Abstract
Transition metals are essential nutrients for all organisms and important players in the host-microbe interaction. During bacterial infection, a tug-of-war between the host and microbe for nutrient metals occurs: the host innate immune system responds to the pathogen by reducing metal availability and the pathogen tries to outmaneuver this response. The outcome of this competition, which involves metal-sequestering host-defense proteins and microbial metal acquisition machinery, is an important variable for whether infection occurs. One strategy bacterial pathogens employ to overcome metal restriction involves hijacking abundant host metalloproteins. The obligate human pathogens Neisseria spp. express TonB-dependent transport systems that capture human metalloproteins, extract the bound metal ions, and deliver these nutrients into the bacterial cell. This Essay highlights structural and mechanistic investigations that provide insights into how Neisseria acquire iron from the Fe(III)-transport protein transferrin, the Fe(III)-chelating host-defense protein lactoferrin, and the oxygen-transport protein hemoglobin, and obtain zinc from the metal-sequestering antimicrobial protein calprotectin.
Introduction
The importance and ubiquity of transition metals in biology is exemplified by the fact that over 30% of all proteins contain at least one transition metal cofactor [1]. These metal ions function as structural components, cofactors essential for catalyzing chemical transformations in metabolism and detoxification, and electron transfer centers. Microbes employ metal uptake, transport, and storage systems to maintain the concentrations of these nutrients required for growth. In the context of the host-pathogen interaction, microbial pathogens must acquire metal-ion nutrients from the host environment to replicate and cause infection. Because the mammalian host typically maintains “free” transition metals at low concentrations, microbes are faced with the challenge of obtaining these nutrients from a limited supply. Moreover, when confronted with pathogenic invaders, the host mounts a metal-withholding response and the innate immune system further limits the availability of nutrient metal ions. This process is often termed “nutritional immunity” [2–5]. Pathogens employ a number of sophisticated metal acquisition strategies to overcome the metal-limited host environment. In particular, several bacterial pathogens, including Neisseria spp.[6–8] and Staphylococcus spp.[9], capture host metalloproteins and use these biomolecules as sources of nutrient metals (Figure 1). In this Essay, we summarize structural and mechanistic investigations that illuminate how Neisseria utilize TonB-dependent transporters to acquire iron and zinc from the human proteins transferrin, lactoferrin, hemoglobin, and calprotectin.
Figure 1.
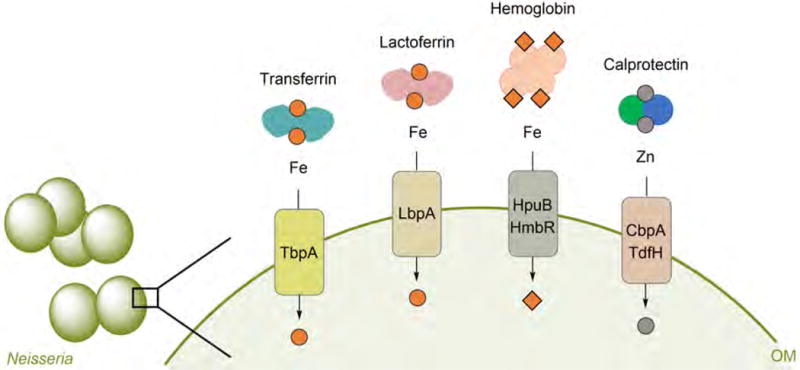
Overview of human metalloproteins and bacterial TBDTs discussed in this review. The cartoon depicts the competition between human host and Neisseria for iron and zinc. Metal withholding by human transferrin (Fe(III)), lactoferrin (Fe(III)), hemoglobin (heme Fe), and calprotectin (Zn(II)) occurs during infection. Neisseria hijack these host proteins for metal acquisition by expressing the TonB-dependent transporters TbpA, LbpA, HpuB, HmbR, CbpA, and TdfH. Calprotectin also binds Mn(II) and Fe(II), and Neisseria might be able to extract these metal ions from the protein in a similar fashion to that of Zn(II) acquisition.
Neisseria spp
Neisseria meningitidis and N. gonorrhoeae are obligate human pathogens that colonize mucosal surfaces.[10] N. meningitidis typically colonizes the upper respiratory tract asymptomatically, but this strain can cause severe infections including meningitis and sepsis. N. gonorrhoeae preferentially colonizes the urogenital tract, which results in inflammation, and causes the sexually-transmitted disease gonorrhea. N. gonorrhea is a major public health concern because it has developed resistance to various antibiotic classes, including the fluoroquinolones and cephalosporins [11]. Moreover, vaccine development for infections caused by Neisseria is complicated because Neisseria readily outmaneuver the host immune system. In particular, Neisseria undergo phase variation and capsule switching, which cause modifications to the cell surface [10,12]. Although vaccines are available for some N. meningitides serotypes, currently there are no vaccines for N. gonorrhoeae. Because metal acquisition is an essential component of microbial virulence, understanding how Neisseria acquire nutrient metals in the host may provide new approaches for antibiotic and vaccine development [12,13].
