Abstract
Purpose of review
Manganese (Mn) is critical for neurodevelopment but also has been implicated in the pathophysiology of several neurological diseases. We discuss how Mn requirements intersect with Mn biology and toxicity, and how these requirements may be altered in neurological disease. Furthermore, we discuss the emerging evidence that the level of Mn associated with optimal overall efficiency for Mn-biology does not necessarily coincide with optimal cognitive outcomes.
Recent findings
Studies have linked Mn exposures with urea cycle metabolism and autophagy, with evidence that exposures typically neurotoxic may be able to correct deficiencies in these processes at least short term. The line between Mn-dependent biology and toxicity is thus blurred. Further, new work suggests that Mn exposures correlating to optimal cognitive scores in children are associated with cognitive decline in adults.
Summary
This review explores relationships between Mn-dependent neurobiology and Mn-dependent neurotoxicity. We propose the hypothesis that Mn levels/exposures that are toxic to some biological processes are beneficial for other biological processes and influenced by developmental stage and disease state.
Keywords: Mn biology, neurotoxicity, neurological disease, aging
Introduction
Mn is an essential metal for human health, found naturally within the environment and necessary for several enzymes regulating oxidative stress(1), anti-oxidant status(2), mitochondrial function(3), and neurotransmitter synthesis(4). More recent work on metal biology has identified a role for Mn in urea metabolism(5) and autophagy (6). Several recent reviews on Mn-biology describe in detail the role of Mn as a co-factor for these biologic and metabolic pathways(7). However, fewer studies have addressed the direct connection between increased Mn exposure and increased enzyme activity in the context of neurological disease. This review examines the evidence that Mn-dependent biology within the urea cycle and autophagy are critical mediators of several neurological diseases including Parkinson’s Disease (PD), Alzheimer’s Disease (AD) and Huntington’s Disease (HD). Additionally, we discuss the potential discrepancies between optimal Mn levels for biological and cognitive function relative to Mn neurotoxicity. It is clear from epidemiologic studies that low and high Mn exposures in both children and adults result in cognitive deficits(8). Although there is debate over the accuracy of biomarkers for Mn exposure, there are several biomarkers positively associated with impaired cognitive development and function(9,10). Thus far, there is a prevailing view that Mn exposure which results in efficacious Mn-dependent biology is equally well suited for optimal cognitive development and function, however this has yet to be directly tested and several in vivo and in vitro studies provide evidence to the contrary. We postulate here that there is a growing body of evidence for a discordance between the Mn levels associated with optimal efficiency of several Mn-dependent biological processes and the Mn levels which provides the maximal cognitive benefit. We further discuss the possiblity that these beneficial effects of Mn, may indeed overlap with neurotoxic effects of Mn exposure.
Mn exposure increases Mn-dependent enzyme expression and activity
It is reasonably assumed that neuronal deficits in bioavailable Mn may be associated with deficits in Mn-dependent enzyme activity. Further, there are several clinical and translational studies that demonstrate a deficit in metabolic pathways requiring Mn are associated with neurological disease(5,11,12). Until recently, it was unknown whether Mn exposure could reverse deficits in Mn-dependent enzymatic reactions associated with disease, by increasing the bioavailability of the essential cofactor for these reactions. On one hand, there are reports that chronic Mn exposure ultimately decreases the expression of Mn-dependent enzymes(13) although these findings could also represent neuroprotective/homeostatic responses to restore enzymatic activity due to excess availability of the essential cofactor, especially in conditions where Mn may be rate limiting. Recent work from our lab provides evidence of acute systemic Mn exposure correcting deficits in Mn-dependent biology that have been associated with the pathophysiology of Huntington’s disease (HD).
