared to PBS. Wounding of keratinocytes led to the release of opioid peptide beta-endorphin (β-END) in conditioned medium, which stimulated the proliferation of endothelial cells. MOPr-selective (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2, CTOP) and non-selective OPr antagonist naloxone inhibited endothelial proliferation induced by wounded keratinocyte conditioned medium. Additionally, accelerated wound area closure in vitro by morphine was suppressed by methylnaltrexone, a non-selective OPr antagonist with high affinity for MOPr. Morphine and its congeners stimulated the proliferation of endothelial cells from WT mice but not those from MOPr-KO mice. Furthermore, morphine-induced mitogen-activated protein kinase/extracellular signal regulated kinase (MAPK/ERK) phosphorylation in endothelial cells was significantly decreased in MOPr-KO mice as compared to WT mice. Collectively, these data suggest that MOPr plays a critical role in the proliferation phase with the formation of granulation tissue during wound healing.
Introduction
Wound healing is an orderly and timely process resulting in the formation of new skin that is similar in strength and integrity to normal skin. However, under several pathophysiological conditions such as diabetes and sickle cell disease, the healing process is impaired, leading to chronic non-healing wounds, often associated with pain.1 Therefore, therapies that target the multicellular processes to promote wound healing and alleviate pain are required.
Experimental and clinical studies suggest that opioids and opioid receptors (OPrs) expressed in the skin are integral to normal skin homeostasis and repair.2–4 Opioids and OPrs are expressed in a wide variety of tissues including the central nervous system, vasculature, tumors, skin keratinocytes, and immune cells. Opioid primarily interact with three classical OPrs: mu, delta, and kappa (MOPr, DOPr, and KOPr, respectively). These G-protein coupled receptors bind to both endogenous opioid ligands including endorphins, enkephalins, and dynorphins and exogenous ligands such as morphine and its congeners. Best known for its anti-nociceptive activity, the opioid signaling axis is also implicated in wound healing.2,3,5,6 Opioids offer a unique advantage in treating pathophysiological wounds because of their ability to ameliorate pain via peripheral OPrs, since pain is often a serious manifestation of non-healing chronic wounds.3,5
Cutaneous wound healing cascade involves several phases involving hemostasis, inflammation, proliferation and remodeling.7 Soluble factors including growth factors and cytokines are released during the earlier phases from platelets and inflammatory cells to initiate recruitment and proliferation of cells required for the formation of new tissue. Opiods are also released from the inflammatory cells at the site of injury, while, opioid receptors are known to be activated in the periphery and dorsal root ganglion (DRG) neurons in painful conditions.3 Additionally, neuropeptides released from the nerve terminals upon injury have been shown to stimulate neurogenic inflammation, angiogenesis and wound healing.8 Release of neuropeptides during the proliferation phase may have a beneficial effect on wound healing but their sustained increase may lead to amplified inflammation and contribute to impaired healing.
Atrophy of the epidermis in MOPr and DOPr knockout mice suggests a controlling role for OPrs on keratinocyte proliferation and differentiation.9 Angiogenesis and cell proliferation with formation of granulation tissue are fundamental contributors to the wound healing process.10 Morphine and other MOPr agonists stimulate angiogenesis in tumors and wounds and accelerate wound closure in normal and diabetic rats.5,6 Therefore, we examined the contribution of MOPr on the early phase of proliferation involving endothelial proliferation and revascularization in chronic wounds.
We hypothesized that MOPr promotes angiogenesis and endothelial cell proliferation and concomitantly modulates pro-inflammatory and vasoactive neuropeptides, substance P (SP) and calcitonin gene-related peptide (CGRP) in the wound microenvironment involving the inflammatory and proliferation phases of wound healing. To examine our hypothesis, we used a full thickness delayed ischemic wound model that mimics features of chronic pathological wounds and MOPr, DOPr, and KOPr knockout (KO) mice and their wild-type (WT) control mice. Our data demonstrate that MOPr and the opioid system contribute to the healing of ischemic wounds via multicellular interactions.
METHODS
Opioid receptor KO mice
MOPr/DOPr/KOPr-KO mice and their WT control 129S6 mice were used as described by us earlier.11 All experiments were performed following approved protocols from the University of Minnesota’s Institutional Animal Care and Use Committee and conform to the statutes of the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Delayed ischemic wound model
A highly reproducible full thickness delayed ischemic wound healing model without necrosis was used.5,12 Mice were anaesthetized with 2.6% isoflurane. A rectangular template was placed on the spine between the base of the scapulae and the iliac crest. The skin was marked with a pair of uniform circles. The dorsal skin was then folded to align the two circles opposite each other, and a wooden dowel was placed against one side of skin. A sterile 6-mm skin biopsy punch was used to create a pair of full-thickness wounds centered on the midline. Two parallel incisions expanding through the panniculus carnosus were made along the long side of the template. Elevation of the skin between the incisions by blunt dissection was used to create an ischemic skin flap. Incisions were closed with autoclips.
Drug administration in vivo
Wounds were topically treated twice a day with Eucerin cream (Beiersdorf) containing morphine sulfate (Baxter Esilerderle Mfd.; 3 mg/g) or PBS. Treatment was initiated immediately after wounding. Wounds were gently cleaned with sterile saline before applying the treatments.