TonB-dependent metal transport
Neisseria are Gram-negative bacteria and therefore possess an outer membrane (OM) and an inner membrane (IM) enclosing the periplasm. Thus, uptake of nutrient metals from the extracellular environment into the cytoplasm involves transport across two membranes, and typically occurs through specific energy-dependent membrane transporters. Like other Gram-negative bacteria, Neisseria express TonB-dependent transporters (TBDTs, Figures 2 and 3) in the OM. These transporters deliver nutrients (e.g. metals, vitamins, amino acids, and carbohydrates) from the extracellular space across the OM into the periplasm using energy provided by the proton motive force established across the IM [14]. Energy transduction to the OM is accomplished by TonB–ExbB–ExbD, a three-protein complex anchored in the IM [15]. TBDTs have been extensively studied in the context of siderophore uptake (e.g. enterobactin transporter FepA [16], Figure 3) [17], and an increasing number of TBDT crystal structures have been reported recently [15]. All known TBDTs share a general domain architecture consisting of a C-terminal 22-stranded β-barrel spanning the OM and an N-terminal plug domain inserted inside the barrel (Figure 3) [15,18,19]. The plug domain occludes the barrel pore, contributes to the specificity of ligand binding, and interacts with TonB to initiate transport. The extracellular loops of the TBDT and the walls of the β-barrel, as well as residues from the extracellular side of the plug domain, provide sites of ligand recognition and binding. A conserved TonB box located in the N-terminal region of the plug domain is critical for energy transduction and transport. The TonB box is oriented on the periplasmic side of the TBDT and, upon ligand binding, conformational changes in the plug domain expose the TonB box such that it can interact with TonB (Figure 2). Subsequent energy transduction from TonB–ExbB–ExbD causes further conformational change of the plug domain and transport of the ligand into the periplasm. A recent study demonstrated that the TonB–ExbB–ExbD complex is composed of a pentamer of ExbB, a dimer of ExbD, and at least one TonB, and provided new hypotheses for the molecular basis of how this complex utilizes the proton motif force to undergo conformational change and transduce energy to the OM [20].
Figure 2.
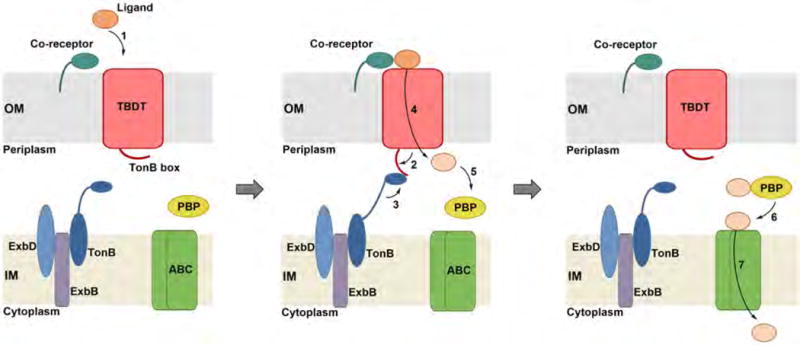
TonB-dependent transport by Gram-negative bacteria. Ligand binding at the TBDT (1), which can be mediated by a co-receptor (shown), results in conformational changes in the transporter. An interaction between the TonB box of the TBDT and the TonB–ExbB–ExbD complex occurs (2). Subsequently, the TonB complex provides energy for ligand transport across the OM (3, 4). After entry into the periplasm, most ligands are bound by a PBP (5). The PBP shuttles the ligand to an ABC transporter (6) that mediates transport across the IM (7). Abbreviations: OM, outer membrane; IM, inner membrane; TBDT, TonB-dependent transporter; PBP, periplasmic binding protein; ABC, ATP-binding cassette.
Figure 3.
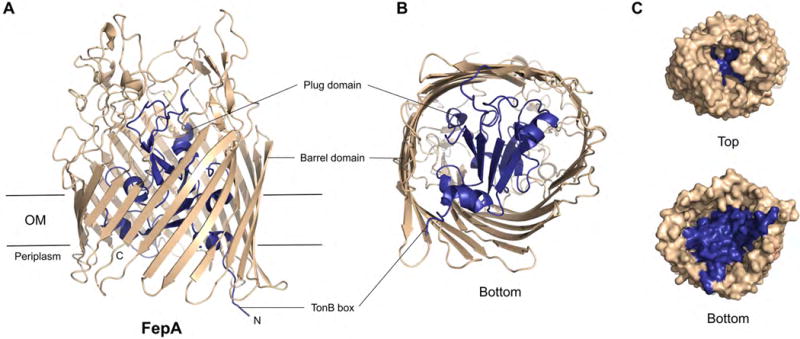
General structure of a TBDT exemplified with the ferric enterobactin transporter FepA. (A) Crystal structure of FepA (PDB: 1FEP) [16]. The membrane view is shown. (B) View from the periplasm (bottom) into the barrel. (C) View from the extracellular space (top) or periplasm (bottom) into the barrel, respectively, depicted as surface model. The barrel domain is shown in tan, and the plug domain in blue.
