Abstract
An altered intestinal microbiota composition is associated with insulin resistance and type 2 diabetes mellitus. We previously identified increased intestinal levels of Eubacterium hallii, an anaerobic bacterium belonging to the butyrate-producing Lachnospiraceae family, in metabolic syndrome subjects who received a faecal transplant from a lean donor. To further assess the effects of E. hallii on insulin sensitivity, we orally treated obese and diabetic db/db mice with alive E. hallii and glycerol or heat-inactive E. hallii as control. Insulin tolerance tests and hyperinsulinemic-euglycemic clamp experiments revealed that alive E. hallii treatment improved insulin sensitivity compared control treatment. In addition, E. hallii treatment increased energy expenditure in db/db mice. Active E. hallii treatment was found to increase faecal butyrate concentrations and to modify bile acid metabolism compared with heat-inactivated controls. Our data suggest that E. hallii administration potentially alters the function of the intestinal microbiome and that microbial metabolites may contribute to the improved metabolic phenotype.
Introduction
The prevalence of obesity and type 2 diabetes mellitus is expected to rise to 1 in 3 adult subjects having type 2 diabetes mellitus in 2050.1 The pathophysiology of these metabolic disorders is complex, involving both environmental (dietary) and genetic factors affecting altered intestinal microbiota composition.2 Insulin resistant subjects are characterised by reduced levels of short-chain fatty acid (SCFA)-producing bacteria.3,4 Moreover, daily oral supplementation with the SCFA butyrate exerts beneficial effects on insulin resistance and dyslipidemia in diet-induced obese mice.5 Transplantation of lean healthy microbiota in both murine and human models of insulin resistance has been shown to significantly improve insulin sensitivity and to increase levels of butyrate-producing bacteria in the gut.6,7 With regards to the latter, we identified a specific increase in the butyrate-producer Eubacterium hallii in small intestinal biopsies of human obese and insulin resistant subjects upon lean donor faecal transplantation,7 which was associated with improved (peripheral) insulin sensitivity.
E. hallii is an anaerobic, Gram-positive, catalase-negative bacterium belonging to the Lachnospiraceae family of the phylum Firmicutes that is present in both murine and human faeces.8 E. hallii is a butyrate-producing species. Interestingly, in contrast to other intestinal bacterial isolates like Roseburia and Faecalibacterium that produce butyrate from monosaccharides, E. hallii has the capacity to also produce butyrate from lactate and acetate in a low pH environment such as the proximal small intestine.9 However, in vivo treatment with oral E. hallii has never been performed. We therefore performed a study in obese and insulin resistant db/db mice to investigate whether oral administration (by gavage) of E. hallii would have beneficial effects on insulin sensitivity. Upon identification of the optimal E. hallii treatment dose of 108 CFU per day, we used this dose to subsequently investigate the effect of active and heat-inactivated E. hallii treatment on insulin sensitivity and energy metabolism using hyperinsulinemic-euglycemic clamp and metabolic cage approaches.
We found that oral treatment with active E. hallii improved insulin sensitivity in severely insulin resistant db/db mice and significantly increased energy expenditure. Furthermore, our data indicate that E. hallii mildly modifies SCFA production and bile acid composition, which potentially contributes to the beneficial effects of E. hallii treatment on insulin sensitivity in obese and diabetic db/db mice.