Mn and urea cycle metabolism
The neuronal urea cycle is associated with two Mn-dependent enzymes, suggesting that it could be particularly vulnerable to alterations in Mn bioavailability. The most abundant of the Mn-dependent urea cycle enzyme is arginase, which hydrolyzes arginine into ornithine and urea. Though evidence for urea cycle defects in the brain of HD patients had not been previously identified, a recent study found urea was elevated in post-mortem brain samples of HD patients (5). However, the mechanism for this defect in urea cycle metabolism is unclear. In patients with HD, citrulline is increased in plasma, further evidence that the urea cycle is impacted in the periphery(14) as well as in the central nervous system.
Tight control of arginine homeostasis is critical for neuronal health. Arginine can be converted into nitric oxide and citrulline by nitric oxide synthase and although nitric oxide is an essential neurotransmitter, in excess, nitric oxide has known deleterious effects on cell signaling molecules and regulates neuronal survival(15). Nitric oxide levels are altered in both HD and Alzheimer’s Disease (AD) and arginine levels are altered in AD (16,17). In HD, further evidence that the urea cycle is dysregulated comes from animal models, in which there is increased citrulline in brain tissue, suggesting a deficiency in arginase activity (18). Additionally, HD animals supplemented with excess L-arginine had significantly earlier symptom onset and cognitive impairments compared to control animals(19) which also provides support for impaired arginine metabolism in HD. It should be acknowledged that elevated brain urea in HD post-mortem tissue suggests either excess urea cycle activity, or a deficit in urea clearance.
In contrast with many enzymes that require metal ion co-factors, Mn cannot be replaced with other common divalent ions as a co-factor for arginase(20). A reduction in the bioavailability of the co-factor Mn, either globally, or within specific brain regions, would be expected to slow arginase kinetics, and indeed, animals fed a Mn-deficient diet have reduced arginase activity(21). For a more detailed description of arginase activity and localization within the brain, refer to a recent review and chapter by our lab(7).
Given the impact of arginase on the urea cycle and on nitric oxide homeostasis, alterations in its activity could significantly contribute to the pathophysiology of neurological disease. In fact, recent work in a prodomal HD mouse model found there is a basal deficiency in striatal arginase activity which is corrected with acute Mn exposure, suggesting that lack of activity was due to reduced Mn bioavailability and not to reductions in the arginase protein(22). Interestingly, there was an elevation in the urea cycle metabolites, specifically arginine, citrulline and ornithine, in the striatum of HD mice, which was reduced to the levels of control animals after Mn exposure. The return of urea cycle homeostasis in the striatum of HD mice after Mn treatment further implicates Mn-dependent biology, and in particular, arginase activity, as a mechanism to delay or prevent the pathophysiology of HD as well as other neurological diseases. To our knowledge, this is the first evidence to directly tie impairment in Mn homeostasis to altered Mn-dependent enzymatic activity, and indicates that Mn regulation may be crucial to proper long-term neuronal function.
A role for Mn in autophagy
Another more recently described role for Mn in neuronal health has been in the regulation of autophagy. Autophagy, the process by which a cell “self-digests” cytosolic components for degradation and recycling, plays a critical role in neuronal homeostasis. Dysregulation of autophagy has been heavily implicated in neurological diseases such as Parkinson’s disease (PD), Alzheimer’s Disease (AD) and HD (23–25). In fact, one argument for the importance of autophagy in the pathophysiology of neurological disorders is the ability for the autophagosome to degrade protein aggregates often associated with these diseases. A study by Martinez-Vicente et al found that although autophagosomes are able to form normally within cells of HD patients, they are devoid of any cargo, in particular Huntingtin protein aggregates, suggesting that perhaps the disturbances in autophagy may be a mechanism of toxicity within HD(25). Conversely, when autophagy genes are ‘turned off,’ protein aggregation increases and neurodegeneration ensues(26).