Some TBDTs have a co-receptor anchored to the extracellular side of the OM that mediates ligand binding to the transporter (Figure 2). Following entry into the periplasm, further transport to the cytoplasm often involves a periplasmic binding protein (PBP) that binds the molecule or ion of interest and delivers it to an ATP-binding cassette (ABC) transporter located in the IM. Transport across the IM is thus driven by ATP hydrolysis (Figure 2) [21].
Neisseria express TBDTs under conditions of metal limitation [22], which allow Neisseria to utilize abundant human metalloproteins present at infection sites and in the bloodstream as nutritional sources. In particular, Neisseria employ TBDTs to extract nutrient metal ions from human transferrin (hTF) [23], lactoferrin (hLF) [24], hemoglobin (hHb) [25], and calprotectin (hCP) [7,8]. Among the different TBDTs employed by pathogenic Neisseria for metal uptake, the iron acquisition systems are the most well characterized to date and are discussed in several extensive reviews [6,26–28]. In the following sections, we present recent work that has advanced understanding of how Neisseria use TBDTs to acquire nutrient iron and zinc. These studies afford important structural and mechanistic insights into how Neisseria hijack host non-heme and heme iron proteins, describe the discovery of zinc piracy from the metal-sequestering host-defense protein CP, and provide inspirations for future work that includes fundamental investigations and initiatives directed towards therapeutic development.
Neisseria pirate iron from transferrin, lactoferrin and hemoglobin
Iron is an essential nutrient for humans and almost all microbial pathogens [28–31]. It is a co-factor for proteins involved in primary metabolism, DNA synthesis, and electron transfer. Despite its high abundance (e.g. a ≈70-kg human contains ≈4 g of Fe[32]), the pool of “free” iron is low. Iron exhibits low solubility in the ferric oxidation state (Ksp = 10−18 M), which is predominant under aerobic conditions. Moreover, iron participates in Fenton chemistry and thereby induces the formation of harmful reactive oxygen species, which damage lipids, DNA and proteins. As a result, the uptake, transport, storage and utilization of iron are tightly regulated in humans. Although labile iron pools have been detected, most iron is tightly bound to proteins, evidenced by the low “free” iron concentration in serum (≈10−24 M) [29,33]. During infection, the human host further lowers iron availability at sites of infection by deploying iron-sequestering host-defense proteins such as lactoferrin (LF) and lipocalin-2 [34,35], and increasing production of the peptide hormone hepcidin to inhibit iron transport into the bloodstream [36].
In this section, we consider how Neisseria overcome host-imposed iron restriction by using systems composed of a TBDT and its co-receptor to extract this nutrient from hTF, hLF, and hHb, three abundant metalloproteins with different biological functions [6,25,27,28]. Neisseria can acquire iron from human TF and LF, but not from other mammalian orthologues; this specificity is hypothesized to restrict the host range of these bacteria to humans [37,38]. Moreover, clinical research indicates that Neisseria must express either a TF or LF receptor to cause infections in humans [39,40].
Iron removal from transferrin
TF is a bilobal glycoprotein (≈80 kDa) that functions in iron transport. It can also be classified as a host-defense protein because it restricts iron availability [31,41]. TF is synthesized in the liver and secreted into the bloodstream where it binds and transports Fe(III). Iron delivery into cells occurs via receptor-mediated endocytosis. The TF receptor (TFR) binds holo-TF and, following cellular uptake, iron release occurs in the acidic endosomes [42]. Subsequently, the TFR-apoTF complex is recycled, and apo-TF is released into the extracellular space.
TF consists of two homologous lobes (N- and C-lobe) that are connected by a short peptide linker (Figure 4A). Each lobe can be divided into two subdomains, which form a cleft that provides the iron-binding site [43]. Within each cleft, one Fe(III) ion is bound in a six-coordinate geometry by one aspartic acid, one histidine and two tyrosine residues, and two oxygen atoms from a bound carbonate ion complete the octahedral coordination sphere (Figure 4B) [44]. hTF undergoes conformational changes with metal binding and release; holo-hTF is more compact than the apo-hTF [46]. At neutral pH, hTF coordinates Fe(III) with high affinity at both lobes (Kd ≈10−20 M−1) [47]. Crystallographic studies of the N-lobe of hTF indicated that the bound carbonate ion is stabilized by hydrogen-bonding interactions with an adjacent arginine residue [45]. In acidic endosomes, protonation of this arginine may facilitate dissociation of the carbonate and thus release of Fe(III).
Figure 4.
