Abstract
Background
Potential differences in efficacy of different BCG strains are of importance for daily practice, especially in the era of BCG shortage.
Objective
To retrospectively compare the outcome with BCG Connaught and BCG Tice in a large study cohort of pT1 high grade non muscle-invasive bladder cancer (NMIBC) patients.
Design, setting, and participants
Individual patient data were collected for 2451 patients with primary T1G3 tumors from 23 centers who were treated with BCG for the first time between 1990 and 2011.
Outcome measurements and statistical analysis
Using Cox multivariable regression and adjusting for the most important prognostic factors in this non randomized comparison, BCG Connaught and TICE were compared for time to recurrence, progression and the duration of cancer specific (CSS) and overall survival (OS).
Results and limitations
Information on the BCG strain was available for 2099 patients: 957 on Connaught and 1142 on TICE. 765 (36%) patients received some form of maintenance BCG, 560 (59%) on Connaught and 205 (18%) on TICE. Without maintenance, Connaught was more effective than TICE only for the time to first recurrence (HR = 1.48, 95% CI: 1.20 – 1.82, p < 0.001). With maintenance, TICE was more effective than Connaught for the time to first recurrence (HR = 0.66, 95% CI: 0.47 – 0.93, p=0.019) with a trend for CSS (HR = 0.36, 95% CI: 0.14 – 0.92, p = 0.033). For time to progression and OS, Connaught and TICE had a similar efficacy. Compared to no maintenance therapy, maintenance BCG significantly reduced the risk of recurrence, progression and death, both overall and disease specific, for TICE but not for Connaught.
Conclusions
We found that BCG Connaught results in a lower recurrence rate as compared to BCG Tice when no maintenance is used. However, the opposite is true when maintenance is given.
Patient summary
Since there is currently a BCG shortage, information on the efficacy of different BCG strains is important. In this non randomized retrospective comparison in over 2000 patients, we found that BCG Connaught reduces the recurrence rate compared to BCG Tice when no maintenance is used, but the opposite is true when maintenance is given.
Introduction
BCG remains an important treatment option after TURBT in non-muscle-invasive bladder cancer (NMIBC) patients. It is recommended in intermediate and high risk patients, although intravesical chemotherapy also has a role in intermediate risk patients [1].
Several different BCG strains have been used for treating NMIBC, all originating from the original vaccine for tuberculosis. They have been distributed across countries and continents since many decades, resulting in an accumulation of mutations [2]. Whether these differences have resulted in differences in efficacy or toxicity for either tuberculosis vaccination or NMIBC treatment remains largely unknown [2].
In NMIBC, the current BCG shortage has made the comparison of different strains and a strain's potential replacement by another strain particularly important. There are, however, only few RCT's comparing different BCG strains. The two largest are a 1995 Dutch trial and a 2014 Swiss trial. The Dutch study showed that an induction course of BCG RIVM reduced recurrences compared to an induction course of BCG Tice [3]. The Swiss trial [2] found an induction course of BCG Connaught to be significantly better in reducing recurrences than an induction course of BCG Tice. These authors suggested that the superiority of BCG Connaught was due to a superior immunogenicity, as demonstrated in mice, and genetic differences. A recent Japanese trial compared BCG Tokyo172 and BCG Connaught in 129 NMIBC patients [4]. CR rates in (concomitant) CIS and 2 year RFS were similar in both arms. In our study, a large European cohort of high risk NMIBC patients, we have retrospectively compared the two most widely used BCG strains, Connaught and Tice, with respect to recurrence, progression, cancer specific survival (CSS) and overall survival (OS).
Patients and methods
Database
Patients, methods and results have been previously reported [5]. Individual patient data were retrospectively collected for patients with high grade/G3 T1 urothelial carcinoma who received at least an induction course of BCG which started between 1990 and 2011. Patients with a previous NMIBC that was not T1 high grade/G3 were eligible if they did not receive BCG for that tumor. In addition to patient and tumor characteristics, information on BCG strain, dose, total number of instillations, and reasons for stopping BCG were recorded. Instillations beyond six were defined as maintenance BCG. Endpoints were time to first recurrence (any stage or grade), progression to muscle-invasive disease, duration of cancer specific survival (CSS) and overall survival (OS).