A role for Mn exposure elevating autophagic processes has recently been discovered. It is potentially possible then, that chronic elevated Mn exposure may be a viable option to restore autophagy for neurological diseases in which impairment of this pathway contributes to the pathophysiology. Both acute and chronic Mn exposures have been shown to increase autophagy both in vitro and in vivo, but over the course of several days post-exposure there is a significant drop of autophagic activity (27–29). These findings suggest two possibilities: 1) that Mn could potentially be a mechanism to rescue impaired autophagy; and/or 2) that the increase in autophagy is a neuroprotective/homeostatic response mechanism against the toxic effects of excess intracellular Mn. Further study needs to be done to understand the effects of Mn on autophagy and whether Mn could be a potential therapeutic target to rescue macroautophagy.
Relationships between Mn neurotoxicity and neurodegeneration
One particularly difficult aspect of understanding Mn toxicity in the context of neurological disease is a significant overlap in symptoms that makes it difficult to differentiate biochemical and physiologic changes due to Mn toxicity or disease. The most well-known consequence of excess Mn exposure is Manganism, which closely resembles the physical characteristics of PD. Interestingly, elevated Mn levels were found in the cerebral spinal fluid of PD patients(30) and non-human primates exposed to Mn exhibited PD- and AD-like features in the brain(31,32). To further complicate understanding of Mn toxicity, it appears that the need for Mn and Mn-dependent enzymes changes with age making it difficult to assign dietary intake or exposure limits. For instance, a recent study in fibroblast cell culture found that as cells ‘age’ (increase in passage), they utilize incrementally more Mn and achieve greater activity in the Mn-dependent enzyme Sodium Oxide Dismutase 2(SOD2)(33). It is feasible that this increase in Mn utility represents a homeostatic mechanism within the cell to compensate for increased oxidative stress which accumulates throughout the natural aging process(33,34) and it thought to be a critical component of the pathophysiology of many neurological disorders.
Connections between Mn biology and cognition
Epidemiologic studies show clear associations between Mn exposure and cognitive impairment in children and adults. These associations, as typically observed with essential metals, follow an inverted-U shape when assessing the relationship between exposure and neurocognitive outcome(8) where insufficient as well as excess Mn results in reduced cognitive ability. Regardless of life stage, it is clear that both high and low levels of Mn exposure biomarkers in humans have been negatively associated with cognitive function(8). This same trend is observed in Mn-dependent biology, where both excessive and insufficient Mn in both in vivo and in vitro studies has deleterious consequences on oxidative stress levels(35), anti-oxidant capacity(2), mitochondrial function(3) and the metabolic pathways discussed previously.
To further complicate our understanding of Mn biology, it is also clear that genetic variants prevalent in the general population (between 17%–48%) alter blood Mn levels(36). For instance there are two non-coding genetic variants in the Mn-transporter SLC30A10 which significantly alter blood Mn levels in healthy populations(36). Interestingly, one variant was associated with increased blood Mn, lower expression of SLC30A10 and impaired postural balance as well as other neurological parameters. While the other variant was associated with lower blood Mn, increased SLC30A10, and evidence of neurological impairment(36). Similarly, the zinc-transporter ATP13A2 (PARK9) has shown a sensitivity to Mn and has several known polymorphisms which alter the association between soil Mn content and cognition(37). Perhaps more importantly, there was no significant relationship between ATP13A2 variant and cognition independent of Mn exposure(37). With increasing Mn exposure, one variant was linked to reduced motor coordination while there was no association with other genotypes(37). The recent evidence that genetic variants with high prevalence alters blood Mn levels and neurological function has direct clinical relevance as the management of individuals may change significantly depending on their genetic variant.