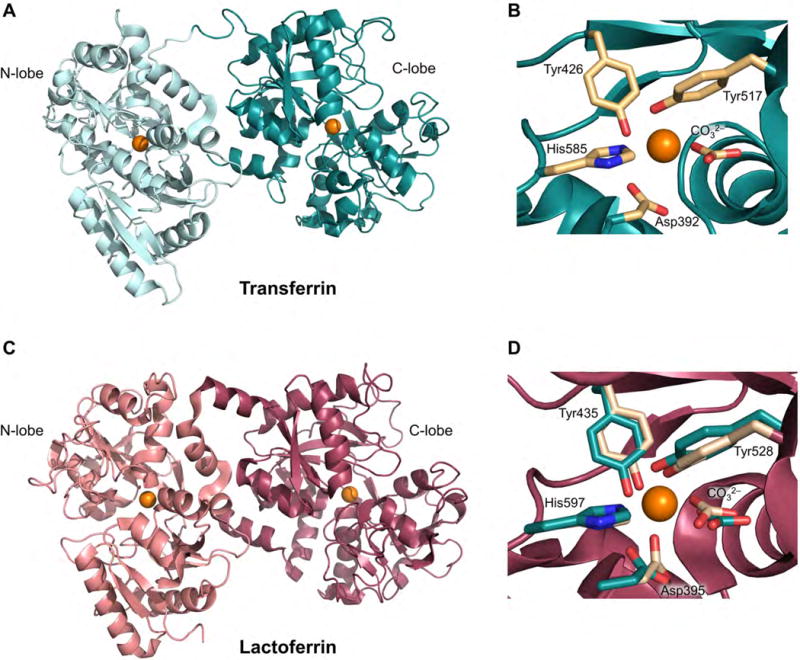
Structures of diferric hTF and hLF. (A) Crystal structure of hTF with Fe(III) ions bound at both lobes (PDB: 3V83)[55]. (B) Iron-binding site of the C-lobe of hTF; the metal-binding residues are shown in tan. (C) Crystal structure of hLF with Fe(III) ions bound at both lobes (PDB: 1B0L) [63]. (D) Iron-binding site of the C-lobe of hLF (red) in superposition with the coordinating residues from the C-lobe of hTF (teal). The Fe(III) ions are shown as orange spheres.
Neisseria employ the TbpAB system (Tbp = TF-binding protein) to acquire iron from hTF[48–50]. TbpAB recognizes and binds holo-hTF, removes Fe(III) from the protein, and transports the metal ion into the cell. TbpA (≈100 kDa) is a TBDT and TbpB (≈80 kDa) is its bilobal co-receptor. TpbA binds apo- and holo-hTF with similar affinities, and extracts Fe from hTF in the absence of TpbB. Nevertheless, iron removal from holo-hTF occurs more rapidly when the co-receptor is present [51,52]. TbpB preferentially binds holo-hTF and thereby facilitates capture of the holo-form and, following Fe(III) extraction, release of apo-hTF [53,54].
Recent crystallographic and cryo-electron microscopy studies provided structures of TbpA—hTF and TbpB—hTF, a model for the TbpA—TbpA—hTF ternary complex, and remarkable insight into the molecular basis of iron piracy by TbpAB (Figure 5) [55–57]. Co-crystal structures of TbpA—hTF and TbpB—hTF revealed that both TbpA and TbpB bind the C-lobe of hTF, but at different sites (Figure 5A,B). In the current model, the N-lobe of TbpB captures holo-hTF, and the resulting TbpB–holo-TF complex forms a transient ternary complex with TbpA (Figure 5B), which allows transfer of holo-hTF to TbpA [56]. It has been proposed that formation of the holo-hTF—TbpB complex locks hTF in the closed Fe(III)-bound conformation until delivery to TbpA occurs, thereby increasing the efficiency of metal import. Long extracellular loops of TbpA and an extended loop from the plug domain interact with the C-lobe of holo-hTF (Figure 5B) [55]. A helix finger within one of the extracellular loops is postulated to facilitate iron release from holo-hTF by inserting a lysine residue into the cleft of the hTF C-lobe and thereby destabilizing the coordination site [55]. Moreover, binding of hTF to TbpA causes conformational changes in the plug domain of TbpA, initiating energy transduction via interaction with TonB–ExbB–ExbD and transport of the Fe(III) ion through the β-barrel. Unfolding of the plug domain creates a pathway in the β-barrel lined by multiple oxygen donor atoms from adjacent amino acid residues (EIEYE motif, Figure 5B). These oxygen donors have been proposed to transiently bind Fe(III) during its transport through TpbA.
Figure 5.
