Abstract
Salmonella Typhimurium, engineered to express flagellin B, recently demonstrated unprecedented tumor control through a TLR-dependent mechanism. Here, we review new observations that support the potential of utilizing modified bacteria to enhance antitumor immunity. We also discuss the implications of these findings for clinical applications, including immune checkpoint blockade therapies.
Recent work published in Science Translational Medicine (Zheng et al., 2017) provides the first demonstration that tumor-targeting Salmonella serotype Typhimurium (S. Typhimurium) engineered to overexpress bacterial flagellin B (FlaB), results in a remarkable delay of tumor outgrowth against murine colon and melanoma models when compared to previous bacterial therapies testing the effects of cancer treatment(s) in immunocompetent mice (Rosenberg et al., 2002). The unique capacity of this approach in transforming the immune-suppressive and/or -exclusive tumor microenvironment into an immune-permissive setting, has important clinical considerations, including the possibility of improving patient responsiveness to immune checkpoint blockade.
Over the past 20 years, attenuated intravenously-injected S. Typhimurium strains have demonstrated an impressive capacity to colonize solid tumors at a ~1000-fold increased level compared to normal organs (Rosenberg et al., 2002). Bacteria induce central tumor necrosis that facilitates nutrient release, thus contributing to their tropism and tumor-specific replication (Leschner et al., 2009). Previous work has also demonstrated that bacteria can induce the recruitment of tumor-infiltrating neutrophils that create a “wall” around the central necrotic core; this in turn, prevents further bacterial infection of cancer cells in the peripheral rim of viable tumor cells (Westphal et al., 2008). In line with restricted bacterial localization to necrotic tumor areas, previous Phase I clinical trials investigating the use of attenuated S. Typhimurium for metastatic melanoma demonstrated a high degree of patient safety, but unfortunately, no antitumor efficacy (Toso et al., 2002).
A recent report has reinvigorated the bacterial field by using attenuated S. typhimurium to deliver exogenous FlaB into tumors. Unprecedented tumor rejection or delay- durable for up to 4 months in the experimental monitoring period- was observed against MC38 and B16F10 tumors in C57BL/6 mice, as well as in human HCT116-luc2 lung xenograft tumors in nude mice, following treatment with attenuated ΔppGpp S. Typhimurium; this bacterial strain was triggered to generate FlaB through an arabinose-inducible plasmid (Zheng et al., 2017). FlaB is normally produced by another bacterium, Vibrio vulnificus, and the genetic modification in ΔppGpp S. Typhimurium enhances inoculation-induced toll-like receptor (TLR) activation as a way of amplifying immune-mediated anti-tumor effects elicited by S. Typhimurium (Zheng et al., 2017). Specifically, using genetically-deficient TLR4−/−, TLR5−/−, and MyD88−/− mice, the study demonstrated that, following tumor bacterial colonization, FlaB-mediated activation of TLR4, and MyD88, and to some extent, TLR5, contributed to antitumor activity and to the transformation of the tumor microenvironment, from an immunosuppressive to a pro-inflammatory state. This was reflected by reducing the percentage of intratumoral resident-macrophages with an immunosuppressive M2 phenotype, while increasing the percentage of pro-inflammatory M1 macrophages that likely accounted for the elevated anti-tumor cytokine levels (IL1β, TNFα) observed in tumors treated with FlaB-expressing S. Typhimurium (Zheng et al., 2017). However, despite being a safe and effective treatment for the animals, almost half of the tumors eventually relapsed, highlighting the obvious need to further optimize this bacterial approach.