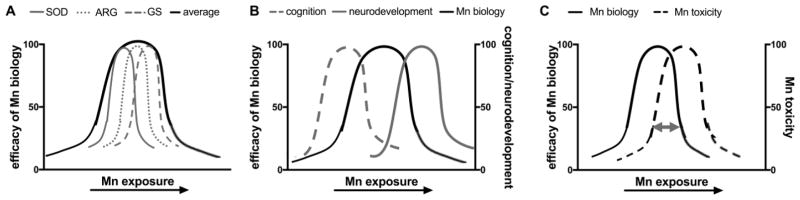
What ultimately remains unclear is whether the amount of Mn exposure/intake that makes a given Mn-dependent biological processes most efficient coincides with the optimal exposure for another process or functional domain of Mn biology, such as cognitive function. Thus comparing neurodevelopment in infants and children versus cognitive ability in adults, it is possible that Mn levels needed for optimal development of cognitive function, may overlap with those causing Mn-toxicity. In other words, the efficacy of biological processes such as Mn-dependent enzymatic or autophagic pathways, may have different optimal levels for an organism depending on the specific conditions. Further these optimal levels could theoretically be different for distinct Mn-dependent processes. Different Mn-dependent enzymes have distinct Mn-binding requirements, and it is unclear whether the appropriate intracellular concentrations necessary for optimal binding can be achieved simultaneously. Specifically, Glutamine synthetase requires 4 Mn2+ per subunit for optimal enzymatic activity(38), while Arginase requires 2 Mn2+ per subunit(39) and Superoxide dismutase requires only 1 Mn2+ per monomer(40). The affinity for Mn binding sites within and between these Mn-dependent enzymes are not identical, and the occupancy of Mn in these binding sites in vivo is not well understood. Even for enzymes in the same sub-cellular compartment – it is unclear whether optimal binding, yet avoidance of toxicity, is achieved at the same concentration of Mn. Further complicating our understanding of Mn biology and toxicology is the possibility that the optimal efficiency of Mn-dependent processes changes with age and neurodevelopment. The potential discordance between optimal Mn-biology and cognitive function and how this relationship changes with age and disease is depicted in Figure 1.
Figure 1.
Discrepancies between efficacy of Mn-dependent biology and Mn toxicity in the context of neurological disease and age. Discrepancies between A) select Mn-dependent enzymes, B) the efficacy of Mn biology and optimal neurodevelopment and cognition, and C) the efficacy of Mn biology and Mn toxicity. The gray double-sided arrow in (C) indicates that the degree of overlap between optimal Mn biology and Mn toxicity may change with age or disease state. Superoxide dismutase (SOD); Arginase (ARG); Glutamine synthetase (GS), the relationship between efficacy and Mn exposure in vivo is not clear, thus individual curves are arbitrary.
Mn requirements during infant and child neurodevelopment are significantly higher compared to adults despite having a similar recommended daily intake (RDI) of approximately 1.5–5 mg/day(41). This discrepancy is in part to accommodate potential differences in gastrointestinal absorption and the greater efficiency at which Mn crosses the blood-brain barrier in children(1). Mn is essential for several enzymes involved in neurodevelopment and given the short time in which these molecular developmental milestones must occur, it is likely that Mn-dependent biological processes are more active and thus require greater Mn, which we propose may acutely have toxic effects on non-neuronal cells, but ultimately leads to peak neurodevelopment (Figure 1B,1C). Conversely, in healthy adults, the need for Mn-dependent biology is lower than that of developing children, and thus the Mn exposure/intake which results in optimal cognition is lower. Although assessing the molecular kinetics in humans would be challenging, there is evidence that the highest IQ levels in children correspond to blood and hair Mn levels associated with cognitive decline in adults(9,42). There are at least two possible mechanisms at play. First, early exposures associated with higher cognitive function in children may contribute later in life to cognitive decline. Second, levels associated with optimal function of the underlying Mn-dependent processes in children, may be associated with cognitive neurotoxicity in adults. Further work is needed to parse these possibilities.
Lastly, the Mn requirement for optimal health may increase in neurological diseases to compensate for the increased oxidative stress, mitochondrial dysfunction, and inflammation often associated with their pathophysiology. Thus, in the setting of neurological disease, the overlap between Mn biology and toxicity at a given Mn exposure could shift to compensate for an increased need for Mn biological processes (Figure 1C) that function in neuroprotection. This shift could also occur in the context of age and neurodevelopment.