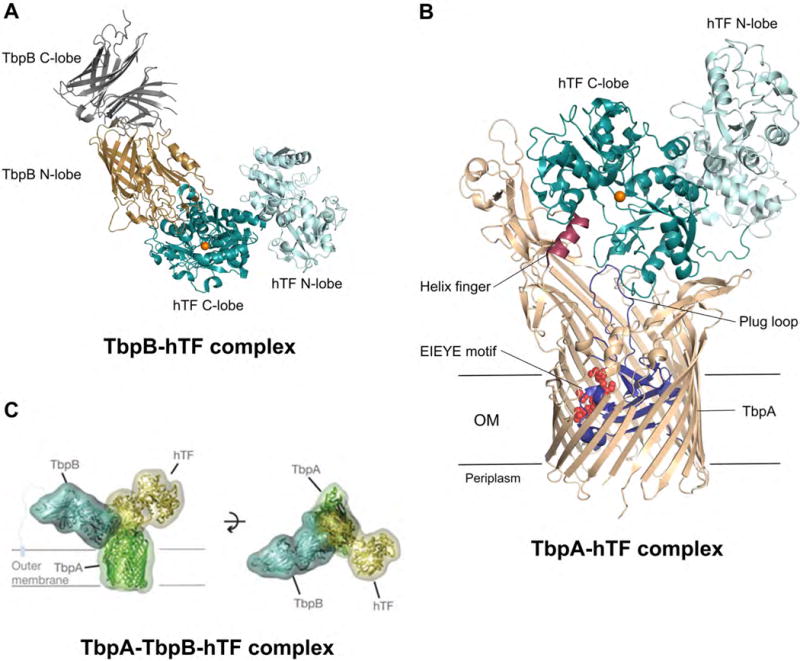
Interaction of hTF with TbpAB of N. meningitidis. (A) Crystal structure of hTF bound to the co-receptor TbpB (PDB: 3VE1)[56]. TbpB is shown in gray/brown and TF is shown in teal/cyan. (B) Crystal structure of hTF bound to the TBDT TbpA (PDB: 3V8X)[55]. TF is shown in teal/cyan, TbpA in tan with the plug domain depicted in blue, and the helix finger and EIEYE motif in red; a Fe(III) ion has been modeled into the hTF C-lobe and is shown as an orange sphere. (C) Model of the ternary complex as determined by negative stain electron microscopy [55]. TF is shown in yellow, TbpA in green, and TbpB in cyan. The figure is reproduced with permission from Nature Publishing Group [55] Abbreviations: hTF, human transferrin; OM, outer membrane.
Following transport across the OM and entry into the periplasm, FbpA (Fe-binding protein, a PBP) coordinates the Fe(III) ion and delivers it to the ABC transporter FbpBC for cytosolic delivery (Figure 2) [58,59]. FbpA is described as “bacterial transferrin” because of its structural and functional similarities with hTF [60]. Lastly, FbpBC is also required for cytosolic iron delivery from other non-heme sources that include iron extracted from hLF and ferric citrate [61].
Iron removal from lactoferrin
Lactoferrin (LF) is a member of the transferrin protein family primarily found in mucosal secretions, including breast milk in lactating females, and as a cytoplasmic component of neutrophils [34]. Whereas the main physiological role of TF is iron transport, LF is a component of the innate immune system and primarily serves a protective function at mucous membranes [62]. It contributes to the metal-withholding response by sequestering Fe(III) from invading microbes (Figure 1) [34,62]. hLF (≈80 kDa) shares 60% sequence identity with hTF, exhibits a bilobal structure, and coordinates one Fe(III) ion at each lobe with the same ligand set as TF (Figure 4C,D) [43,63,64]. Nevertheless, equilibrium dialysis studies revealed that hLF exhibits ≈260-fold higher Fe(III) affinity than hTF, and Fe(III) dissociation occurs less readily from Fe(III)-hLF than from Fe(III)-hTF under acidic conditions [64,65]. The latter feature presumably allows LF to retain bound iron in low pH environments such as the stomach and at sites of inflammation [64].
Neisseria utilize LbpAB (LF-binding protein) to extract Fe(III) from hLF. Structural data for this system is limited and, to the best of our knowledge, only a crystal structure for the N-lobe of LbpB has been reported to date [66]. Nevertheless, this system exhibits similarities to TbpAB, and bioinformatics and homology modeling afforded a proposal for how LbpAB extract Fe(III) from hLF [67]. The TBDT LbpA shares ≈40% sequence identity with TbpA, and a homology model indicated that LpbA displays the extended plug loop for ligand binding, the helix finger involved in Fe(III) extraction, and the EIEYE motif for Fe(III) transport through the β-barrel as observed for TbpA (Figure 5B). The co-receptor LbpB shares ≈30% sequence identity with its hTF homologue TbpB and is predicted to have a similar bilobal structure and preferentially bind holo-hLF [50,67]. Biophysical and structural investigations will inform the working model for LbpAB, and illuminate whether this system employs the same Fe(III) release mechanism as TbpA or whether a modified strategy is required because of differences in the Fe(III)-binding affinities of hLF.
In addition to enabling Neisseria to use hLF as an iron source, LbpB has been reported to protect N. meningitidis from the bactericidal effects of lactoferricins [68], which are small, cationic peptides that form as a result of proteolytic degradation of LF. Lactoferricins are found at sites of infection and exhibit antibacterial activity [69]. It appears that LbpB captures lactoferricins by electrostatic interactions between the negatively charged amino acid residues in its C-lobe and the cationic peptides [68].