Statistical analysis
As the two strains were not assigned by randomization and there are imbalances in the distribution of the prognostic factors, Cox proportional hazards multivariable regression analyses were carried out to adjust for the number of the adverse prognostic factors using the most important variables that had been previously identified: age (< 70 years, > 70 years) for progression and survival, number of tumors (single, multiple) for recurrence, tumor size (< 3 cm, > 3 cm) for recurrence, progression and survival and concomitant CIS (no, yes) for progression [5]. A second confirmatory analysis which included each of these variables in the models instead of the number of adverse factors provided similar results. Adjustment for the use of maintenance, tests for interaction of BCG strain and maintenance, and separate analyses in patients receiving and not receiving maintenance were done.
Times to events were calculated taking the date of starting BCG as time zero. OS was estimated using the Kaplan-Meier technique. To take into account patients who died before the event of interest (competing risk), times to the other events were estimated using cumulative incidence functions. Patients without an event were censored at the last date of follow-up.
Results
In this cohort of 2451 primary T1G3 patients, information on treatment with Connaught or TICE was available in 18 of 23 centers with 2099 patients: 957 on Connaught and 1142 on TICE. Maintenance BCG was given in 765 patients (36%), 560 of 957 patients (59%) on Connaught and 205 of 1142 patients (18%) on TICE. The distribution of patient and tumor characteristics is given in Table 1. More patients on TICE had multiple tumors, large tumors, restaging TURs and one or more adverse risk factors for survival. Fewer TICE patients received maintenance.
Table 1. Patient and tumor characteristics.
| BCG Connaught (n=957) | BCG Tice (n=1142) | Total (n = 2099) | ||
|---|---|---|---|---|
| Age | <70 | 556 (58.1%) | 638 (55.9%) | 1194 (56.9%) |
| >70 | 401 (41.9%) | 504 (44.1%) | 905 (43.1%) | |
| Gender | Male | 792 (82.8%) | 928 (81.3%) | 1720 (81.9%) |
| Female | 165 (17.2%) | 214 (18.7%) | 379 (18.1%) | |
| Tumor status | Primary | 835 (87.3%) | 1024 (89.7%) | 1859 (88.6%) |
| Recurrent | 122 (12.8%) | 118 (10.3%) | 240 (11.4%) | |
| Previous intravesical chemotherapy | No | 913 (95.4%) | 1066 (93.4%) | 1979 (94.3%) |
| Yes | 44 (4.6%) | 76 (6.6%) | 120 (5.7%) | |
| Number of tumors | Single | 381 (45.5%) | 420 (36.9%) | 801 (40.5%) |
| Multiple | 457 (54.5%) | 719 (63.1%) | 1176 (59.5%) | |
| Missing | 119 | 3 | 122 | |
| Largest tumor size | <3cm | 557 (75.0%) | 440 (70.3%) | 997 (72.8%) |
| >3cm | 186 (25.0%) | 186 (29.7%) | 372 (27.2%) | |
| Missing | 214 | 516 | 730 | |
| Concomitant CIS | No | 731 (76.4%) | 841 (73.6%) | 1572 (74.9%) |
| Yes | 226 (23.6%) | 301 (26.4%) | 527 (25.1%) | |
| Restaging TUR before BCG | No | 582 (60.8%) | 482 (42.2%) | 1064 (50.7%) |
| Yes | 303 (31.7%) | 558 (48.9%) | 861 (41.0%) | |
| Missing | 72 (7.5%) | 102 (8.9%) | 174 (8.3%) | |
| Risk factors for recurrence | 0 | 278 (37.5%) | 260 (41.5%) | 538 (39.3%) |
| 1 | 347 (46.8%) | 284 (45.4%) | 631 (46.1%) | |
| 2 | 117 (15.8%) | 82 (13.1%) | 199 (14.6%) | |
| Missing | 215 | 516 | 731 | |
| Risk factors for progression | 0 | 234 (31.5%) | 162 (25.9%) | 396 (28.9%) |
| 1 | 340 (45.8%) | 294 (47.0%) | 634 (46.3%) | |
| 2 | 142 (19.1%) | 136 (21.7%) | 278 (20.3%) | |
| 3 | 27 (3.6%) | 34 (5.4%) | 61 (4.5%) | |
| Missing | 214 | 516 | 730 | |
| Risk factors for survival | 0 | 332 (44.7%) | 212 (33.9%) | 544 (39.7%) |
| 1 | 324 (43.6%) | 319 (51.0%) | 643 (47.0%) | |
| 2 | 87 (11.7%) | 95 (15.2%) | 182 (13.3%) | |
| Missing | 214 | 516 | 730 | |
| Maintenance | No | 397 (41.5%) | 937 (82.1%) | 1334 (63.6%) |
| Yes | 560 (58.5%) | 205 (17.9%) | 765 (36.4%) |
Median follow-up was 5.2 yr with a maximum follow-up of 18.7 yr. The number of patients who recurred, progressed and died is summarized in Tables 2 – 4.