There is recent evidence in young rodents that Manganese exposure results in biochemical changes indicative of Mn toxicity; however, this exposure does not correspond to any cognitive impairment(43,44). Interestingly, at these same doses, aged adult animals experience decline in several behavioral and cognitive outcomes(45). Unfortunately, the current rodent and non-human primate studies examining the effects of Mn on cellular toxicity and behavioral and cognitive changes utilize Mn exposures that preclude the possibility of observing any beneficial effects.
Conclusions
The studies discussed here provide evidence that in addition to Mn requirements changing over development, there are likely differences across tissue type and under certain neurological conditions. Indeed, a loss of homeostatic control over Mn levels may underscore some neurodegenerative diseases. A better understanding of the concordance between Mn-dependent biology and neurodevelopment and cognition would better inform our understanding of how Mn toxicity changes with age and neurological disease, and the limits of how much Mn intake/exposure is optimal for health. The vast majority of the literature investigating the relationship between Mn exposure and neurologic disease supports a role for Mn-toxicity being mechanistically linked to the pathophysiology of some neurological diseases. However, we posit here that in certain developmental periods, short-term toxicity in non-neuronal tissue or perhaps even the brain itself, may ultimately result in optimal neurodevelopment due to overlap in optimal levels for Mn-biology and neurotoxic thresholds.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Anna C. Pfalzer and Aaron B. Bowman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Michalke B, Fernsebner K. New insights into manganese toxicity and speciation. J Trace Elem Med Biol. 2014;28(2):106–16. doi: 10.1016/j.jtemb.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Buettner GR, Ng CF, Wang M, Rodgers VGJ, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med [Internet] 2006 Oct 15;41(8):1338–50. doi: 10.1016/j.freeradbiomed.2006.07.015. [cited 2016 Oct 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunter TE, Gerstner B, Lester T, Wojtovich AP, Malecki J, Swarts SG, et al. An analysis of the effects of Mn2+ on oxidative phosphorylation in liver, brain, and heart mitochondria using state 3 oxidation rate assays. Toxicol Appl Pharmacol [Internet] 2010 Nov 15;249(1):65–75. doi: 10.1016/j.taap.2010.08.018. [cited 2016 Oct 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul [Internet] 1984;24:153–69. doi: 10.1016/b978-0-12-152824-9.50021-6. [cited 2016 Oct 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/6149889. [DOI] [PubMed] [Google Scholar]
- 5•.Patassini S, Begley P, Reid SJ, Xu J, Church SJ, Curtis M, et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington’s disease. Biochem Biophys Res Commun. 2015;468(1–2):161–6. doi: 10.1016/j.bbrc.2015.10.140. This study is the first to demonstrate an alteration in urea cycle metabolism in the brain of HD patients. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Miah M, Culbreth M, Aschner M. Autophagy in Neurodegenerative Diseases and Metal Neurotoxicity. Neurochem Res [Internet] 2016 Feb 11;41(1–2):409–22. doi: 10.1007/s11064-016-1844-x. [cited 2016 Oct 5] Available from: http://link.springer.com/10.1007/s11064-016-1844-x. [DOI] [PubMed] [Google Scholar]
- 7.Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annu Rev Nutr [Internet] 2015;35:71–108. doi: 10.1146/annurev-nutr-071714-034419. [cited 2016 Aug 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25974698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vollet K, Haynes EN, Dietrich KN. Manganese Exposure and Cognition Across the Lifespan: Contemporary Review and Argument for Biphasic Dose–Response Health Effects. Curr Environ Heal Reports [Internet] 2016 Oct 8;:1–13. doi: 10.1007/s40572-016-0108-x. [cited 2016 Oct 17] Available from: http://link.springer.com/10.1007/s40572-016-0108-x. [DOI] [PMC free article] [PubMed]