Iron removal from hemoglobin
Approximately 70% of iron in the human body exists in the form of heme [32]. Most heme is bound to the oxygen-carrying protein hHb. Neisseria and many other bacterial pathogens exploit Hb as an iron source [3–5,28]. N. gonorrhoeae and N. meningitidis express the TBDT HpuB and its co-receptor HpuA to acquire heme from Hb and the Hb-haptoglobin complex [70–72]. N. meningitidis also expresses a second Hb-binding receptor, HmbR [70,73]. Both systems extract heme from Hb and transport it into the periplasm. Heme is subsequently transported to the cytoplasm where heme degradation, catalyzed by the heme oxygenase HemO, affords iron release [74,75]. The mechanism of heme transport into the cytoplasm requires further investigation [27]. The neisserial Hb receptors show some similarity to the Tf and Lf receptors, but less molecular details are known to date. A structural model of the co-receptor HpuA from N. gonorrhoeae in complex with Hb, which was based on a crystal structure of the C-terminal portion of HpuA, was reported recently [REF].
Neisseria pirate zinc from calprotectin
Zinc is an essential nutrient for all organisms [76]. This abundant and ubiquitous metal ion is a structural or catalytic component of several thousand proteins in the human genome [76,77]. In humans, Zn(II) homeostasis is controlled by Zn(II) transporters (ZnTs, ZIPs) and metallothioneins, the latter of which are cysteine-rich peptides that act as cellular Zn(II) buffers by maintaining low free Zn(II) concentrations and providing Zn(II) when needed by the cell. The total intracellular Zn(II) concentration is estimated to be ≈200 μM [78]. Most intracellular Zn(II) is tightly bound to proteins, and the levels of “free” Zn(II) are estimated to be ≈10−10 M for most cell types [77,78]. Zn(II) is an important player in the immune response to invading pathogens; the host can either enhance or restrict Zn(II) availability to cause zinc intoxication or zinc starvation of the pathogen, respectively [79]. To restrict zinc availability at infection sites, the human innate immune system deploys Zn(II)-sequestering host-defense proteins, including the abundant neutrophil protein calprotectin (CP) [80]. hCP is a component of neutrophil extracellular traps (NETs), which form when neutrophils release proteins and chromatin to capture and kill microbes [81–82]. In this section, we consider the competition between the host and Neisseria for zinc by highlighting two recent studies reporting that Neisseria overcome zinc limitation by expressing TBDTs that capture Zn(II)-hCP and use this innate immune factor as a zinc source [7,8].
hCP is a heterooligomer of the proteins S100A8 (10.8 kDa) and S100A9 (13.2 kDa) (Figure 6A) [83,84]. Each S100 subunit contains two Ca(II)-binding EF-hand domains [84,85], and Ca(II) binding causes hCP to convert from a low-M(II) to a high-M(II) affinity form [86–88]. Both forms coordinate transition metals and display antibacterial activity; however, hCP exhibits enhanced transition-metal affinities and enhanced antimicrobial activity in the presence of excess Ca(II) ions [86–88]. Because cytoplasmic Ca(II) levels are typically low (i.e. nanomolar range) and extracellular Ca(II) levels are high (≈2 mM) [89], the current model states that CP responds to local Ca(II) concentrations and morphs into the high M(II)-affinity form following release into the extracellular space [86–88]. The hCP heterodimer has two transition-metal-binding sites that form at the S100A8/S100A9 interface. Site 1 is a His3Asp motif (Figure 6B) and site 2 is a His6 motif (Figure 6C) [84,85,90–93]. Both sites coordinate Zn(II) with high affinity (Kd < 1 × 10−12 M, +Ca(II)) and contribute to Zn(II) sequestration [94].
Figure 6.