Furthermore, although not specifically addressed in the above study, the antitumor efficacy of FlaB-expressing S. Tyhphimurium might relate to the extent of induced anti-tumor T cell responses. Indeed, prior studies have reported that CD8+ T cells are required for durable tumor control in mice (Wainwright et al., 2014), which also directly applies to studies using S. Typhimurium (Binder et al., 2016; Binder et al., 2013). Indeed, unsurpisingly, unmodified intravenous S. Typhimurium failed to control tumor progression (Binder et al., 2013; Zheng et al., 2017), and this lack of efficacy- even against immunogenic tumors- correlated with a lack of antitumor cytotoxic T cell responses (Binder et al., 2013). However, when bacteria were engineered to express tumor-associated antigen, enhanced CD8+ T cell responses and eradication of well-established murine tumors (at ≥14 days post-engraftment and ≥100 mm3 in size) were achieved (Binder et al., 2013). In support of these findings, monotherapeutic intratumoral injection of heat-killed S. Typhimurium alone showed no impact on immune-mediated control of tumors in mouse models of large established fibrosarcoma; yet, when the intratumoral heat-killed bacteria were combined with adoptively-transferred CD8+ T cells, durable tumor eradication was achieved (Binder et al., 2016). Collectively, these studies demonstrate that, in mice, bacteria and/or bacterial cell products, fail to achieve significant antitumor effects when administered alone, but under the right conditions, might effectively synergize with antitumor T cell immunity to achieve tumor eradication for certain tumor types.
How might FlaB-expressing bacteria induce a tumor-specific T cell response? Possible mechanisms might include: i) activation of multiple TLR pathways in lymphoid tissues resulting in enhanced T cell priming; ii) induction of S. Typhimurium-specific CD4+ T cells (Bergman et al., 2005) that presumably traffic to bacteria-colonized tumors, providing enhanced effector functions to tumor-specific CD8+ T cells; and/or iii) transformation of the tumor microenvironment into a pro-inflammatory one, thus presumably facilitating the antitumor functions of innate immune cells as well as effector CD8+ T cells. Because efficient antitumor T cell responses require both T cell priming and maintenance of T cell effector function in tumors, FlaB-expressing bacteria might likely provide unique immune cell activation in lymphoid tissues and the tumor.
Future experiments testing T cell-mediated mechanisms and therapeutic efficacy will be required in the context of treating large, well-established tumors in immunocompetent animals. In the current study, mice were treated with FlaB-expressing S. Typhimurium at approximately 10 days post-inoculation (Zheng et al., 2017), a time associated with the enrichment of artificial tumor-specific CD8+ T cells primed by the engraftment of tumor cells into animal subjects (Binder et al., 2013; Kline et al., 2008). One must consider that the current study’s monotherapeutic treatment of mice at an early time point might have potentially contributed to the impressive preclinical findings, and thus, may not be accurately predictive of a clinical response that could be extended to human cancer patients. Given the minimum requirement of two weeks post-inoculation of cancer cells to avoid this remnant response, future studies using FlaB-expressing S. Typhimurium should treat longer-established MC38 or B16F10 tumors which could be modeled by inoculating less numbers of cancer cells into mice, thereby extending the phase in which tumors become established. Further exploration of this bacterial therapy in terms of mechanistic insight as well as efficacy against established preclinical tumors of different immunogenicities and tissue types might address some of these translationally-relevant questions.
With the demonstration that tumor-trophic bacteria can facilitate remarkable and durable tumor control in immunocompetent rodents in a TLR-activating FlaB-dependent manner, the study by Jung-Joon Min’s laboratory has important implications for cancer immunotherapy. For instance, given its tumor-trophic and TLR-activating potential, FlaB-expressing S. Typhimurium may possess unique characteristics enabling them to synergize with the effects of immune checkpoint blockade treatment (e.g. anti-PD-1, anti-CTLA-4, etc.). Specifically, FlaB-expressing bacteria might induce TLR-mediated CD8+ and CD4+ T cell infiltration in otherwise poorly-infiltrated tumors that fail to respond to checkpoint blockade (Topalian et al., 2012). The bacteria, directly or indirectly, might also provide secondary activation to effector CD8+ T cells in the tumor that might otherwise be too dysfunctional to be rescued by checkpoint blockade alone. Thus, we argue that FlaB-expressing S. Typhimurium or other next-generation bacterial therapies, in combination with existing immunotherapies such as checkpoint blockade, bear great potential in cancer treatment approaches and should be explored in depth.