Table 2. Multivariable Adjusted Comparison of BCG Connaught to BCG TICE: All Patients.
| BCG Connaught (n=957) N, (%) | BCG Tice (n=1142) N, (%) | Hazard Ratio (95% CI) | P value | P value Interaction with Maintenance | ||
|---|---|---|---|---|---|---|
| Recurrence | No | 518 (54.1) | 517 (45.3) | 1.18 (1.00 – 1.39) | 0.047 | < 0.001 |
| Yes | 439 (45.9) | 625 (54.7) | ||||
| Progression | No | 779 (81.4) | 940 (82.3) | 1.08 (0.84 – 1.39) | 0.55 | 0.16 |
| Yes | 178 (18.6) | 202 (17.7) | ||||
| Survival | Alive | 713 (74.5) | 868 (76.0) | 1.02 (0.81 – 1.28) | 0.87 | 0.13 |
| Dead | 244 (25.5) | 274 (24.0) | ||||
| Death Bladder Cancer | No | 864 (90.3) | 1054 (92.3) | 0.73 (0.50 – 1.06) | 0.10 | 0.12 |
| Yes | 93 (9.7) | 88 (7.7) |
HR > 1 favors Connaught, HR < 1 favors TICE
Table 4. Multivariable Adjusted Comparison of BCG Connaught to BCG TICE: Patients with BCG maintenance.
| BCG Connaught (n=560) N, (%) | BCG Tice (n=205) N, (%) | Hazard Ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Recurrence | No | 319 (57.0) | 136 (66.3) | 0.66 (0.47 – 0.93) | 0.019 |
| Yes | 241 (43.0) | 69 (33.7) | |||
| Progression | No | 466 (83.2) | 176 (85.9) | 0.79 (0.49 – 1.29) | 0.35 |
| Yes | 94 (16.8) | 29 (14.1) | |||
| Survival | Alive | 423 (75.5) | 167 (81.5) | 0.72 (0.46 – 1.12) | 0.14 |
| Dead | 137 (24.5) | 38 (18.5) | |||
| Death Bladder Cancer | No | 509 (89.9) | 199 (97.1) | 0.36 (0.14 – 0.92) | 0.033 |
| Yes | 51 (9.1) | 6 (2.9) |
HR > 1 favors Connaught, HR < 1 favors TICE
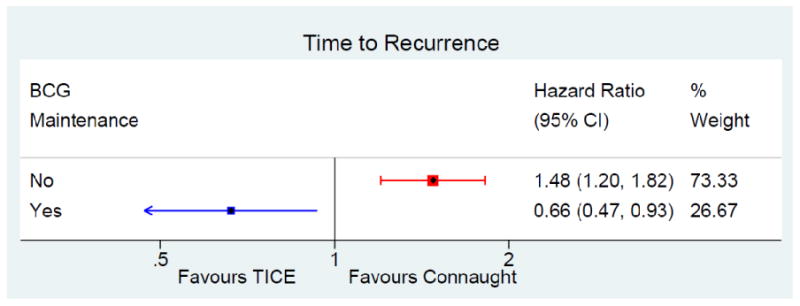
Time to First Recurrence
439 patients (46%) on Connaught and 625 (55%) on TICE recurred, 50% on Connaught and 59% on TICE without maintenance, 43% and 34% with maintenance, respectively (Tables 2-4).