Wound healing measurement in vivo
Wounds were examined every day in situ. Gross observations were recorded. Each wound was measured periodically by tracing twice onto transparent plastic sheets. Wound area was calculated using Adobe Photoshop and NIH Image software using standard diameter circles for calculating wound areas. Initial (day 1 post wounding) wound area was used as a baseline and for each time point. The remaining area of each wound at each time point was calculated as a percent of wound compared to its baseline as follows: (1− {[Areai − Arean]/Areai}) × 100, where Ai is the initial and An is the area at day n.5
Laser Scanning Confocal Microscopy (LSCM)
At 21 days post-wounding, the closed wound area, including both epidermal and dermal layers of skin, were retrieved and fixed in Zamboni’s Fixative.13 Control intact dorsal skin from unwounded mice was collected and fixed. The cryosections mounted in Tissue-Tek® O.C.T™ compound were cut in 100-micron sections and blocked in 5% normal donkey serum in PBS containing 1% TritonX-100 with gentle agitation overnight at room temperature. Immunofluorescent staining was performed with either 1) rabbit anti-SP (1:200, Abcam) and sheep anti-CGRP (1:500, AbD serotec) or 2) Phycoerythrin (PE)-conjugated anti-mouse CD31 (1:50, BD Biosciences), guinea pig anti-MOPr (1:200 Millipore) and DAPI to stain nuclei. Corresponding donkey secondary antibodies conjugated with fluorescein isothiocyanate (FITC) or PE were used. To demonstrate specificity of staining, only secondary antibodies were used in the absence of either primary Ab. All samples were mounted in 1.35–1.5% noble agar and were observed with a LSCM FV 500 confocal microscope (Olympus Corporation) and processed with FluoView software (Olympus). SP immunoreactivity in the skin was quantitated in optical sections of 1.68-μ (30 steps) captured with a 20X objective (UPLAPO Olympus BX61). CGRP immunoreactivity was quantitated in optical sections 0.48-μ (38 steps) captured with a 40X objective (UPLFLN Laser 40XO Olympus BX61). A threshold intensity corresponding to the average intensity of labeled regions was measured using Image J (NIH). Data was collected from three sections, averaged, and expressed as number of neuropeptide ir-pixels as a percentage of total pixels as described.14 All images were acquired at the same magnification and size.
Cell culture
Human keratinocytes (HaCaT cell line, provided by Dr. Carol Lange, University of Minnesota) were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies, Thermo Fisher Scientific Inc) containing 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 units/ml streptomycin. Human dermal microvascular endothelial cells (HDMECs) from neonatal human foreskins were isolated as described15 and cultured in MCDB 131 medium (Life Technologies) supplemented with 1 μg/ml hydrocortisone acetate, 5 × 10−4 M dibutyryl cyclic AMP, 10 mM L-glutamine, 100 units/ml penicillin, 100 units/ml streptomycin, 0.25 mg/ml amphotericin B, 0.004% heparin, 10 μg/L epidermal growth factor as well as 20% heat inactivated male human serum. Human umbilical vein endothelial cells (HUVECs) from post-partum human umbilical cords were isolated as described.15 Blood outgrowth endothelial cells (BOECs) were isolated as described by us16 and cultured on fibronectin-coated wells in EGM-2 complete medium (Clontech) with 10% FBS and 0.15 g/L dibutyryl cAMP.
Scratch wound assay in vitro
HUVECs were grown in 6-well plates to confluence. A scratch wound was created by scraping the monolayer using a cell scraper. Boundaries of the scraped wound were marked on the underside of the well. Cells were incubated with 1 μM morphine, 1 μM morphine + 1 μM methylnaltrexone (MNTX) or PBS. Wound width was measured by Image J at 3 different places along the length of the wound at different time points. Percent wound closure was calculated for each measurement in comparison to time point ‘0’.17
Cell proliferation assay
BOECs or HDMECs seeded on 96-well plate at 5000 cells/well and grown in regular culture medium overnight were serum and growth factor depleted for 24 h.15 BOECs were incubated with MOPr agonists (0.1 μM fentanyl, 1 μM morphine or 0.3 μM hydromorphone) or PBS, whereas HDMECs were incubated with conditioned medium from unwounded and wounded keratinocytes with or without the non-selective OPr antagonist or MOPr-selective antagonist naloxone or D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), respectively. Cells were cultured for another 48 h, and cell proliferation was evaluated by detection of bromodeoxyuridine incorporation using the commercially available cell proliferation ELISA (Roche Diagnostics) according to manufacturer’s protocol. Absorbance at 370 nm was obtained with a VersaMax microplate reader (Molecular Devices) 18
Beta-endorphin (β-END) release
Cultured human keratinocytes were seeded on 6-well plates to reach 80–90% confluence at 24 h. Cells were serum starved for 24 h. Cells were scratched with a 26-gauge needle, and cell-free supernatant was collected immediately after wounding within one minute and at 0.5, 1, 2, 4, and 24 h. An immunoassay kit (Phoenix Pharmaceuticals Inc.) specific for the detection of β-END was used to analyze β-END levels in the wounded keratinocyte conditioned medium.
Western immunoblotting
Whole tissue lysates of intact unwounded skin or margins of the wounded skin, with epidermal and dermal layers, post 30 min wounding were used to analyze MOPr. Additionally, whole cell lysate of serum- and growth factor-starved BOECs from WT and MOPr-KO mice stimulated with 1 μM morphine for the indicated time, were used to analyze signal pathway activation, Either tissue or BOECs lysates (30 μg protein/lane) were resolved on a 3–15% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore). Antibodies against MOPr (Santa Cruz) and total and phospho-mitogen-activated protein kinase/extracellular signal regulated kinase (MAPK/ERK, Thr202/tyr204) (1:500, Cell Signaling Technology) were used. Immunoreactive proteins were visualized with the ECF™ system (Amersham Life Sciences), and chemifluorescence signals were acquired using Storm 860 Phosphorimager (Molecular Dynamics) as described.19
Statistical analysis
All data were analyzed using Prism software (v 6.0a, GraphPad Prism Incorporation). A one-way ANOVA with Bonferroni’s multiple comparison correction and t-test was used to compare treatments. A two-way ANOVA with Bonferroni’s multiple comparison correction was used to compare the pain behavioural response or between treatments. A p-value of < 0.05 was considered significant. All data are presented as mean ± SEM.