- 9•.Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, et al. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA) Environ Health Perspect [Internet] 2015 Oct;123(10):1066–71. doi: 10.1289/ehp.1408993. [cited 2016 Oct 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25902278This study demonstrates the bi-phasic relationship between select Mn exposure biomarkers and child IQ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Agustín R, Rodríguez-Agudelo Y, Schilmann A, Solís-Vivanco R, Montes S, Riojas-Rodríguez H, et al. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res [Internet] 2013 Feb;121:39–44. doi: 10.1016/j.envres.2012.10.007. [cited 2016 Oct 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23141434. [DOI] [PubMed] [Google Scholar]
- 11.Chiang MC, Chen HM, Lee YH, Chang HH, Wu Y-C, Soong B-W, et al. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum Mol Genet [Internet] 2007;16(5):483–98. doi: 10.1093/hmg/ddl481. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17213233. [DOI] [PubMed] [Google Scholar]
- 12.Chiang M-C, Chen H-M, Lai H-L, Chen H-W, Chou S-Y, Chen C-M, et al. The A2A adenosine receptor rescues the urea cycle deficiency of Huntington’s disease by enhancing the activity of the ubiquitin-proteasome system. Hum Mol Genet [Internet] 2009 Aug 15;18(16):2929–42. doi: 10.1093/hmg/ddp230. [cited 2016 Oct 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19443488. [DOI] [PubMed] [Google Scholar]
- 13.Morello M, Zatta P, Zambenedetti P, Martorana A, D’Angelo V, Melchiorri G, et al. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: an immunohistochemical study. Brain Res Bull [Internet] 2007 Nov 1;74(6):406–15. doi: 10.1016/j.brainresbull.2007.07.011. [cited 2016 Oct 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17920449. [DOI] [PubMed] [Google Scholar]
- 14.Gruber B, Kłaczkow G, Jaworska M, Krzysztoń-Russjan J, Anuszewska EL, Zielonka D, et al. Huntington’ disease--imbalance of amino acid levels in plasma of patients and mutation carriers. Ann Agric Environ Med [Internet] 2013;20(4):779–83. [cited 2016 Oct 31] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24364452. [PubMed] [Google Scholar]
- 15.Deckel AW, Tang V, Nuttal D, Gary K, Elder R. Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington’s disease transgenic mice. Brain Res. 2002;939(1):76–86. doi: 10.1016/s0006-8993(02)02550-7. [DOI] [PubMed] [Google Scholar]
- 16.Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, et al. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci [Internet] 2001 Dec;14(12):1961–7. doi: 10.1046/j.0953-816x.2001.01820.x. [cited 2016 Oct 31] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11860491. [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Fleete MS, Jing Y, Collie ND, Curtis MA, Waldvogel HJ, et al. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol Aging. 2014;35(9):1992–2003. doi: 10.1016/j.neurobiolaging.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Chiang M-C, Chen H-M, Lee Y-H, Chang H-H, Wu Y-C, Soong B-W, et al. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum Mol Genet [Internet] 2007 Mar 1;16(5):483–98. doi: 10.1093/hmg/ddl481. [cited 2016 Oct 5] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17213233. [DOI] [PubMed] [Google Scholar]
- 19.Deckel AW, Volmer P, Weiner R, Gary KA, Covault J, Sasso D, et al. Dietary arginine alters time of symptom onset in Huntington’s disease transgenic mice. Brain Res [Internet] 2000 Sep 1;875(1–2):187–95. doi: 10.1016/s0006-8993(00)02640-8. [cited 2016 Oct 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/10967315. [DOI] [PubMed] [Google Scholar]