After adjustment for the risk of recurrence, maintenance BCG prolonged the time to first recurrence (HR = 0.60, 95% CI: 0.51 – 0.70, p < 0.001). The difference was mainly found in patients receiving TICE (HR = 0.37, 95% CI: 0.26 – 0.51, p < 0.001) rather than in patients receiving Connaught (HR = 0.85, 95% CI: 0.69 – 1.06, p = 0.15).
The difference in time to first recurrence between strains, after adjustment for the risk of recurrence and use of maintenance, was significant (HR = 1.18, 95% CI: 1.00 – 1.39, p = 0.047) in favor of Connaught. However, the test for interaction between strain and maintenance BCG was significant (p < 0.001). Adjusting for the risk of recurrence, there was a significantly longer time to first recurrence on Connaught as compared to TICE when no maintenance was given (HR = 1.48, 95% CI: 1.20 – 1.82, p < 0.001), whereas with maintenance there was a significantly longer time to first recurrence on TICE (HR = 0.66, 95% CI: 0.47 – 0.93, p = 0.019) (Tables 3-4, figure 1).
Table 3. Multivariable Adjusted Comparison of BCG Connaught to BCG TICE: Patients without BCG maintenance.
| BCG Connaught (n=397) N, (%) | BCG Tice (n=937) N, (%) | Hazard Ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Recurrence | No | 199 (50.1) | 381 (40.7) | 1.48 (1.20 – 1.82) | < 0.001 |
| Yes | 198 (49.9) | 556 (59.3) | |||
| Progression | No | 313 (78.8) | 764 (81.5) | 1.21 (0.88 – 1.65) | 0.24 |
| Yes | 84 (21.2) | 173 (18.5) | |||
| Survival | Alive | 290 (73.1) | 701 (74.8) | 1.18 (0.89 – 1.56) | 0.25 |
| Dead | 107 (26.9) | 236 (25.2) | |||
| Death Bladder Cancer | No | 355 (89.4) | 855 (91.2) | 0.90 (0.57 – 1.40) | 0.63 |
| Yes | 42 (10.6) | 82 (8.8) |
HR > 1 favors Connaught, HR < 1 favors TICE
Figure 1. forest plot time to first recurrence.
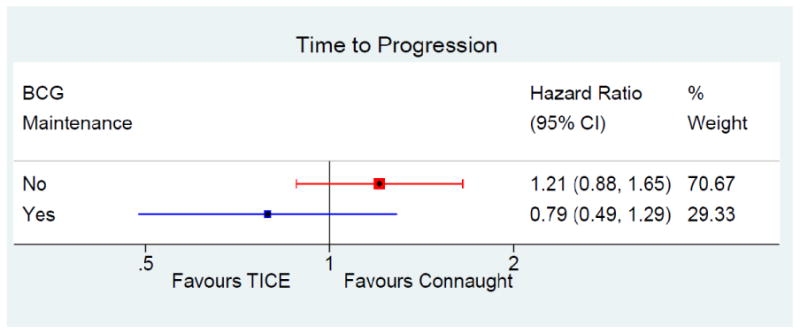
Time to Progression
178 patients (19%) on Connaught and 202 (18%) on TICE progressed, 21% on Connaught and 18% on TICE without maintenance, 17% and 14% with maintenance, respectively (Tables 2-4).
After adjustment for the risk of progression, maintenance BCG prolonged the time to progression for TICE (HR = 0.59, 95% CI: 0.36 – 0.94, p = 0.03) but not for Connaught patients (HR = 0.94, 95% CI: 0.67 – 1.31, p = 0.71).
When adjusted for the risk of progression (age > 70, size > 3 cm, CIS) and use of maintenance, there wasn't a significant difference in the time to progression between the strains (HR = 1.08, 95% CI: 0.84 – 1.39, p = 0.55) (Table 2, figure 2). The test for interaction between strain and maintenance BCG was not significant (p = 0.16).
Figure 2. forest plot time to progression.