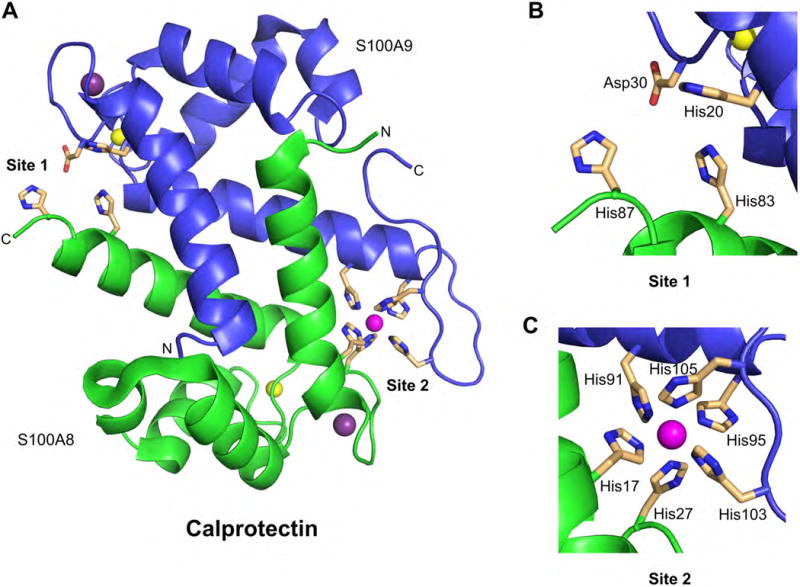
Structure of the human CP heterodimer showing the sites for transition metals. (A) Crystal structure of Mn(II)-, Ca(II)-, and Na(I)-bound hCP (PDB: 4XJK)[93]. A heterodimer taken from a heterotetramer structure is shown. The S100A8 subunit is shown in green, the S100A9 in blue; the Mn(II) ion is a magenta sphere, the Ca(II) ions are yellow spheres, and the Na(I) are purple spheres. (B) Site 1 is a His3Asp motif. (C) Site 2 is a His6 motif and is shown with a bound Mn(II) ion. No structure of Zn(II)-hCP is available; however, solution studies show that both the His3Asp and His6 sites coordinate Zn(II), resulting in a 2:1 stoichiometry per heterodimer[86,94].
A recent microbiology study examined a ≈100-kDa TBDT of unknown function expressed by N. meningitidis that was originally named TdfH [7]. This TBDT is of similar size to TpbA and LpbA, and it was predicted to bind an as-yet unidentified protein. Moreover, TdfH expression was induced under Zn(II)-limited conditions and controlled by the zinc-dependent repressor Zur. It was subsequently discovered that N. meningitidis binds hCP in a TdfH-dependent manner [7]. This interaction was enhanced in the presence of Zn(II), suggesting that TdfH preferentially binds Zn(II)-hCP over the apo form. When cultured under Zn(II)-limited conditions in the presence of hCP, N. meningitidis grew whereas a mutant strain that could not express TdfH was unable to grow. Taken together, these data afford a new model where N. meningitidis responds to Zn(II) starvation by expressing a TBDT that captures Zn(II)-hCP and uses this protein as a Zn(II) source. This discovery resulted in re-naming of TdfH to CbpA (CP-binding protein) A [7].
N. gonorrhoeae have a homologous TBDT, hereafter called CbpA [8]. This transporter is also expressed under condition of Zn(II) limitation and allows N. gonorrhoeae to use Zn(II)-CP as a Zn(II) source. Moreover, CbpA enhanced N. gonorrhoeae resistance to NET-mediated killing, which suggests that N. gonorrhoeae binds Zn(II)-CP and obtains nutrient Zn(II) from this metal-sequestering protein when entangled by NETs.
These two studies uncovered that metal acquisition from hCP is an adaptive strategy that Neisseria employs to overcome Zn(II) restriction. All sequenced strains of N. meningitidis and ≈81% of sequenced N. gonorrhoeae strains harbor the cbpA gene, suggesting an important role for CbpA in host colonization [7,8]. The discovery of this Zn(II) acquisition system provides inspiration for future work addressing the molecular basis of CbpA in metal acquisition, including how CbpA binds hCP and extracts Zn(II). Along these lines, studies with N. meningitidis indicated that CbpA also binds Mn(II)-hCP [7]. This observation suggests that metal acquisition by CbpA may extend beyond Zn(II). hCP has a remarkable ability to sequester multiple first-row transition metal ions at its His6 site [88,93], and it is plausible that Neisseria exploits this property to ensure that each M(II)-hCP-CbpA binding event results in productive metal acquisition. Although hijacking calprotectin for Zn(II) acquisition has only been observed for Neisseria to date, other pathogens may express homologs of CbpA or exploit other members of the S100 protein family (e.g. S100A7, S100A12) for nutrient metal acquisition [7]. The mechanism of Zn(II) is transport into the bacterial cytoplasm after uptake by CbpA has not been specifically addressed; however, involvement of ZnuABC (Zn(II)uptake) is likely [REF1]. Neisseria also express a TBDT named ZnuD for Zn(II) acquisition. In contrast to CpbA, this TBDT has been proposed to mediate the transport of “free” zinc ions [REF2,REF3].
Summary and Concluding Remarks
In this Essay, we present Neisseria as a case study of how a human pathogen overcomes metal-limitation in the host environment by hijacking abundant host metalloproteins to obtain iron and zinc. The highlighted work exemplifies how coordination chemistry underlies the host-microbe interaction, and how fundamental studies in bioinorganic chemistry, structural biology, and microbiology advance understanding of microbial virulence and infectious disease.
We note briefly that Neisseria employ many additional strategies for obtaining nutrient metals in the host environment [7,23,95–98]. An arsenal of metal transport systems allows Neisseria to readily adapt to changes in metal availability [99]. For instance, although Neisseria do not biosynthesize siderophores, microbial metabolites that are produced under nutrient-limited conditions for Fe(III) acquisition, these microbes express TBDTs that recognize and transport “xenosiderophores,” i.e. siderophores made by other bacterial species [95–98]. Thus, in addition to the host-microbe interaction, TBDTs allow Neisseria to participate in microbe-microbe interactions and compete for metal nutrients with other species occupying the same niche.