Results
E. hallii treatment dose-dependently improves insulin-mediated glucose clearance
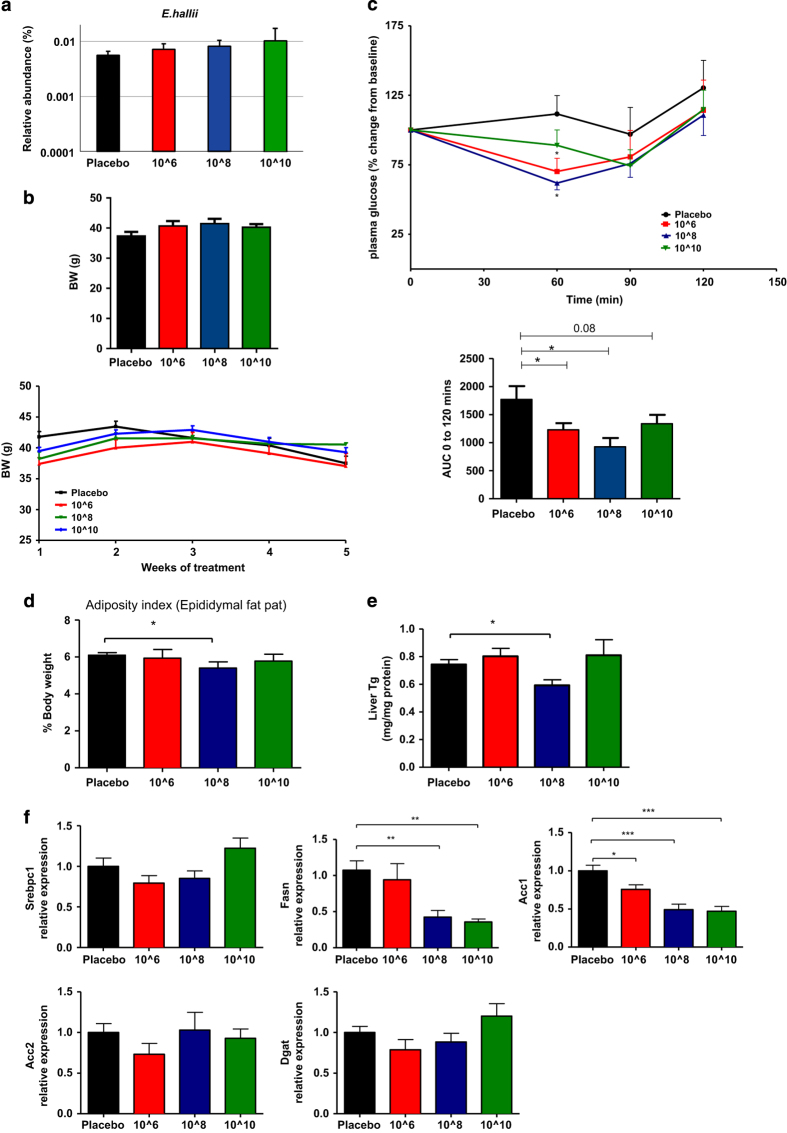
Oral butyrate supplementation has been previously reported to regulate insulin sensitivity.5 As E. hallii is a butyrate-producing bacterium, we assessed whether administration of E. hallii could have beneficial effects on insulin sensitivity in a mouse model for diabetes. We therefore explored the effects of oral administration of increasing dosages of E. hallii on basal parameters (i.e., body weight and food intake) and insulin responsiveness in severely obese and diabetic db/db mice. We found a dose-dependent increase in caecal E hallii concentrations upon treatment with 100 μl of active 106, 108 and 1010CFU E. hallii (once daily for four weeks) (Figure 1a). Nevertheless, global analysis showed no major effect on the intestinal communities (data not shown). Importantly, body weight remained stable in all treatment groups compared with glycerol-treated controls (106 CFU: 38±1.5 g, 108 CFU: 40±0.3 g and 1010 CFU: 41±0.3 g versus placebo: 39±1.3 g. NS; Figure 1b). We then set out to assess insulin responsiveness by performing intraperitoneal insulin tolerance tests (ITT) in all treatment groups. Interestingly, E. hallii-treated groups displayed significantly improved insulin-mediated reduction in blood glucose levels (106 CFU: −32±7%, 108 CFU: −39±9% and 1010 CFU; −34±7% P<0.05) after 4 weeks of treatment compared with glycerol-treated controls (−2±7% P<0.05; Figure 1c). Altogether, these data indicate that E. hallii treatment improves insulin-mediated reduction in glucose levels without affecting food intake and body weight in severely obese and diabetic mice. The 108 CFU E. hallii-treated mice exhibited the most remarkable response to insulin at all time points (t=60, 90 and 120 min). In addition, 108 CFU E. hallii administration resulted in significantly reduced epididymal fat pad weight (Figure 1d) and hepatic triglyceride levels (Figure 1e), which was also reflected in the expression pattern of genes involved in lipogenesis (Fasn and Acc1 were significantly reduced, (Figure 1f) and gluconeogenesis (trend towards reduction of G6Pc, Pk, Pck1 were noticed), (Supplementary Figure S1). This to us suggested that 108 CFU of E. hallii would be the optimal dosage to perform further investigations.
Figure 1.
E. hallii treatment dose-dependently improves insulin sensitivity. Male db/db mice (n=8 per group) were daily treated with vehicle or increasing doses of E. hallii by gavage for 4 weeks. Figures depict effect of E. hallii treatment on (a) relative abundance of E. hallii in caecum, (b) body weight (showing average body weight per treatment group after 4 weeks of treatment and weekly weight gain throughout treatment period), (c) insulin tolerance test (showing insulin-mediated glucose clearance on t=60, 90 and 120 min after insulin administration and corresponding area under the curve (AUC)), (d) adiposity index (epididymal fat pad as % of body weight), (e) hepatic triglyceride (TG) content, (f) expression levels of hepatic lipogenic genes. Data are mean±s.d. Statistical analysis was performed using Student’s t-test. *P<0.05; **P<0.01.
E. hallii treatment improves insulin sensitivity and increases energy expenditure
On the basis of the results from the dose–response study, we chose 108 CFU E. hallii as daily therapeutic dose and repeated the study using active and heat-inactivated E. hallii as control. In line with observations from the dose–response study, body weight and food intake (Figure 2a,b) were similar in active and heat-inactivated E. hallii-treated mice. In addition, lean and fat mass (as % of body weight) were similar in both treatment groups (Figure 2c).
Figure 2.