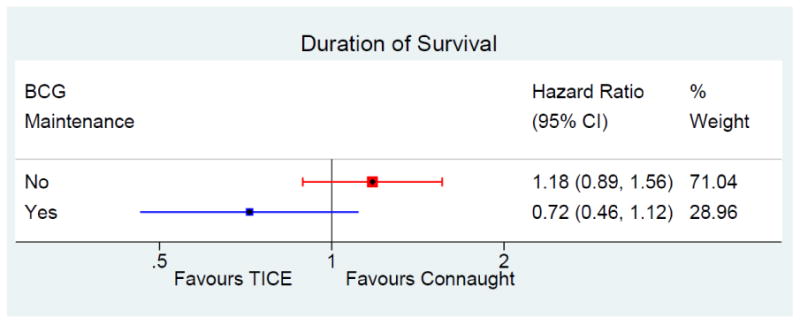
Duration of Survival
244 patients (26%) on Connaught and 274 (24%) on TICE died due to any cause, 27% on Connaught and 25% on TICE without maintenance, 24% and 18% with maintenance, respectively.
After adjustment for the risk of death, maintenance BCG significantly prolonged the duration of survival in TICE patients (HR = 0.63, 95% CI: 0.41 – 0.97, p = 0.04) but not in Connaught patients (HR = 0.94, 95% CI: 0.70 – 1.25, p = 0.65).
There wasn't a significant difference in survival between strains (Table 2, figure 3) after adjusting for the risk of death and use of maintenance (HR = 1.02, 95% CI: 0.81 – 1.28, p = 0.87). The test for interaction between strain and maintenance was not significant (p = 0.13).
Figure 3. Forest plot duration of survival.
Time to Death due to Bladder Cancer
93 patients (10%) on Connaught and 88 (8%) on TICE died due to bladder cancer, 11% on Connaught and 9% on TICE without maintenance, 9% and 3% with maintenance, respectively.
After adjustment for the risk of death, maintenance BCG prolonged the time to death due to bladder cancer in patients treated with TICE (HR = 0.37, 95% CI: 0.15 – 0.93, p = 0.034) but not in patients treated with Connaught (HR = 0.79, 95% CI: 0.50 – 1.23, p = 0.30).
There was no significant difference in the time to death due to bladder cancer according to strain when adjusted for the risk of death and the use of maintenance (HR = 0.73, 95% CI: 0.50 – 1.06, p = 0.10). The test for interaction between strain and maintenance BCG was not statistically significant (p = 0.12), however when maintenance was used, there was a suggestion of a significantly longer time to death due to bladder cancer with TICE (Table 4, figure 4).
Figure 4. forest plot time to death due to bladder cancer.

Discussion
The current dataset remains the largest ever reported on patients with pT1G3 bladder tumors who received BCG as primary treatment.
Our results showed that without maintenance therapy, Connaught was more effective than TICE for time to first recurrence. However, when maintenance was given, TICE was more effective than Connaught for time to first recurrence with a positive trend for time to death due to bladder cancer. For time to progression and overall survival, Connaught and TICE had similar efficacy. Compared to no maintenance therapy, maintenance BCG significantly reduced the risk of recurrence, progression and death for TICE but not for Connaught.
Why is Connaught better when no maintenance is given whereas Tice seems to be better with longer treatment? Mice experiments from Rentsch showed a stronger immune response to Connaught than to Tice BCG [2]. This might explain the initial advantage of Connaught. However, we also know that immunotherapy, in contrast to chemotherapy, is more likely to have an optimal biologically effective dose than a maximal effective dose. This, for example, was shown for intravesical BCG by de Reijke et al. [6]. Looking at urinary interleukin-2 (IL-2) kinetics, IL-2 tended to be higher during the first course and lower during the second and third course of BCG, suggesting a decreasing immune response with time. Combining these findings, one might hypothesize that Connaught reaches an earlier cumulative optimal dose and clinical effect as compared to Tice due to a stronger immune response. On the other hand, Connaught might lose some of its efficacy during maintenance therapy when Tice has reached its optimum.
Without maintenance, our lower risk of recurrence on Connaught is in line with the study by Rentsch [2] who reported a significantly higher 5 year recurrence free survival rate, 74% (62.8% – 87.2%) for Connaught compared to 48% (35.5% – 65.1%) for Tice, p=0.01. Their 5 year progression rates were 5.9% for Connaught and 12.9% for Tice (p=0.34). In a multivariate analysis, BCG strain remained the only significant variable for recurrence (HR 2.91). Their mice experiments showed less Tice bacilli in lymph nodes and a stronger immune response to Connaught in spite of similar numbers of live bacilli in both strains. It is noteworthy that the increased immune response in the bladder did not cause more local bladder symptoms. Connaught patients had even less dysuria, 13%, versus 30% with Tice, p=0.015. Limitations of their study are the low patient numbers (142 versus a goal of 300) and the long recruitment period (12 years).