RESULTS
OPrs contribute to healing of ischemic wounds
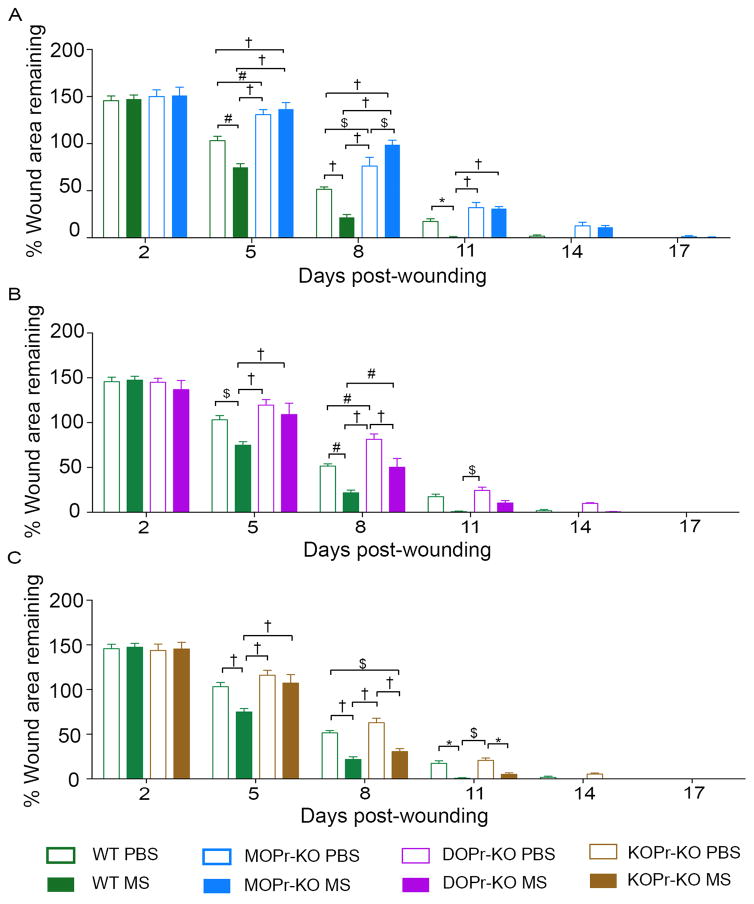
Wound healing improved significantly in morphine-treated wounds as compared to PBS-treated wounds in WT mice during the study period (Figure 1A–C). MOPr-KO mice consistently showed poorer healing compared to WT mice in PBS-treated groups (Fig 1A). Moreover, morphine-treatment did not improve healing in MOPr-KO mice. In DOPr-KO mice healing was significantly delayed on day 8 but not other days compared to WT mice, whereas no significant difference was observed in healing in KOPr KO mice compared to WT, in PBS treated groups (Figures 1B and C). In DOPr-KO and KOPr-KO wounds, morphine did not have a significant effect on wound closure on day 5, but on day 8 and 11, morphine promoted wound closure, as compared to PBS. All wounds closed nearly completely by day 14. Thus, MOPr may contribute to the proliferation phase with revascularization and granulation tissue formation, whereas DOPr may mediate the later stages of wound healing such as epithelialization phase.
Figure 1. Wound closure in opioid receptor KO mice.
Ischemic wound healing in WT control or opioid receptor-KO mice—MOPr-KO (A), DOPr- KO (B), and KOPr-KO (C)—treated with PBS or morphine (MS) was measured on days 2, 5, 8, 11, 14, 17, and 20. Percent of wound remaining compared to day 1 is reported. *p<0.05, $p < 0.01, #p < 0.001, †p < 0.0001. Significance was determined by two-way ANOVA with Bonferroni’s multiple comparison (A-C). Each value represents mean ± SEM (n = 8 per group from 4 mice each of anterior and posterior wounding area). Abbreviations: WT, wild-type; KO, knockout; MOPr, mu-opioid receptor; DOPr, delta-opioid receptor; KOPr, kappa-opioid receptor; MS, morphine.
MOPr is involved in neuropeptide expression in wound tissue
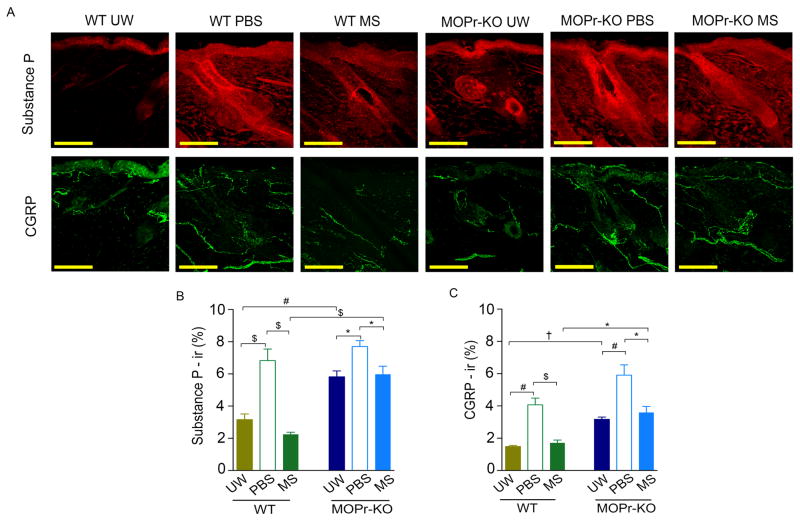
The intact skin of MOPr-KO mice showed significantly increased SP and CGRP immunoreactivity as compared to WT mice (p<.001 and p<.0001, respectively; Figure 2A–C). Both neuropeptides showed a significantly higher immunoreactivity in the wounds of WT and MOPr-KO mice, as compared to intact skin. Wounds treated with PBS did not show a significant difference in neuropeptide immunoreactivity between WT and MOPr-KO mice. However, expression of SP and CGRP was significantly reduced in morphine-treated ischemic wounds as compared to PBS-treated wounds in both WT (p<.01) and MOPr-KO (p<.05) mice. Notably, both SP and CGRP immunoreactivity remained significantly higher in morphine-treated MOPr-KO as compared to WT mice (p<.01 and p<.05, respectively). Together, these data suggest that MOPr-KO may have a regulatory effect on neuropeptides in the skin and that morphine treatment mediates reductions in neuropeptide expression independent of MOPr.
Figure 2. Downregulation of neuropeptides with morphine via MOPr.
Expression of substance P and CGRP in unwounded (UW) or wounded skin (A) after treatment with PBS or MS in MOPr-KO and WT mice on day 21 was quantified as % immunoreactivity (ir, B and C). *p<0.05, $p < 0.01, #p < 0.001, †p < 0.0001. Significance was determined by t-test (unpaired, two-tailed). Each value represents mean ± SEM (n = 8 per group from 4 mice each of anterior and posterior wounding area). Scale bar: 100 μm. Abbreviations: CGRP, calcitonin gene-related peptide; WT, wild-type; UW, unwounded; KO, knockout; MOPr, mu-opioid receptor; MS, morphine; ir, immunoreactivity.