- 20.Ash DE. Structure and function of arginases. J Nutr [Internet] 2004 Oct;134(10 Suppl):2760S–2764S. doi: 10.1093/jn/134.10.2760S. [cited 2016 Oct 31] discussion 2765S–2767S. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15465781. [DOI] [PubMed] [Google Scholar]
- 21.Brock AA, Chapman SA, Ulman EA, Wu G. Dietary manganese deficiency decreases rat hepatic arginase activity. J Nutr [Internet] 1994 Mar;124(3):340–4. doi: 10.1093/jn/124.3.340. [cited 2016 Oct 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/8120652. [DOI] [PubMed] [Google Scholar]
- 22.Bichell TJV, Wegrzynowicz M, Grace Tipps K, Bradley EM, Uhouse MA, Bryan M, et al. Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington’s disease mouse model. Biochim Biophys Acta [Internet] 2017 Feb 14; doi: 10.1016/j.bbadis.2017.02.013. [cited 2017 Feb 20]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0925443917300546. [DOI] [PMC free article] [PubMed]
- 23.Martinez-Vicente M. Autophagy in neurodegenerative diseases: From pathogenic dysfunction to therapeutic modulation. Semin Cell Dev Biol [Internet] 2015 Apr;40:115–26. doi: 10.1016/j.semcdb.2015.03.005. [cited 2016 Nov 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25843774. [DOI] [PubMed] [Google Scholar]
- 24.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. Autophagy Induction and Autophagosome Clearance in Neurons: Relationship to Autophagic Pathology in Alzheimer’s Disease. J Neurosci. 2008;28(27) doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, et al. Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci [Internet] 2010 May 11;13(5):567–76. doi: 10.1038/nn.2528. [cited 2016 Oct 24] Available from: http://www.nature.com/doifinder/10.1038/nn.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res [Internet] 2014 Jan;24(1):92–104. doi: 10.1038/cr.2013.153. [cited 2016 Oct 24] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24281265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Cao R, Cai T, Aschner M, Zhao F, Yao T, et al. The role of autophagy dysregulation in manganese-induced dopaminergic neurodegeneration. Neurotox Res [Internet] 2013 Nov;24(4):478–90. doi: 10.1007/s12640-013-9392-5. [cited 2016 Oct 10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23604964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorojod RM, Alaimo A, Porte Alcon S, Pomilio C, Saravia F, Kotler ML. The autophagic-lysosomal pathway determines the fate of glial cells under manganese- induced oxidative stress conditions. Free Radic Biol Med [Internet] 2015 Oct;87:237–51. doi: 10.1016/j.freeradbiomed.2015.06.034. [cited 2016 Oct 20] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26163003. [DOI] [PubMed] [Google Scholar]
- 29.Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol [Internet] 2011 Nov 1;256(3):227–40. doi: 10.1016/j.taap.2011.07.018. [cited 2016 Oct 20] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21856324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci [Internet] 2011 Apr 15;303(1–2):95–9. doi: 10.1016/j.jns.2011.01.003. [cited 2016 Oct 27] Available from: http://www.ncbi.nlm.nih.gov/pubmed/21292280. [DOI] [PubMed] [Google Scholar]
- 31.Guilarte TR. APLP1, Alzheimer’s-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology [Internet] 2010 Sep;31(5):572–4. doi: 10.1016/j.neuro.2010.02.004. [cited 2016 Oct 27] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20188756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, et al. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem [Internet] 2008 Jun;105(5):1948–59. doi: 10.1111/j.1471-4159.2008.05295.x. [cited 2016 Oct 27] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18284614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Ghneim HK. The kinetics of the effect of manganese supplementation on SOD2 activity in senescent human fibroblasts. Eur Rev Med Pharmacol Sci [Internet] 2016 May;20(9):1866–80. [cited 2016 Nov 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/27212182This study demonstrates that human fibroblasts increase Mn requirements with age. [PubMed] [Google Scholar]