Only a few studies have previously studied different BCG strains. In 1987, Kaisary compared BCG Glaxo and BCG Pasteur, initiated because results with BCG in Great Britain were disappointing [7]. In this pilot study with 21 patients, results between the strains were similar.
In a 1994 Medical Research Council randomized marker lesion study, Fellows compared BCG Pasteur and Evans BCG [8]. In 99 patients, all papillary tumors but one were resected and a course of 6 BCG instillations was given. In 51 eligible patients on Evans BCG, 27 (53%) still had a marker lesion or lesions at other sites at the 3 months evaluation compared to 16 (37%) of 46 eligible patients in the Pasteur group. The difference was only “statistically suggestive”.
In 1995, a Dutch study compared 6 weeks of BCG Tice, 6 weeks of BCG RIVM and 6 months of Mitomycin-C (MMC) in 437 NMIBC patients [3]. 5 year recurrence free rates with BCG Tice were the lowest (36+5%) and significantly less than with MMC (57+5%), p=0.01. 5 year recurrence free rates with BCG RIVM were also better (54+5%) than those with Tice BCG, but the difference was not statistically significant. So again, results with BCG Tice were suboptimal without maintenance.
A recent study compared a 6 to 8 week course of BCG Tokyo172 and BCG Connaught [4]. The study was stopped in 2012 because Connaught production was suspended. From 2004 until 2012, 178 patients were enrolled, of which 66 Tokyo172 and 63 Connaught patients were analyzed. After a median follow up of almost 2.5 years, the compete response rates of concomitant CIS patients were 90.3% and 85.0% for Tokyo172 and Connaught, respectively (p = 0.896). Two year recurrence free survival rates weren't significantly different: 73.2% and 68.8%, respectively. These two strains have also been compared in a mouse model [9]. Tumor cells with various doses of both BCG strains were injected in the flank of syngenic mice.Tokyo172 achieved similar tumor suppression at a lower dose than Connaught. However, although Tokyo172 had about half the bacilli compared to Connaught, the colony forming unit content was about 13-fold higher for Tokyo172. Tokyo172 bacilli were also better dispersed after reconstitution and Tokyo172 bacilli showed better attachment to tumor cells in vitro. The authors concluded that these differences in favor of Tokyo172 were the main reasons for a lower dose of Tokyo172 to be clinically effective (18 vs 81 mg dry weight).
Other non-clinical studies have addressed potential differences between different BCG strains. Secanella et al. used 3 evolutionary early and 5 late BCG sub-strains, with genetic and antigenic differences, in bladder cancer cell lines [10]. As in the use of BCG vaccines, antitumor effect and IL-6 and IL-8 response differed between BCG strains and cell line used. For T24 and J82 cells, for example, Russian and Connaught BCG strains (an evolutionary early and late sub-strain) were more effective in cell proliferation inhibition and cytokine response as compared to 6 other BCG strains, including Tice BCG.
It is conceivable that serial passages have resulted in genetic mutations and alterations, which have resulted in differences in phenotype, antigenicity and subsequent clinical efficacy and toxicity of the different sub-strains that are currently used for intravesical treatment.
The strength of this study is that it is a large homogeneous group of T1G3 patients previously untreated with BCG. A limitation is that it is a retrospective study in a selected patient group without central pathology review. A subset of patients (41%) had a second resection, however adjustment for a reTUR did not change the overall conclusions. Neither the BCG strain nor the decision to give maintenance treatment were randomized. Finally, BCG maintenance schedules and their duration were not standardized. However, approximately one third of patients receiving maintenance were treated with the SWOG three year maintenance schedule in both strains. A prospective randomized trial with sufficient patients and adequate and clearly defined treatment schedules is needed to confirm these results.