MOPr expression is upregulated in wounds
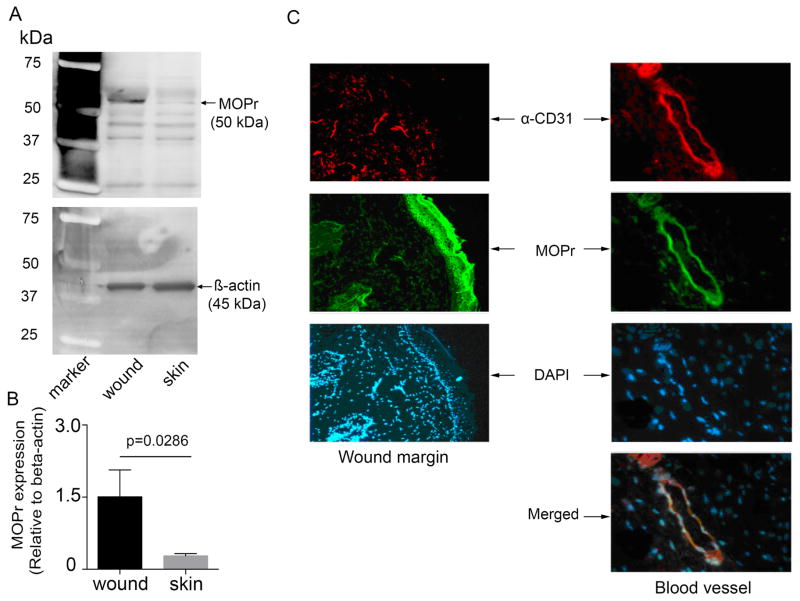
We next examined whether wounding stimulated MOPr expression. MOPr expression was significantly higher in wounds as compared to intact skin (p=0.0286; Figure 3A, B). Co-localization of CD31 and MOPr demonstrated co-expression of MOPr on blood vessels in wounds and on wound margins (Figure 3C). Thus, wounding leads to an increase in MOPr expression, which may contribute to the normal healing process via vascular and epithelial-specific effects.
Figure 3. Upregulation of MOPr expression in response to wounding.
(A) MOPr protein expression is shown in unwounded and wounded skin from WT mice (n=4 per group). (B) Bar represents band density of MOPr relative to β-actin in normal and wounded skin. (C) Representative confocal images show co-expression of MOPr (green) and CD31 (red), a marker of vascular endothelial cells, in WT mice. Nuclei were visualized with DAPI (blue). Scale bar: 100 μm. Each image represents 6 separate, reproducible experiments. Significance was determined by t-test (B, unpaired, two-tailed). Abbreviations: MOPr, mu-opioid receptor, WT, wild-type.
Keratinocytes release endogenous opioid peptide β-END upon wounding
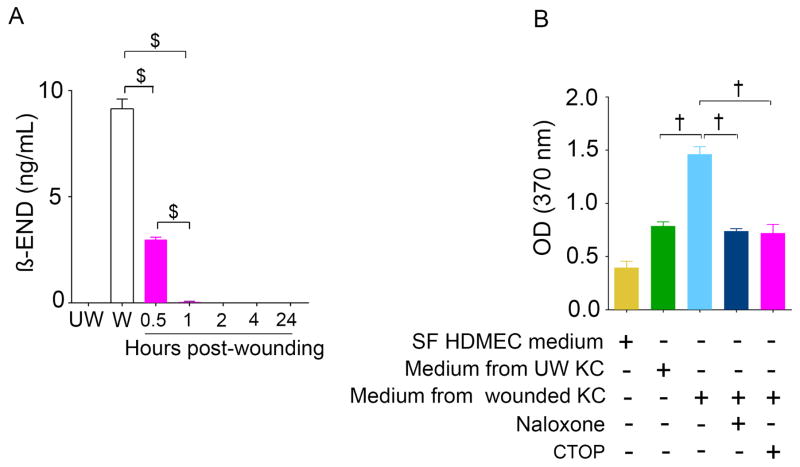
Wounding of HaCat keratinocytes instantly led to the release of β-END in the medium (0 min), which declined to barely detectable levels 60 min post-wounding (p=0.0024, Figure 4A). Furthermore, we observed that the addition of conditioned cell medium from scratched HaCat cells stimulated 2-fold proliferation of endothelial cells (p<.0001; Figure 4B). Notably, naloxone and a MOPr-selective antagonist CTOP significantly inhibited HaCat conditioned medium-induced endothelial proliferation (p<.0001). These data suggest that wounding activates the opioid system by leading to an increase in OPrs and a concomitant increase in β-END from keratinocytes, which in turn may stimulate angiogenesis by activating endothelial proliferation. It is likely that morphine accelerates healing by binding to the increased OPrs on wound endothelium.
Figure 4. β-END release from scratch wounded keratinocytes.
(A) The opioid peptide, β-END, released from HaCaT keratinocytes was measured in culture medium from monolayers at unwounded (UW), wounded (W) and 0.5, 1, 2, 4, and 24 hours after wounding. (B) HDMECs proliferation was assessed by cell proliferation ELISA after treatment with conditioned medium from wounded HaCaT cell medium. Significance was determined by t-test (A, unpaired, two-tailed) or one-way ANOVA (B). Abbreviations: MOPr, mu-opioid receptor; WT, wild-type; β-END, beta-endorphin; SF, serum free; UW, unwounded; W, wounded; KC, keratinocytes; HaCaT, human keratinocytes; HDMECs, Human dermal microvascular endothelial cells; CTOP, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2.