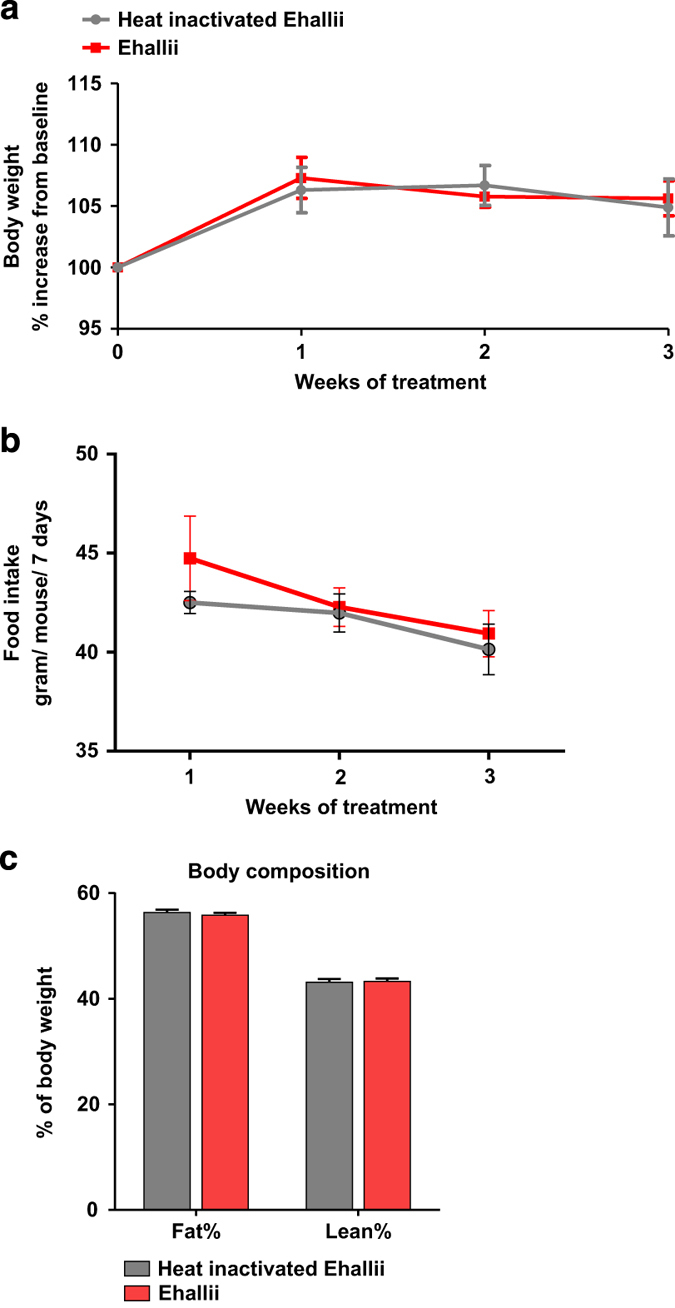
Effect of active and heat-inactivated E. hallii on body weight, food intake and body composition. Male db/db mice (n=7–10 per group) were daily treated with active or heat-inactivated E. hallii (108 CFU) for 4 weeks. Figures depict effect of active or heat-inactivated E. hallii treatment on (a) body weight. (b) food intake and (c) body composition (as determined by magnetic resonance imaging). Data are mean±s.d. Statistical analysis was performed using Student’s t-test *P<0.05.
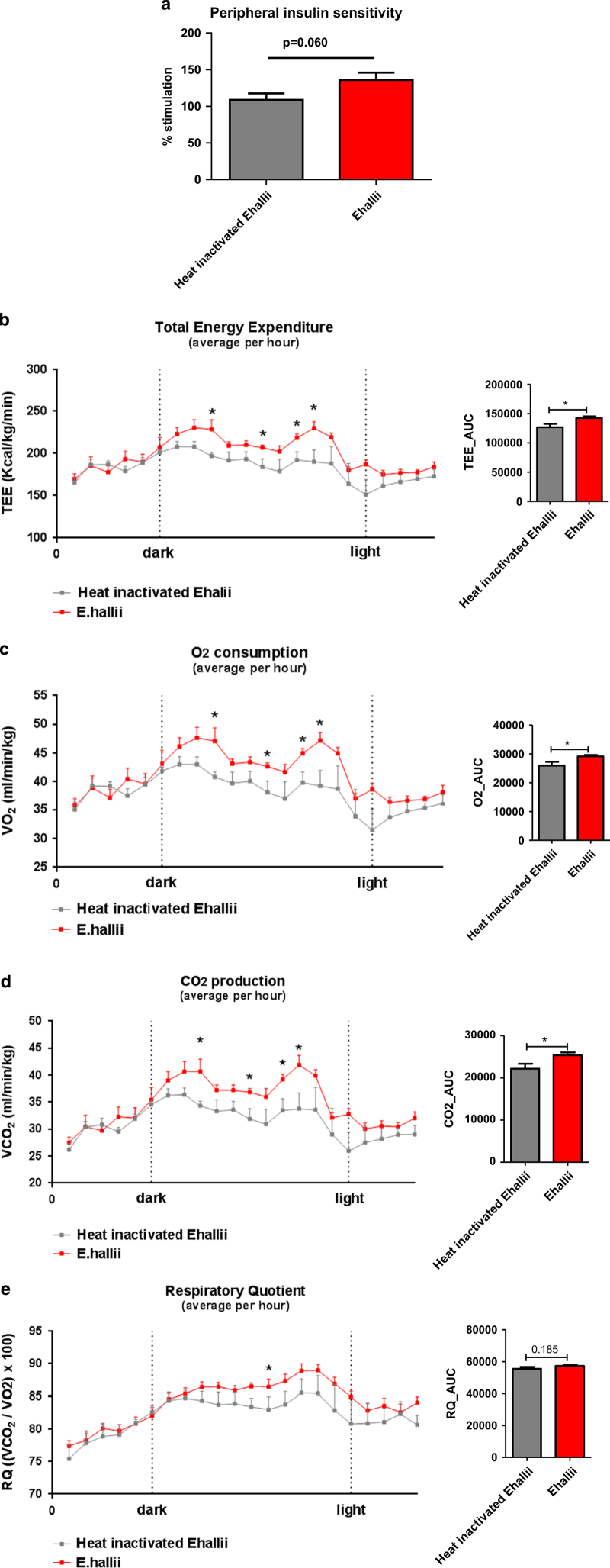
Considering the effects of E. hallii treatment on insulin-mediated reduction in glucose levels as assessed by ITT, we moved forward with an in-depth assessment of insulin sensitivity by performing hyperinsulinemic-euglycemic clamp experiments in conscious, unrestrained mice. We assessed the ability of insulin to suppress endogenous Ra (endogenous rate of appearance, a marker of hepatic glucose production) and whole-body glucose disappearance (Rd; Supplementary Table S1). Although endogenous glucose production was not significantly altered (active: −33.9±3.7% versus heat-inactivated: −41.1±5.4%, P=0.299), treatment with E. hallii led to a close-to-significant increase in the ability of insulin to stimulate Rd (active: 136% versus heat-inactivated: 109%, P=0.060; Figure 3a). Considering the fact that db/db mice are severely insulin resistant, the improved Rd following 4 weeks of E. hallii treatment is of significant biological relevance.