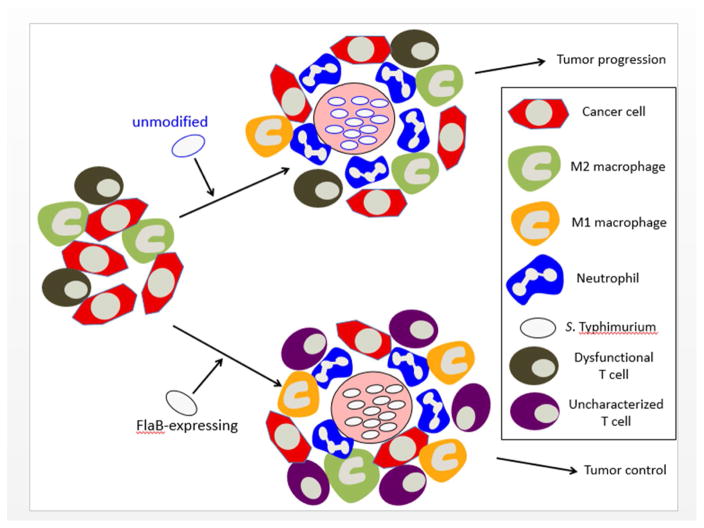
Figure 1. Tumor-Trophic FlaB-Expressing S. Typhimurium Induce Changes in the Tumor Microenvironment, Contributing to Antitumor Activity.
Upon intravenous injection in mice bearing tumors, S. Typhimurium bacteria induce a central necrotic core in tumors where they reside, recruiting high numbers of tumor-infiltrating neutrophils. The neutrophils form a “wall” such that the bacteria are separated from the viable tumor rim. Unmodified bacteria fail to control growth of the viable tumor rim, which is associated with the impaired effector functions of tumor-infiltrating CD8+ T cells (dysfunctional T cells). However, when attenuated bacteria express FlaB, an additional significant proinflammatory change occurs, where immunosuppressive M2 macrophages are replaced with immune-activating M1 macrophages. This treatment has been associated with durable tumor control; however, the precise role of T cells in this model remains to be defined (uncharacterized T cells).
Acknowledgments
This work was supported NIH grants K99 NS082381 (D.A.W.), R00 NS082381 (D.A.W.), and R01 NS097851-01 (D.A.W.), Robert H. Lurie Comprehensive Cancer Center – Zell Scholar Program of the Zell Family Foundation Gift (D.A.W.) and Cancer Research Institute – Clinic and Laboratory Integration Program.
Footnotes
Authors’ contributions: Writing, review, and/or revision of the manuscript D.C. Binder and D.A. Wainwright
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun. 2005;73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DC, Arina A, Wen F, Tu T, Zhao M, Hoffman RM, Wainwright DA, Schreiber H. Tumor relapse prevented by combining adoptive T cell therapy with Salmonella typhimurium. Oncoimmunology. 2016;5:e1130207. doi: 10.1080/2162402X.2015.1130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DC, Engels B, Arina A, Yu P, Slauch JM, Fu YX, Karrison T, Burnette B, Idel C, Zhao M, et al. Antigen-specific bacterial vaccine combined with anti-PD-L1 rescues dysfunctional endogenous T cells to reject long-established cancer. Cancer Immunol Res. 2013;1:123–133. doi: 10.1158/2326-6066.CIR-13-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- Leschner S, Westphal K, Dietrich N, Viegas N, Jablonska J, Lyszkiewicz M, Lienenklaus S, Falk W, Gekara N, Loessner H, et al. Tumor invasion of Salmonella enterica serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. PLoS One. 2009;4:e6692. doi: 10.1371/journal.pone.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother. 2002;25:218–225. doi: 10.1097/01.CJI.0000014623.45316.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, Shin MG, Chung IJ, Hong Y, Bom HS, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]