MOPr is involved in endothelial cell proliferation and migration
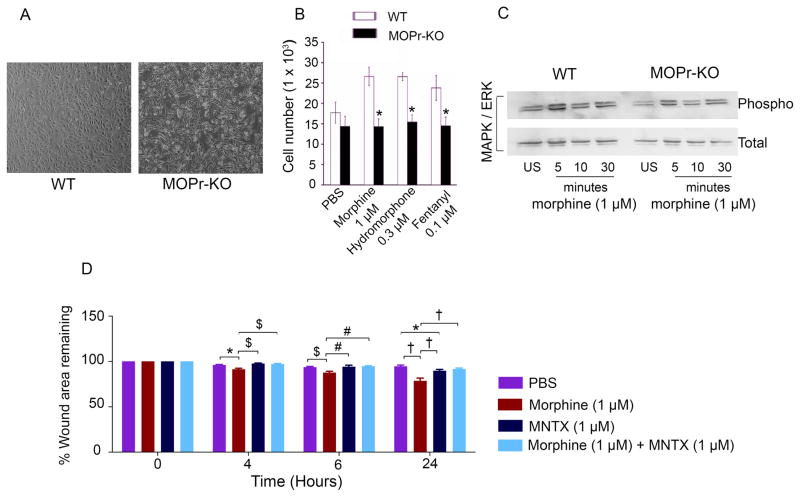
We next examined whether MOPr contributes to endothelial proliferation and migration, two critical processes involved in angiogenesis and wound healing. BOECs derived from MOPr-KO mice showed a stringy and atypical endothelial phenotype as compared to the typical cobblestone morphology of BOECs from WT mice (Figure 5A). Cell proliferation promoted by MOPr agonists, morphine, hydromorphone, and fentanyl was attenuated in MOPr-KO-derived BOECs as compared to those from WT mice (p<.05 for each agonist; Figure 5B). MAPK/ERK phosphorylation, which plays a central role in endothelial proliferation, was stimulated by morphine in BOECs derived from WT mice, but was diminished appreciably in MOPr-KO BOECs (Figure 5C). Morphine also promoted cell migration, another critical step in angiogenesis and wound healing. In vitro scratched wound area in an endothelial monolayer was significantly reduced with morphine treatment as compared to PBS (p<.05, p<.01 and p<.0001 at 4, 6, and 24 hours post-wounding; Fig 5D). Moreover, morphine’s effect on scratched endothelial area could be abolished by a single application of the OPrs antagonist MNTX, which has a relatively high affinity for MOPr (p<.01, p<.001 and p<.0001 of morphine vs morphine + MNTX at 4, 6 and 24 hours post-wounding respectively).
Figure 5. Critical role of MOPr for endothelial phenotype, proliferation, and signaling.
(A) Endothelial cell morphology of BOECs isolated from WT (left panel) and MOPr-KO (right panel) mice. (B) BOEC proliferation was quantified after treatment with MOPr agonists (morphine/hydromorphone/fentanyl) in MOPr-KO or WT mice. (C) Morphine-induced activation of phospho-p42/p44 MAPK/ERK in BOECs from WT and MOPr-KO mice. (D) Percentage of remaining wound area was calculated after incubation of scratch wounded HDMECs in the presence or absence of morphine, MOPr antagonist MNTX, or both. *p < 0.05, $p < 0.01, #p < 0.001, †p < 0.0001. Significance was determined by t-test (B; unpaired, two-tailed) as well as two-way ANOVA with Bonferroni’s multiple comparison (D). Abbreviations: MOPr, mu-opioid receptor; BOECs, blood outgrowth endothelial cells; WT, wild-type; KO, knockout; MAPK/ERK, mitogen activated protein kinase/extracellular signal regulated kinase; MNTX, methylnaltrexone.
DISCUSSION
Wound healing occurs in distinct yet overlapping phases, including, hemostasis, inflammation, proliferation and maturation. Ischemia and vasculopathy are mostly the underlying pathophysiological cause of chronic non-healing wounds, but the peripheral and central nociceptor activation leading to pain is also initiated upon injury. OPrs modulate skin homeostasis, angiogenesis and wound healing.17,20 The contribution of OPr targeted therapies therefore is 2-fold because OPr agonists can potentially influence the inflammatory and proliferation phase of wound healing and also ameliorate pain. Therefore, we examined the contribution of MOPr on proliferation phase encompassing revascularization, on healing of full thickness ischemic wounds using MOPr-KO mice and endothelial cells derived from the blood of MOPr-KO mice. We demonstrate that MOPr contributes to wound healing via the activation of an endogenous opioid system upon wounding; our data suggest that this system includes the release of β-END and upregulation of MOPr, endothelial-specific effects on proliferation, migration and MAPK/ERK signaling in early phase of wounding, as well as regulation of neuropeptides SP and CGRP. Since MOPr agonists are also used as analgesics, topical opioids may concomitantly reduce pain while improving healing of painful chronic wounds.
OPr activity is complex due to multiple OPrs and their expression on inflammatory, vascular and epithelial cells associated with distinct phases of wound healing and neural activity in the skin. Therefore, the use of MOPr-, DOPr-, and KOPr-specific KO mice, allowed us to examine the contribution of MOPr on the distinct phase(s) of the healing process. Additionally, ischemic model of delayed healing of full thickness cutaneous wounds offers the advantage to examine the different phases of the healing cascade while recapitulating the features of pathological chronic wounds.
Earlier studies have examined the effect of opioids on wound closure and the role of DOPr on reepithelialization phase of healing. Our data on wound closure are in agreement with earlier observations on the contribution of MOPr and DOPr in wound healing process.5,6,17,21 Healing of punch biopsy cutaneous wounds, burn wounds and cage fight wounds was demonstrated to be delayed in DOPr-KO mice.22 Delayed wound healing in DOPr-KO is accompanied by an aberrant epithelial phenotype with an atrophic epidermal layer and hypertrophic wound edges.22,23 Using the converse approach of targeted upregulation of DOPr in keratinocytes, these authors showed that DOPr contributes to epidermal homeostasis and keratinocyte function via MAPK/ERK signaling.24 The timing of keratinocyte differentiation and proliferation in the wound may be critical; hence, the healing effect of opioids may be influenced by the phase-dependent expression of individual OPrs in the wound.