Figure 3.
E. hallii treatment improves insulin sensitivity and energy expenditure. Male db/db mice (n=7–10 per group) were daily treated with active or heat-inactivated E hallii (108 CFU) for 4 weeks. Figures depict (a) effect on peripheral insulin sensitivity as assessed by hyperinsulinemic-euglycemic clamp, (b) total energy expenditure, (c) O2 consumption, (d) CO2 production and (e) respiratory exchange ratio. Data are mean±s.d. Statistical analysis was performed using Student’s t-test *P<0.05.
Butyrate supplementation has previously been shown to improve energy expenditure in diet-induced obese mice.5 Altogether with our data on the beneficial effects of E. hallii, a butyrate producer, on insulin sensitivity in db/db mice, this motivated us to assess the effect of E. hallii on energy expenditure in this mouse model. Energy expenditure, oxygen consumption and CO2 production were monitored in metabolic chambers. Interestingly, active E. hallii treatment significantly increased total energy expenditure (active: 214±4 kcal/kg/min versus heat-inactivated: 191±9 kcal/kg/min, P<0.05; Figure 3b), oxygen consumption (active: 44.1±0.9 ml/min/kg versus heat-inactivated: 39.6±1.8 ml/min/kg, P<0.05; Figure 3c) and CO2 production (active: 38.0±1.0 ml/min/kg versus heat-inactivated: 33.4±1.8 ml/min/kg, P<0.05; Figure 3d) in the dark cycle. Respiratory quotient (expressed as VCO2/VO2) was not significantly altered (Figure 3e). In addition, to assess potential E. hallii-mediated changes in energy absorption, we analysed genes involved in glucose and lipid absorption in proximal part of the intestine. E. hallii treatment reduced intestinal genes involved in glucose (Sglt1 and Glut2) transport and lipid absorption (Cd36 and Fatp4; Supplementary Figure S2).
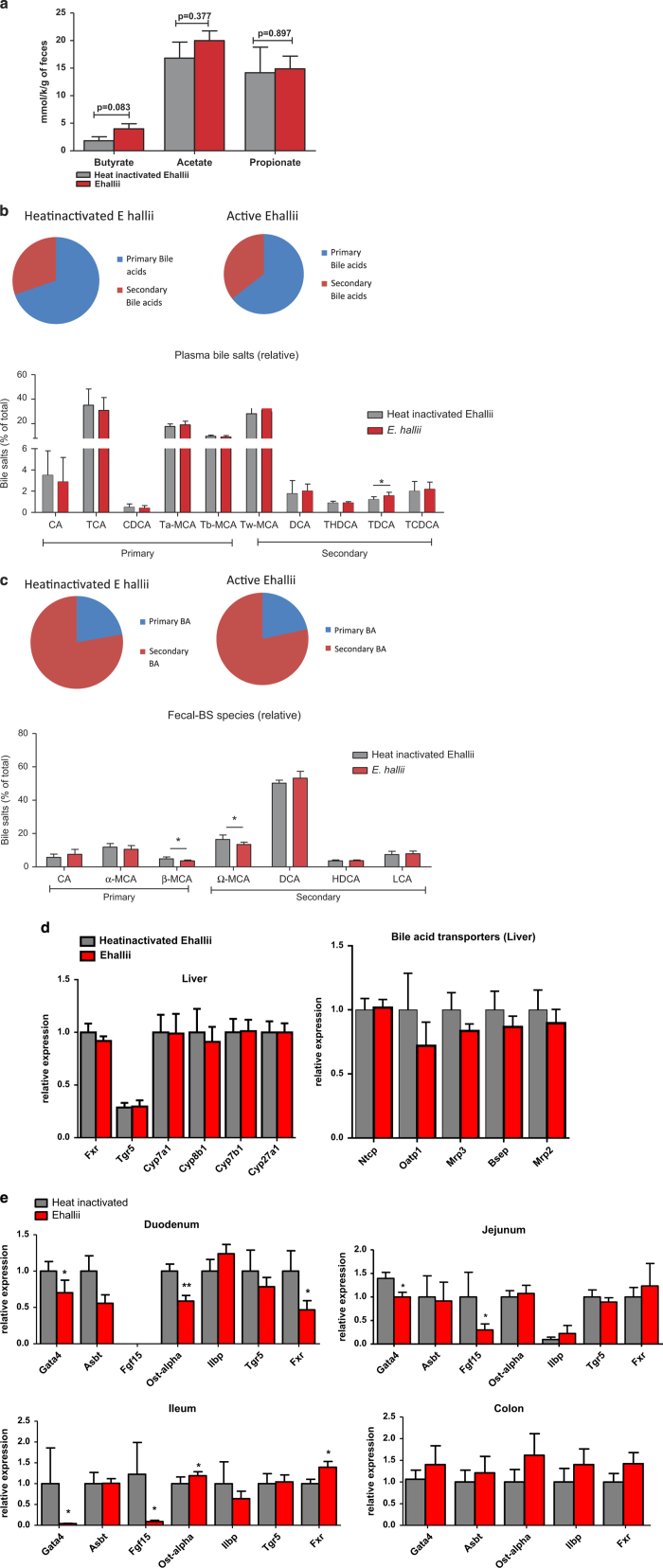
To assess whether treatment with E. hallii increased SCFA levels, potentially providing insight into E. hallii-mediated effects on energy metabolism, we collected faeces (24 h) and measured concentrations of the SCFA’s butyrate, acetate and propionate. Active E. hallii treatment moderately increased faecal butyrate concentrations compared with heat-inactivated controls while propionate and acetate concentrations remained unaffected (Figure 4a).