Conclusion
We report on the difference in efficacy between the two most commonly used BCG strains. In a large retrospective dataset of T1 high grade patients previously untreated with BCG, our results show that without maintenance, Connaught was more effective than TICE for the time to first recurrence potentially due to a stronger initial immune response caused by Connaught BCG. However, when maintenance was given, TICE was more effective than Connaught for the time to first recurrence.
Highlights.
Without maintenance, Connaught is more effective than TICE for the time to first recurrence
This superiority is potentially due to a stronger initial immune response caused by Connaught BCG
When maintenance was given, TICE is more effective than Connaught for the time to first recurrence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamat AM, Witjes JA, Brausi M, Soloway M, Lamm D, Persad R, Buckley R, Böhle A, Colombel M, Palou J. Defining and Treating the Spectrum of Intermediate-Risk NMIBC. J Urol. 2014;192:305–15. doi: 10.1016/j.juro.2014.02.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rentsch CA, Birkhäuser FD, Biot C, Gsponer JR, Bisiaux A, Wetterauer C, Lagranderie M, Marchal G, Orgeur M, Bouchier C, Bachmann A, Ingersoll MA, Brosch R, Albert ML, Thalmann GN. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol. 2014;66:677–88. doi: 10.1016/j.eururo.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 3.Vegt PDJ, Witjes JA, Witjes WPJ, Doesburg WH, Debruyne FMJ, Meijden APMvd. A randomized study of intrasvesical Mitomycin-C, Bacillus Calmette-Guerin Tice and Bacillus Calmette-Guerin RIVM in pTa-pT1 papillary carcinoma and carcinoma in situ of the bladder. J Urol. 1995;153:929–933. [PubMed] [Google Scholar]
- 4.Sengiku A, Ito M, Miyazaki Y, et al. A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol. 2013;190:50–4. doi: 10.1016/j.juro.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 5.Gontero P, Sylvester R, Pisano F, Joniau S, Vander Eeckt K, Serretta V, Larré S, Di Stasi S, Van Rhijn B, Witjes AJ, Grotenhuis AJ, Kiemeney LA, Colombo R, Briganti A, Babjuk M, Malmström PU, Oderda M, Irani J, Malats N, Baniel J, Mano R, Cai T, Cha EK, Ardelt P, Varkarakis J, Bartoletti R, Spahn M, Johansson R, Frea B, Soukup V, Xylinas E, Dalbagni G, Karnes RJ, Shariat SF, Palou J. Prognostic Factors and Risk Groups in T1G3 Non-Muscle-invasive Bladder Cancer Patients Initially Treated with Bacillus Calmette-Guérin: Results of a Retrospective Multicenter Study of 2451 Patients. Eur Urol. 2015;67:74–82. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 6.de Reijke TM, D deBoer EC, Kurth KH, Schamhart DHJ. Urinary Interleukin-2 monitoring during prolonged BCG treatment: can it predict the optimal number of instillations? J Urol. 1999;161:67–71. doi: 10.1097/00005392-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Kaisary AV. Intravesical BCG therapy in the management of multiple superficial bladder carcinoma. Comparison between Glaxo and Pasteur strains. Br J Urol. 1987;59:554–8. doi: 10.1111/j.1464-410x.1987.tb04876.x. [DOI] [PubMed] [Google Scholar]
- 8.Fellows GJ, Parmar MK, Grigor KM, Hall RR, Heal MR, Wallace DM. Marker tumour response to Evans and Pasteur bacille Calmette-Guérin in multiple recurrent pTa/pT1 bladder tumours: report from the Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party) Br J Urol. 1994;73:639–44. doi: 10.1111/j.1464-410x.1994.tb07548.x. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda N, Honda I, Yano I, Koyama A, Toida I. Bacillus calmette-guerin Tokyo172 substrain for superficial bladder cancer: characterization and antitumor effect. J Urol. 2005;173:1507–12. doi: 10.1097/01.ju.0000154354.06892.ba. [DOI] [PubMed] [Google Scholar]
- 10.Secanella Fandos S, Luquin M, Julian E. Connaught and Russian strains showed the highest direct antitumor effects of different bacillus Calmette-Guérin substrains. J Urol. 2013;189:711–18. doi: 10.1016/j.juro.2012.09.049. [DOI] [PubMed] [Google Scholar]