Our data show that KOPr may not contribute to wound healing nor mediate the effect of morphine. The role of KOPr on cutaneous wound healing has not been examined previously, but its activation has been shown to inhibit angiogenesis by suppressing the expression of vascular endothelial growth factor receptor 2 (VEGFR2) on the endothelium.25 However, contradictory effects of KOPr have been demonstrated on tissue injury in the central nervous system.26,27 KOPr agonists dynorphin A, U50,488H, and U69,593 administered centrally or systemically worsened neurological outcomes after fluid-percussion traumatic brain injury in rats.26 Conversely, KOPr-selective U50,488H was demonstrated to have a beneficial effect by improving cerebral blood flow after acute spinal and brain injury in mice and cats.27 None of these were direct measures of wound healing, but the anti-angiogenic effect of KOPr activation may negatively influence the proliferation phase with revascularization and granulation tissue formation. Therefore, the role of KOPr in skin homeostasis and wound healing needs to be further investigated.
Keratinocytes play a critical role in skin homeostasis and epithelialization phase via DOPr. However, angiogenesis for revascularization and vascular physiology are critical for the healing process. Vasculopathy is one of the most common causes of non-healing leg ulcers.1 Considering the critical role of MOPr and its ligands in angiogenesis and the neural system, we examined the mechanism(s) involving revascularization and neuroinflammation that are orchestrated by OPrs and their ligands. Endorphins and analgesic opioids including morphine have been demonstrated to stimulate endothelial proliferation, survival, and angiogenesis in wounds and tumors via MOPr mediated MAPK/ERK signaling.5,6,19,28 The MOPr-specific agonist [D-Arg2,Lys4]dermorphin-(1,4)-amide (DALDA) attenuated 3% dextran sodium sulfate (DSS)-induced intestinal wounds in C57BL/6 mice.21 In this study, DALDA promoted colonocyte proliferation and migration via Stat3-mediated signaling, suggesting that MOPr protects from DSS-induced injury and accelerates mucosal healing. The MOPr antagonist naltrexone inhibited corneal re-epithelialization,29 and fentanyl-induced wound healing in diabetic rats was inhibited by the OPr antagonist naloxone;5 these findings suggest a direct role of MOPr in wound repair. MOPr co-activates phospho-platelet derived growth factor receptor-β (PDGFR-β), epidermal growth factor receptor (EGFR), and VEGFR2 and downstream MAPK/ERK signaling, which stimulate angiogenesis.12,30 Earlier we showed that fentanyl stimulates PDGFR-β phosphorylation in ischemic wounds in diabetic rats.5 Synergistic activity of platelet derived growth factor (PDGF) and other growth factors have been implicated in the wound healing process.31 MOPr may therefore orchestrate revascularization via a synergistic cascade of growth-promoting signaling directly and/or by co-activating growth factor receptor tyrosine kinases in the wounds.
Opioids also modulate vascular physiology. Blood flow was increased in chronic wounds in sickle patients with leg ulcers.32 Systemic morphine reversed hyperemia by vasoconstricting vasa nervorum in nerve trunk injury, but not in contralateral uninjured sciatic nerves.33 In contrast, contralateral uninjured nerve trunk area demonstrated a rise in local perfusion, indicating vasodilation, after morphine treatment. Moreover, in older neuromas on day 7, perfusion was influenced partially, but on day 14 there was no effect on perfusion with morphine. These observations suggest that morphine may promote healing by increasing perfusion in and around the healing wounds.
Injury such as wounds lead to nociceptor activation and release of neuropeptides in the periphery.8,34 SP promotes angiogenesis under ischemic injury.35,36 Upon activation inflammatory cells such as mast cells also release SP.37 The increase in SP during the inflammatory phase may contribute to the healing process by stimulating angiogenesis and granulation tissue formation. However, a sustained increase in neuropeptides in later phase of tissue remodeling may be detrimental because of neurogenic inflammation induced by SP. We observed an increase in CGRP- and SP-ir in the healed chronic wounds of WT and MOPr-KO mice, which were attenuated in morphine treated healed scars. Morphine suppressed SP release at both the central and peripheral levels.38,39 However, in MOPr-KO mice baseline levels of both neuropeptides are raised before morphine treatment, and after treatment the levels return to the levels of unwounded skin in MOPr-KO mice, but remain significantly increased compared to WT wounds treated with morphine. Furthermore, increased neuropeptides in the unwounded skin of MOPr-KO mice compared to WT mice suggest that MOPr regulates neuropeptide expression in the skin perhaps on inflammatory cells and keratinocytes in addition to their release from the peripheral nerve terminals. It is likely that partial reduction in neuropeptides with morphine is a MOPr-independent process and perhaps occurs via DOPr or KOPr. Indeed, peripherally applied MOPr-, DOPr- and KOPr- agonists reduced heat-induced SP release and edema in the rat paw.40 Opioids may inhibit the release as well as function of SP, because MOPr- and DOPr-agonists perfused with SP reduced plasma extravasation and blood flow, in an OPr-dependent manner.41,42 Thus, sustained increase in neuropeptides in the skin of MOPr-KO mice during the maturation phase may interfere with healing by promoting neurogenic inflammation. Taken together these observations suggest that morphine treatment reduces neuroinflammation in the wounds, which may contribute to morphine-induced healing.
β-END has been demonstrated to contribute to healing of painful chronic wounds.20 Keratinocytes are the major source of β-END in skin.43 Seminal observations from Bigliardi/Bigliardi-Qi et al. have demonstrated that OPr ligands and OPrs affect the migration of normal human skin keratinocytes and fibroblasts and HaCaT cells in in vitro wound assays.17,22,23,44 Endogenous OPrs and opioid peptides are differentially implicated in modulating wound healing in acute and chronic stages.17,20 Expression of MOPr was significantly higher in wounds compared to intact skin and co-localized with vasculature and the epidermal layer. Wounding of keratinocytes instantly led to the release of β-END in the medium, which declined to barely detectable levels 60 min post-wounding (Fig. 4A). These data suggest that wounding activates the opioid system by increasing MOPrs and a concomitant increase in β-END from keratinocytes, which acts upon endothelial MOPr and stimulates revascularization in the wounds. It is likely that morphine accelerates healing by binding to the increased OPrs at the wound site. Since endothelial cells from MOPr-KO mice show diminished response to MOPr ligand-induced proliferation and MAPK/ERK phosphorylation, it is likely that morphine stimulates wound angiogenesis via MOPr in the early phase of the healing cascade.