Figure 4.
Effect of E. hallii treatment on short-chain fatty acids (SCFA’s) and bile acids. Male db/db mice (n=7–10 per group) were daily treated with active or heat-inactivated E. hallii (108 CFU) for 4 weeks. Figures depict (a) faecal SCFA levels, (b) plasma primary and secondary bile acids and plasma bile acid composition, (c) faecal primary and secondary bile acids and plasma bile acid composition, (d) hepatic and (e) intestinal (duodenum, jejunum, ileum and colon) expression of genes involved in bile acid metabolism and transport. Data are mean±s.d. Statistical analysis was performed using Student’s t-test *P<0.05; **P<0.01.
Alterations in gut microbiota composition have significant impact on bile acid levels and bile acid composition.10 In addition to their role in solubilising food and uptake of food-soluble vitamins, bile acids are also important regulators of glucose and energy homeostasis.11 We therefore assessed whether active E. hallii treatment affected plasma and faecal bile acid levels and composition. Plasma primary and secondary bile acid levels were similar in active versus heat-inactivated E. hallii-treated mice (Figure 4b, pie chart). Further analysis of primary and secondary bile acid species revealed that the concentration of the secondary bile acid tauro-conjugated deoxycholic acid was significantly increased (Figure 4b, bar graph). Faecal primary and secondary bile acid levels remained unaffected by active E. hallii treatment (Figure 4c, pie chart). Levels of the primary bile acid β-MCA and the secondary bile acid ω-MCA, however, were significantly reduced in active versus heat-inactivated E. hallii -treated mice (Figure 4c, bar graph).
We then assessed expression levels of genes involved in bile acid metabolism and transport in liver and small intestine. Bile acid synthesis is tightly regulated by the bile acid receptor Farnesoid X receptor (Fxr) in liver and intestine. Hepatic Fxr exerts negative feedback control on cholesterol 7 alpha-hydroxylase (Cyp7al), the rate-limiting enzyme in hepatic bile salt synthesis.12 Expression levels of hepatic Fxr and Cyp7a1 were similar in active and heat-inactive E. hallii-treated db/db mice (Figure 4d). Expression of genes encoding bile acid-synthetic genes such as (Cyp7a1, Cyp8b1, Cyp7b1 and Cyp27a1) and bile acid transporters (Ntcp, Oatp1, Mrp3, Bsep and Mrp2) remained unaffected by active E. hallii treatment. Although expression of Fxr in the small intestine was not altered by active E. hallii treatment, levels of fibroblast growth factor 15 (Fgf15), an FXR-target gene, were significantly reduced, which is suggestive of reduced activation of FXR in the intestine (31). We investigated the effect of active E. hallii treatment on genes regulating intestinal bile acid absorption by analysing the transcription factor (Gata4), apical sodium-dependent bile acid transporter (Abst), apical organic solute transporter (Ostα) and ileal lipid binding protein (Ilbp).13 Active E. hallii treatment significantly reduced and increased the expression of Gata4 and Ostα, respectively, compared with heat-inactive E. hallii treatment. Nevertheless, expression levels of Ilbp and Abst remained unaffected (Figure 4e).
Discussion
The current study demonstrates that daily oral administration of E. hallii improves insulin sensitivity and increases energy metabolism in severely obese and diabetic db/db mice. Our observations that administration of increasing dosages of E. hallii did not affect body weight or food intake indicate that E. hallii treatment might be a safe and effective new probiotic strain to improve insulin sensitivity.
In the dose–response study, we found that E. hallii treatment improved insulin sensitivity, yet the highest treatment dose had less effect on insulin sensitivity than the lower dosages. This phenomenon was also found in a human intervention trial using B. infantis and might be explained by the fact that these high concentrations (>1010 CFU of bacterial strains) induce a crowding effect resulting in less efficient dispersion of the bacteria over the (small) intestine.14 Moreover, oral supplementation of heat-inactivated E. hallii had no effect on murine metabolism, which is in line with the previous data studying the role of specific microbial strains on insulin sensitivity.15 Moreover, as we did not see any effect on body weight and the fact that we do not have the data on locomotor activity upon 4 weeks of E. hallii treatment, further studies will have to elucidate the long-term effects of E. hallii on all these parameters.
It has long been recognised that intestinal bacteria affect SCFA concentrations.8,9 Bacterial fermentation of indigestible fibres in the intestine, for example, by the butyrate-producer E. hallii, results in the production of SCFAs such as butyrate. Oral SCFAs administration to mice fed a high-fat diet reduced body weight and improved insulin sensitivity without changing food intake or levels of physical activity.5 SCFAs have been suggested to act on food intake through G-protein-coupled receptors such as GPR41 and GPR43, which subsequently increase release of the satiety hormones PYY and GLP-1. Furthermore, butyrate has been implicated in regulation of intestinal gluconeogenesis thereby improving glucose and energy homeostasis.16 Although oral E. hallii treatment had only minor effects on intestinal E. hallii abundance, levels of the SCFA butyrate, a metabolite of E. hallii, were doubled (~217%, NS) in active E. hallii-treated mice compared with heat-inactivated E. hallii-treated controls. Increased butyrate levels might potentially mediate the observed beneficial effects on peripheral insulin sensitivity and energy expenditure in active E. hallii-treated db/db mice. However, this hypothesis would require further analysis.