- 34.Parmalee NL, Aschner M. Manganese and aging. Neurotoxicology [Internet] 2016 Sep;56:262–8. doi: 10.1016/j.neuro.2016.06.006. [cited 2016 Nov 1] Available from: http://www.ncbi.nlm.nih.gov/pubmed/27293182. [DOI] [PubMed] [Google Scholar]
- 35.Fernsebner K, Zorn J, Kanawati B, Walker A, Michalke B. Manganese leads to an increase in markers of oxidative stress as well as to a shift in the ratio of Fe(II)/(III) in rat brain tissue. Metallomics [Internet] 2014 Apr;6(4):921–31. doi: 10.1039/c4mt00022f. [cited 2016 Oct 6] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24599255. [DOI] [PubMed] [Google Scholar]
- 36.Wahlberg K, Kippler M, Alhamdow A, Rahman SM, Smith DR, Vahter M, et al. Common Polymorphisms in the Solute Carrier SLC30A10 are Associated With Blood Manganese and Neurological Function. Toxicol Sci [Internet] 2016 Feb;149(2):473–83. doi: 10.1093/toxsci/kfv252. [cited 2016 Nov 7] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26628504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rentschler G, Covolo L, Ahmadi Haddad A, Lucchini RG, Zoni S, Broberg K. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology [Internet] 2012 Aug;33(4):697–702. doi: 10.1016/j.neuro.2012.01.007. [cited 2017 Feb 20] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22285144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krajewski WW, Collins R, Holmberg-Schiavone L, Jones TA, Karlberg T, Mowbray SL. Crystal Structures of Mammalian Glutamine Synthetases Illustrate Substrate-Induced Conformational Changes and Provide Opportunities for Drug and Herbicide Design. J Mol Biol [Internet] 2008 Jan;375(1):217–28. doi: 10.1016/j.jmb.2007.10.029. [cited 2016 Nov 9] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022283607013678. [DOI] [PubMed] [Google Scholar]
- 39.Cama E, Colleluori DM, Emig FA, Shin H, Kim SW, Kim NN, et al. Human Arginase II: Crystal Structure and Physiological Role in Male and Female Sexual Arousal†, ‡. Biochemistry [Internet] 2003 Jul;42(28):8445–51. doi: 10.1021/bi034340j. [cited 2016 Oct 31] Available from: http://pubs.acs.org/doi/abs/10.1021/bi034340j. [DOI] [PubMed] [Google Scholar]
- 40.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med [Internet] 2002 Aug 1;33(3):337–49. doi: 10.1016/s0891-5849(02)00905-x. [cited 2016 Nov 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/12126755. [DOI] [PubMed] [Google Scholar]
- 41.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc [Internet] 2001;101(3):294–301. doi: 10.1016/S0002-8223(01)00078-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25057538%5Cnhttp://www.ncbi.nlm.nih.gov/pubmed/11269606. [DOI] [PubMed] [Google Scholar]
- 42.Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med [Internet] 2007 Mar;64(3):167–77. doi: 10.1136/oem.2006.028761. [cited 2016 Oct 27] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17018581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordova FM, Aguiar AS, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, et al. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol [Internet] 2013 Jul 6;87(7):1231–44. doi: 10.1007/s00204-013-1017-5. [cited 2016 Oct 27] Available from: http://link.springer.com/10.1007/s00204-013-1017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu H, Chen W, Yu H, Wei Z, Yu X. The effects of preweaning manganese exposure on spatial learning ability and p-CaMKIIα level in the hippocampus. Neurotoxicology. 2016;52:98–103. doi: 10.1016/j.neuro.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Su C, Chen K, Zou Y, Shen Y, Xia B, Liang G, et al. Chronic exposure to manganese sulfate leads to adverse dose-dependent effects on the neurobehavioral ability of rats. Environ Toxicol [Internet] 2016 Nov;31(11):1571–9. doi: 10.1002/tox.22161. [cited 2016 Oct 25] Available from: http://doi.wiley.com/10.1002/tox.22161. [DOI] [PubMed] [Google Scholar]