These studies suggest that opioids offer a unique advantage in treating wounds because of their ability to concurrently stimulate revascularization, and reduce neuroinflammation. Targeting MOPr-based interventions at the site of injury could also provide analgesia with minimal central or systemic side effects. We show that MOPr contributes to the early phase of wound healing involving revascularization by promoting endothelial proliferation and migration and activating the MAPK/ERK pathway.
Background
Non-healing, painful wounds in conditions including sickle cell disease and diabetes are difficult to treat. Opioids and opioid receptors are expressed in the skin and are integral to normal skin homeostasis and repair. Topical opioids such as morphine and its congeners have been shown to promote ischemic wound healing in rats.
Translational Significance
We show that the mu opioid receptor in mice mediates wound healing in the skin microenvironment by inhibiting neuropeptide expression and promoting endothelial proliferation through the MAPK/ERK pathway. These studies suggest that opioids offer a unique advantage in treating wounds because of their ability to concurrently induce healing and ameliorate pain via peripheral mu opioid receptors.
Acknowledgments
This study was supported by NIH RO1 HL68802 and UO1 HL117664 and Institute for Engineering in Medicine grants to KG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All authors have read the journal’s policy on disclosure of potential conflicts of interest, and the authors confirm that no potential conflicts of interests exist. All authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all authors. We thank Dariel J. Irizarry for help with scratch assays, Michael Franklin and Barb Benson for editorial assistance and Georgi Walberg and Josette Fontana for help with communicating the manuscript.
Abbreviations
- β-END
beta-endorphin
- BOECs
blood outgrowth endothelial cells
- CGRP
calcitonin gene-related peptide
- CTOP
D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2
- DALDA
[D-Arg2,Lys4]dermorphin-(1,4)-amide
- DOPr
delta opioid receptor
- DMEM
Dulbecco’s Modified Eagle Medium
- DSS
dextran sodium sulfate
- DRG
dorsal root ganglion
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HDMECs
human dermal microvascular endothelial cells
- HUVECs
human umbilical vein endothelial cells
- KOPr
kappa opioid receptor
- KO
knockout
- LSCM
laser scanning confocal microscopy
- MAPK/ERK
mitogen-activated protein kinase/extracellular signal regulated kinase
- MNTX
methylnaltrexone
- MOPr
mu opioid receptor
- OPrs
opioid receptors
- PDGF
platelet derived growth factor
- PDGFR-β
platelet derived growth factor receptor-β
- PE
phycoerythrin
- SCD
sickle cell disease
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SP
substance P
- VEGFR2
vascular endothelial growth receptor 2
- WT
wild-type
Footnotes
DISCLOSURE
KG is a consultant for Fera Pharmaceuticals LLC.
AUTHOR CONTRIBUTION
YW, MG and SR wrote the manuscript, YW, TP, MF and YL performed research, FP analyzed data and prepared illustrations, MA and JE provided opioid receptor knockout mice, YW, MG and KG interpreted the data, and KG conceived, supervised and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kerstein MD. The non-healing leg ulcer: peripheral vascular disease, chronic venous insufficiency, and ischemic vasculitis. Ostomy/wound management. 1996;42:19S–35S. [PubMed] [Google Scholar]
- 2.Bigliardi PL, Dancik Y, Neumann C, Bigliardi-Qi M. Opioids and skin homeostasis, regeneration and ageing - What’s the evidence? Experimental dermatology. 2016;25:586–91. doi: 10.1111/exd.13021. [DOI] [PubMed] [Google Scholar]
- 3.Stein C, Kuchler S. Targeting inflammation and wound healing by opioids. Trends in pharmacological sciences. 2013;34:303–12. doi: 10.1016/j.tips.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocrine reviews. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 5.Gupta M, Poonawala T, Farooqui M, Ericson ME, Gupta K. Topical fentanyl stimulates healing of ischemic wounds in diabetic rats. Journal of diabetes. 2015;7:573–83. doi: 10.1111/1753-0407.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poonawala T, Levay-Young BK, Hebbel RP, Gupta K. Opioids heal ischemic wounds in the rat. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13:165–74. doi: 10.1111/j.1067-1927.2005.130207.x. [DOI] [PubMed] [Google Scholar]
- 7.Singer AJ, Clark RA. Cutaneous wound healing. The New England journal of medicine. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 8.Hong HS, Lee J, Lee E, et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nature medicine. 2009;15:425–35. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 9.Bigliardi-Qi M, Bigliardi PL. The role of opioid receptors in migration and wound recovery in vitro in cultured human keratinocytes and fibroblasts. Methods in molecular biology. 2015;1230:273–7. doi: 10.1007/978-1-4939-1708-2_23. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Advances in wound care. 2014;3:647–61. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber ML, Farooqui M, Nguyen J, et al. Morphine induces mesangial cell proliferation and glomerulopathy via kappa-opioid receptors. American journal of physiology Renal physiology. 2008;294:F1388–97. doi: 10.1152/ajprenal.00389.2007. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Schultz GS, Bloch M, Edwards PD, Tebes S, Mast BA. Molecular and mechanistic validation of delayed healing rat wounds as a model for human chronic wounds. Wound Repair Regen. 1999;7:486–94. doi: 10.1046/j.1524-475x.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 13.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–65. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmberg AB, Brandon EP, Idzerda RL, Liu H, McKnight GS, Basbaum AI. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type I regulatory subunit of cAMP-dependent protein kinase. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:7462–70. doi: 10.1523/JNEUROSCI.17-19-07462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K, Ramakrishnan S, Browne PV, Solovey A, Hebbel RP. A novel technique for culture of human dermal microvascular endothelial cells under either serum-free or serum-supplemented conditions: isolation by panning and stimulation with vascular endothelial growth factor. Experimental cell research. 1997;230:244–51. doi: 10.1006/excr.1996.3421. [DOI] [PubMed] [Google Scholar]