After release into the duodenum, bile acids travel the length of the small intestine and are reabsorbed and transported back to the liver mainly in the distal ileum.17,18 Ruminococcaceae and Lachnospiraceae families of the Firmicutes phylum (such as E. hallii) can mediate primary bile acid conversion to secondary bile acids.19 Furthermore, modulation of intrinsic bacterial bile acid hydrolysis significantly impacts bile acid composition and subsequent metabolic processes in the host.20 Although we found only a small effect of E. hallii treatment on intestinal bile acid metabolism, it is tempting to speculate that E. hallii indeed affects energy metabolism and insulin sensitivity via bile acids. Indeed, the E. hallii L2–7 genome contains 2 complete functional bile salt hydrolase (BSH) genes (W.M.d.V., personal communication) and their role in bile acid metabolism is currently under detailed investigation. Although total plasma and faecal secondary bile acid levels were similar in active and heat-inactivated E. hallii-treated db/db mice, active E. hallii treatment increased levels of the secondary bile acid tauro-conjugated deoxycholic acid. Interestingly expression of other FXR targets such as Ilbp and Abst remained unaltered, but expression of the transcription factor Gata4 decreased significantly. A similar association between Gata4 and Fgf15 was recently reported by Out et al.21 and might be a direct interaction of microbiota with Gata4 expression as also suggested in (ref. 13). Furthermore, changes in intestinal bacteria have been shown to primarily affect Fxr targets in the small intestine and not the liver.19,22,23 This is in line with our observation of decreased expression of the intestinal Fxr target gene Fgf15 but not the hepatic Fxr target Shp after active E. hallii treatment. Microbiota modifications using probiotics have been reported to facilitate changes in intestinal bile acid transport,24 which is in line with the appreciable elevation of the bile acid transporter Ostα in the present study.
In conclusion, we show that daily treatment for 4 weeks with E. hallii L2–7 has no adverse effects and exerts beneficial effects on metabolism, potentially via alterations in butyrate formation and bile acid and metabolism.25,26 Our data thus underscore the therapeutic potential of replenishing missing intestinal bacterial strains for the treatment of human insulin resistance.27 Further research to confirm optimal dose and long-term effects of E. hallii on human insulin sensitivity and bile acid metabolism is urgently awaited.
Materials and methods
E. hallii culture
E. hallii strain L2–7 was cultured under anaerobic conditions as described previously.8,9 Purity was identified by cellular morphology and 16S RNA gene sequence analysis. Cultures were grown to the end of the exponential phase, concentrated by anaerobic centrifugation, washed with phosphate-buffered saline, diluted in a solution containing maltodextrin and glucose in 10% glycerol until final concentrations of 106 colony forming units (CFU), 108 CFU and 1010 CFU in 100 μl were reached. Viability was assessed by most probable number analysis by dilution to extinction and confirmed by microscopic analysis. Samples were stored at −80 °C and used within 6 months during which viability was not noticeably affected.
Animals
All animal experiment were conducted in accordance with the principles of the ‘Guide to the Care and Use of Experimental Animals’ and were approved by the local Animal Ethics Committee, Academic Medical Center-University of Amsterdam, and the University of Gothenburg Animal Studies Committee.
The methods were carried out in accordance with the approved guidelines. Male C57Bl6/J db/db mice (12 weeks old) were purchased from the Jackson Laboratories USA. Animals were housed at AMC SPF vivarium in groups of 5 animals/cage and fed ad libitum with regular chow diet (Research Diets, Inc.) and water. Mice were housed under constant temperature and a 12-h light/dark cycle. At 16 weeks of age, the animals were daily given an oral 100 μl gavage of comprising 106, 108 and 1010 CFU of E. hallii in 10% glycerol stock for 4 weeks (n=8 mice per group). As a control, an oral 100 μl gavage of 10% glycerol in phosphate-buffered saline was used (n=8 mice). Twenty-four-hour faeces were collected after 4 weeks of treatment (24 h collection) for bile acid composition analysis. In the last week of treatment and after an overnight fast, mice (n=8 per group) received an intraperitoneal insulin bolus (Actrapid 0.75 U/kg body weight) and blood glucose was measured (Ascensia Elite glucose meter, Bayer, Leverkusen, Germany) at t=0, 60, 90 and 120-min post injection for determination of insulin sensitivity. Thereafter, animals were sacrificed using 100 mg/kg pentobarbital and faeces and caecum were collected.
Hyperinsulinemic-euglycemic clamp
Male C57Bl6/J6 db/db mice (12 weeks old) were purchased from the Jackson Laboratories, Bar Harbor, ME, USA. Animals were housed at University of Gothenburg SPF vivarium and fed ad libitum with regular chow diet (Research Diets, New Brunswick, NJ, USA) and water. Mice were housed under constant temperature and a 12-h light/dark cycle and underwent daily oral 100 μl gavage for 4 weeks with 108 CFU active or heat-inactivated E. hallii (15 min at 70 °C) as control (n=7–10 per group). In the last week of treatment, at least 4 days before the clamp a catheter was surgically placed in the jugular vein for infusion of insulin and glucose under isoflurane anaesthesia. Prior to the clamp, mice were fasted for 4 h and placed in individual plastic containers. Basal blood glucose (Countour Next blood glucose meter, Bayer AB, Solna, Sweden) was used from tail-blood measurements. A bolus injection of [3-3H] glucose (5 μCi; PerkinElmer, Waltham, MA, USA) was given through the jugular vein catheter (t=−80 min prior insulin infusion), followed by a continuous infusion of 0.05 μCi/min for assessment of basal glucose turnover rate. Three consecutive blood samples were taken at steady state (t=−20, −10 and 0 min prior insulin infusion) for the determination of both plasma [3-3H] glucose and glucose concentration. At t=0, a priming dose of insulin (178 mU/kg; Actrapid Penfill, Novo Nordisk, Bagsværd, Denmark) was given, followed by a continuous insulin infusion rate of 20 mU/min/kg. The infusion of [3-3H] glucose was increased to 0.1 μCi/min during clamp to minimise changes in specific activity during insulin infusion. Blood glucose was measured at 10-min intervals, via tail-blood sampling, to adjust the glucose infusion rate (GIR; 30% glucose Fresenius Kabi, Bad Homburg, Germany) to maintain blood glucose concentration at the basal level. At steady state, defined by stable glycemia and GIR (approximately at t=120 m interval), three consecutive blood samples were taken at 10 min intervals to determine whole-body glucose utilisation (Rd) and hepatic glucose production (Ra) under hyperinsulinemic-euglycemic- conditions. Plasma insulin was measured at t=−10 min (basal) and 120 min (clamp). Animals were killed by an overdose of pentobarbital (Apoteket Farmaci AB, Stockholm, Sweden) and tissue was collected. The blood samples were deproteinised, evaporated and resuspended in deionised water for the determination of radioactivity (Beckman LS6500 Multipurpose Scintillation Counter, Providence, RI, USA). Whole-body glucose appearance (Ra) and endogenous glucose production (endogenous Ra), a measure of hepatic glucose production, were calculated as published as previously described.28
Metabolic chamber experiments and body composition
During a parallel experiment, male db/db mice (aged 12 weeks, n=7–10 per group) were treated orally with 100 μl of 108 CFU active or heat-inactivated E. hallii (15 min at 70 °C) as control for 4 weeks. Thereafter, mice were individually housed in Somedic INCA metabolic cages (Somedic AB, Hörby, Sweden) to study total energy expenditure and respiratory quotient. Oxygen consumption (VO2) and CO2 production (VCO2) were recorded every 2 min for 23 h. Temperature in the metabolic chamber was kept constant at 21 °C and animals had free access to food and water. Data from the first hour was discarded to account for animal acclimatisation. The average total energy expenditure per hour was determined using Weir's equation: (3.9×VO2)+(1.1×VCO2) and respiratory quotient was calculated as the VCO2/VO2 ratio. Also, magnetic resonance imaging scanning for body composition was performed as previously described.25
Intestinal microbiota analysis
Abundances of E. hallii were determined in caecal content by using the Mouse Intestine Tract Chip as previously reported.15,29 Total genomic DNA was extracted from the frozen caecum with the QIAamp DNA stool mini-kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. 16S rRNA gene amplification, in vitro transcription and labelling, and hybridisation were carried out as described.30 The data were normalised and analysed using a set of R-based scripts in combination with a custom-designed relational database, which operates under the MySQL database management system. For the microbial profiling, the Robust Probabilistic Averaging signal intensities of 2667 specific probes for the 94 genus-level bacterial groups detected on the MITChip were used.31 Diversity calculations were performed using a microbiome R-script package (https://github.com/microbiome). Multivariate statistics, redundancy analysis, and principal response curves were performed in Canoco 5.0 and visualised in triplots or a principal response curves plots.32
SCFA and bile acid profiling
Twenty-four-hour faecal samples (pooled from each cage) were collected and stored for later analysis. SFCA content was analysed by gas liquid chromatography following conversion to t-butylmethylsilyl derivate as previously described.9 Concentrations of different bile acids were measured twice in 24-h faecal samples collected in week 4 and in plasma. An internal standard was added before extraction with 0.2 mol/l NaOH at 800 °C for 20 min. Bile salt were trimethylsilylated with pyridine, N,O-Bis(trimethylsilyl) trifluoroacetamide and trimethylchlorosilane. Faecal bile acid profile was measured using capillary gas chromatography (Hewlett–Packert gas chromatograph; HP 6890, Mountain View, CA, USA) equipped with a FID and a CP Sil 19 capillary column; length 25 m, internal diameter 250 μm and a film thickness of 0.2 μm (Chrompack BV, Middelburg, The Netherlands). Plasma bile acids were determined using liquid chromatography tandem mass spectrometry as described previously (17). The primary bile acids cholic acid (CA), taurocholic acid, muricholic acid (MCA), tauroalpha muricholic acid and taurobeta muricholic acid as well as the secondary bile acids taurohyodeoxycholic acid, deoxycholic acid, taurodeoxycholic acid and omega murocholic acid were analysed in plasma and 24 faeces.21 The total amount of primary and secondary bile acids was calculated as the sum of the individually quantified bile salts.
Quantitative real-time PCR
Liver and intestinal tissues were homogenised with tissue-magnaLyzer (Roche, Basel, Switzerland). Total RNA was extracted using Tri-pure reagent (Roche). Complementary DNA was prepared by reverse transcription of 1 μg total RNA using reverse transcription kit (BioRad, Hercules, CA, USA). Hepatic genes involved in lipogenesis (Srebp1c, Fasn, Acc1, Acc2 and Dgat) and gluconeogenesis (Gck1, G6Pc, Pk and Pck1) were examined. Genes involved in bile acid metabolism and transport were tested in liver (Cyp7a1, Cyp8b1, Cyp7b1 and Cyp27a, Ntcp, Oatp1, Mrp3, Bsep and Mrp2) and proximal to distal intestinal segments (duodenum, jejunum and ileum) for (Tgr5, Fxr, Gata4, Asbt, Ilbp Ostα and Fgf15).33 Real-time quantitative PCR was performed using Sensifast SYBR master mix (GC biotech, Alphen a/d Rijn, the Netherlands). Gene-specific intron–exon boundary spanning primers were used and all the results were normalised with the house keeping gene 36B4. All samples were analysed in duplicate and data were analysed according to the 2ΔΔCT method. Primer sequences are presented in Supplementary Table S2.