- 16.Bodempudi V, Ohlfest JR, Terai K, et al. Blood outgrowth endothelial cell-based systemic delivery of antiangiogenic gene therapy for solid tumors. Cancer gene therapy. 2010;17:855–63. doi: 10.1038/cgt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigliardi PL, Neumann C, Teo YL, Pant A, Bigliardi-Qi M. Activation of the delta-opioid receptor promotes cutaneous wound healing by affecting keratinocyte intercellular adhesion and migration. British journal of pharmacology. 2015;172:501–14. doi: 10.1111/bph.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber ML, Chen C, Li Y, et al. Morphine stimulates platelet-derived growth factor receptor-beta signalling in mesangial cells in vitro and transgenic sickle mouse kidney in vivo. British journal of anaesthesia. 2013;111:1004–12. doi: 10.1093/bja/aet221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer research. 2002;62:4491–8. [PubMed] [Google Scholar]
- 20.Bigliardi PL, Sumanovski LT, Buchner S, Rufli T, Bigliardi-Qi M. Different expression of mu-opiate receptor in chronic and acute wounds and the effect of beta-endorphin on transforming growth factor beta type II receptor and cytokeratin 16 expression. The Journal of investigative dermatology. 2003;120:145–52. doi: 10.1046/j.1523-1747.2003.12018.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith JR, Uronis JM, Jobin C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. The American journal of pathology. 2011;179:673–83. doi: 10.1016/j.ajpath.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigliardi-Qi M, Gaveriaux-Ruff C, Zhou H, et al. Deletion of delta-opioid receptor in mice alters skin differentiation and delays wound healing. Differentiation; research in biological diversity. 2006;74:174–85. doi: 10.1111/j.1432-0436.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 23.Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M. Opioids and the skin--where do we stand? Experimental dermatology. 2009;18:424–30. doi: 10.1111/j.1600-0625.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 24.Neumann C, Bigliardi-Qi M, Widmann C, Bigliardi PL. The delta-opioid receptor affects epidermal homeostasis via ERK-dependent inhibition of transcription factor POU2F3. The Journal of investigative dermatology. 2015;135:471–80. doi: 10.1038/jid.2014.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamizu K, Furuta S, Hamada Y, et al. small ka, Cyrillic Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Scientific reports. 2013;3:3213. doi: 10.1038/srep03213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh TK, Fernyak S, Yamakami I, Faden AI. Central and systemic kappa-opioid agonists exacerbate neurobehavioral response to brain injury in rats. The American journal of physiology. 1994;267:R665–72. doi: 10.1152/ajpregu.1994.267.3.R665. [DOI] [PubMed] [Google Scholar]
- 27.Hall ED, Wolf DL, Althaus JS, Von Voigtlander PF. Beneficial effects of the kappa opioid receptor agonist U-50488H in experimental acute brain and spinal cord injury. Brain research. 1987;435:174–80. doi: 10.1016/0006-8993(87)91599-x. [DOI] [PubMed] [Google Scholar]
- 28.Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvascular research. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Zagon IS, Sassani JW, McLaughlin PJ. Re-epithelialization of the rabbit cornea is regulated by opioid growth factor. Brain research. 1998;803:61–8. doi: 10.1016/s0006-8993(98)00610-6. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka N, Nguyen J, Chen C, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesthesia and analgesia. 2011;113:1353–64. doi: 10.1213/ANE.0b013e318232b35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7696–700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. American journal of hematology. 2010;85:831–3. doi: 10.1002/ajh.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaafsma L, Sun H, Zochodne D. Exogenous opioids influence the microcirculation of injured peripheral nerves. The American journal of physiology. 1997;272:H76–82. doi: 10.1152/ajpheart.1997.272.1.H76. [DOI] [PubMed] [Google Scholar]
- 34.Yaksh TL. Substance P release from knee joint afferent terminals: modulation by opioids. Brain research. 1988;458:319–24. doi: 10.1016/0006-8993(88)90474-x. [DOI] [PubMed] [Google Scholar]
- 35.Ziche M, Morbidelli L, Masini E, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. The Journal of clinical investigation. 1994;94:2036–44. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amadesi S, Reni C, Katare R, et al. Role for substance p-based nociceptive signaling in progenitor cell activation and angiogenesis during ischemia in mice and in human subjects. Circulation. 2012;125:1774–86. S1–19. doi: 10.1161/CIRCULATIONAHA.111.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–62. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–7. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- 39.Brodin E, Gazelius B, Panopoulos P, Olgart L. Morphine inhibits substance P release from peripheral sensory nerve endings. Acta physiologica Scandinavica. 1983;117:567–70. doi: 10.1111/j.1748-1716.1983.tb07228.x. [DOI] [PubMed] [Google Scholar]
- 40.Yonehara N, Imai Y, Inoki R. Effects of opioids on the heat stimulus-evoked substance P release and thermal edema in the rat hind paw. European journal of pharmacology. 1988;151:381–7. doi: 10.1016/0014-2999(88)90534-1. [DOI] [PubMed] [Google Scholar]
- 41.Khalil Z, Sanderson K, Modig M, Nyberg F. Modulation of peripheral inflammation by locally administered endomorphin-1. Inflammation research: official journal of the European Histamine Research Society [et al] 1999;48:550–6. doi: 10.1007/s000110050502. [DOI] [PubMed] [Google Scholar]
- 42.Khalil Z, Helme RD. Modulation of a peripheral inflammatory response to substance P by locally administered opioid receptor agonists. Neuropeptides. 1990;17:45–53. doi: 10.1016/0143-4179(90)90140-t. [DOI] [PubMed] [Google Scholar]
- 43.Zanello SB, Jackson DM, Holick MF. An immunocytochemical approach to the study of beta-endorphin production in human keratinocytes using confocal microscopy. Annals of the New York Academy of Sciences. 1999;885:85–99. doi: 10.1111/j.1749-6632.1999.tb08667.x. [DOI] [PubMed] [Google Scholar]
- 44.Bigliardi PL, Buchner S, Rufli T, Bigliardi-Qi M. Specific stimulation of migration of human keratinocytes by mu-opiate receptor agonists. Journal of receptor and signal transduction research. 2002;22:191–9. doi: 10.1081/rrs-120014595. [DOI] [PubMed] [Google Scholar]