Statistical analysis
On the basis of distribution of the clinical data, Student t-test or Mann–Whitney tests (two-sided) were used to analyse the difference between clinical groups. Microbiota analyses were done as described above. *P value<0.05 or **P<0.01 were considered statistically significant.
Acknowledgments
F.B. is supported by Swedish Research Council, Swedish Diabetes Foundation, Swedish Heart Lung Foundation, Swedish Foundation for Strategic Research, Knut and Alice Wallenberg foundation, Göran Gustafsson Foundation, Ingbritt and Arne Lundberg’s foundation, Swedish Heart Lung Foundation, Torsten Söderberg’s Foundation, Ragnar Söderberg’s Foundation, NovoNordisk Foundation, AFA insurances, and LUA-ALF grants from Västra Götalandsregionen and Stockholm County Council. F.B. is a recipient of ERC Consolidator Grant (European Research Council, Consolidator grant 615362—METABASE). W.M.d.V. is supported by the Finland Academy of Sciences (grants 137389, 141140 and 1272870 ), the Netherlands Organization for Scientific Research (Spinoza Award and SIAM Gravity Grant 024.002.002) and the European Research Council (ERC Advanced Grant 250172 MicrobesInside). M.N. is supported by a ZONMW-VIDI grant 2013 (016.146.327).
Footnotes
Supplementary Information accompanies the paper on the npj Biofilms and Microbiomes website (http://www.nature.com/npjbiofilms)
M.N. and W.M.d.V. are founders, own equity and are in the Scientific Advisory Board of Caelus Pharmaceuticals, The Netherlands; M.N. is in the Scientific Advisory Board of Seres Health, Boston USA; W.M.d.V. is in the Scientific Advisory Board of Chr Hansen Horsholm Danmark and the Nestle Institute for Health Science (NIHS) Lausanne Switzerland. F.B. is a founder of and owns equity in MetaboGen AB, Sweden. The remaining authors declare no conflict of interest.
References
- Boyle, J. P., Thompson, T. J., Gregg, E. W., Barker, L. E. & Williamson, D. F. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul. Health Metr. 8, 29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. T., Nieuwdorp, M. & Backhed, F. Microbial modulation of insulin sensitivity. Cell Metab. 20, 753–760 (2014). [DOI] [PubMed] [Google Scholar]
- Karlsson, F. H. et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103 (2013). [DOI] [PubMed] [Google Scholar]
- Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013). [DOI] [PubMed] [Google Scholar]
- Gao, Z. et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916 e917 (2012). [DOI] [PubMed] [Google Scholar]
- Louis, P., Young, P., Holtrop, G. & Flint, H. J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314 (2010). [DOI] [PubMed] [Google Scholar]
- Duncan, S. H., Louis, P. & Flint, H. J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microb. 70, 5810–5817 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann, J. R. et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl Acad. Sci. USA 108, 4523–4530 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon, P. B. et al. Bile acids as regulatory molecules. J. Lipid Res. 50, 1509–1520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000). [DOI] [PubMed] [Google Scholar]
- Beuling, E. et al. Conditional Gata4 deletion in mice induces bile acid absorption in the proximal small intestine. Gut 59, 888–895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwell, P. J. et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 101, 1581–1590 (2006). [DOI] [PubMed] [Google Scholar]
- Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder, F. et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 (2014). [DOI] [PubMed] [Google Scholar]
- Dawson, P. A., Lan, T. & Rao, A. Bile acid transporters. J. Lipid Res. 50, 2340–2357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, J. Y. L. Bile acids: regulation of synthesis. J. Lipid Res. 50, 1955–1966 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley, M., Hill, C. & Gahan, C. G. M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microb. 72, 1729–1738 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, S. A. et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl Acad. Sci. USA 111, 7421–7426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Out, C. et al. Prednisolone increases enterohepatic cycling of bile acids by induction of Asbt and promotes reverse cholesterol transport. J. Hepatol. 61, 351–357 (2014). [DOI] [PubMed] [Google Scholar]
- Miyata, M., Takamatsu, Y., Kuribayashi, H. & Yamazoe, Y. Administration of ampicillin elevates hepatic primary bile acid synthesis through suppression of ileal fibroblast growth factor 15 expression. J. Pharmacol. Exp. Ther. 331, 1079–1085 (2009). [DOI] [PubMed] [Google Scholar]
- Li, F. et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4: 2384. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirolamo, C., Rainaldi, S., Bovenga, F., Murzilli, S. & Moschetta, A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 7, 12–18 (2014). [DOI] [PubMed] [Google Scholar]
- Fang, S. et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 21, 159–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos, W. M. & Nieuwdorp, M. GENOMICS: a gut prediction. Nature 498, 48–49 (2013). [DOI] [PubMed] [Google Scholar]
- Cansby, E. et al. Increased expression of STK25 leads to impaired glucose utilization and insulin sensitivity in mice challenged with a high-fat diet. FASEB J. 27, 3660–3671 (2013). [DOI] [PubMed] [Google Scholar]
- Ramirez-Farias, C. et al. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 101, 541–550 (2009). [DOI] [PubMed] [Google Scholar]
- Rajilic-Stojanovic, M. et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ. Microbiol. 11, 1736–1751 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti, L., Elo, L. L., Aittokallio, T. & Kaski, S. Probabilistic analysis of probe reliability in differential gene expression studies with short oligonucleotide arrays. IEEE/ACM Trans. Comput. Biol. 8, 217–225 (2011). [DOI] [PubMed] [Google Scholar]
- Braak, C. J. F. ter & Smilauer, P. Canoco reference manual and user's guide: software for ordination, version 5.0. m. Microcomputer Power - p496. Ithaca, USA (2012).
- Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.