The global community is confronted with the public health problem of antibiotic resistance in hospital and community settings, necessitating new strategies to prevent and treat infectious disease. Structural and mechanistic investigations of microbial metal-ion transport systems provide the foundation for evaluating whether these systems have potential as targets for therapeutic development. This direction is warranted for infections caused by Neisseria because strains are increasingly resistant to antibiotics in clinical use and there is currently no vaccine available for N. gonorrhoeae. Along these lines, TbpAB and LbpAB have been identified as important targets for vaccine development [13,67]. Also noteworthy, a recent study demonstrated that the TbpA-hTF binding surface is the subject of an evolutionary arms race, indicating that rapid variation of host TF contributes to nutritional immunity by allowing the protein to avoid capture by TpbA [100,101]. In closing, transition metals play essential roles in the host-pathogen interaction and microbial pathogenesis. We expect that further elucidation of the systems involved in microbial metal transport and homeostasis for Neisseria and other human pathogens will enhance our fundamental understanding of metals in biology and provide a valuable guide for new approaches to treat infectious disease.
Acknowledgments
We thank Profs. Jeremy Berg and Stephen Lippard for the invitation to contribute this article and potential topics for their new textbook.
Funding Information
We acknowledge the NIH (Grants 1R01AI114625 and 1R21AI126465) and the NSF (Grant CHE-1352132) for supporting our fundamental and applied research on transition metals and the host-microbe interaction. WN is a recipient of a Leopoldina Fellowship of the German National Academy of Sciences Leopoldina (LPDS 2015-08).
Abbreviations
- ABC
ATP-binding cassette
- (h)CP
(human) calprotectin
- Cbp
calprotectin-binding protein
- Fbp
Fe-binding protein
- (h)Hb
(human) hemoglobin
- IM
inner membrane
- Lbp
lactoferrin-binding protein
- (h)LF
(human) lactoferrin
- NET
neutrophil extracellular trap
- OM
outer membrane
- PBP
periplasmic binding protein
- TBDT
TonB-dependent transporter
- Tbp
transferrin-binding protein
- (h)TF
(human) transferrin
- TFR
transferrin receptor
References
- 1.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg ED. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. J Am Med Assoc. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker KW, Skaar EP. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol Rev. 2014;38:1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet. 2016;50:67–91. doi: 10.1146/annurev-genet-120215-035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohde KH, Dyer DW. Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Front Biosci. 2003;8:d1186–d1218. doi: 10.2741/1133. [DOI] [PubMed] [Google Scholar]
- 7.Stork M, Grijpstra J, Bos MP, Mañas Torres C, Devos N, Poolman JT, Chazin WJ, Tommassen J. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLoS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jean S, Juneau RA, Criss AK, Cornelissen CN. Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH. Infect Immun. 2016;84:2982–2994. doi: 10.1128/IAI.00319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassat JE, Skaar EP. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin Immunopathol. 2012;34:215–235. doi: 10.1007/s00281-011-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virji M. Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7:274–286. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 11.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu W, Chen C-J, Thomas CE, Anderson JE, Jerse AE, Sparling PF. Vaccines for gonorrhea: can we rise to the challenge? Front Microbiol. 2011;2:124. doi: 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cash DR, Noinaj N, Buchanan SK, Cornelissen CN. Beyond the crystal structure: insight into the function and vaccine potential of TbpA expressed by Neisseria gonorrhoeae. Infect Immun. 2015;83:4438–4449. doi: 10.1128/IAI.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer K, Rodionov DA, de Reuse H. New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg’? Trends Biochem Sci. 2008;33:330–338. doi: 10.1016/j.tibs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 17.Braun V. Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB–ExbB–ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 18.Braun V, Endriß F. Energy-coupled outer membrane transport proteins and regulatory proteins. Biometals. 2007;20:219–231. doi: 10.1007/s10534-006-9072-5. [DOI] [PubMed] [Google Scholar]
- 19.Postle K, Larsen RA. TonB-dependent energy transduction between outer and cytoplasmic membranes. Biometals. 2007;20:453–465. doi: 10.1007/s10534-006-9071-6. [DOI] [PubMed] [Google Scholar]
- 20.Celia H, Noinaj N, Zakharov SD, Bordignon E, Botos I, Santamaria M, Barnard TJ, Cramer WA, Lloubes R, Buchanan SK. Structural insight into the role of the Ton complex in energy transduction. Nature. 2016;538:60–65. doi: 10.1038/nature19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 2008;1778:1781–1804. doi: 10.1016/j.bbamem.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Cornelissen CN, Hollander A. TonB-dependent transporters expressed by Neisseria gonorrhoeae. Front Microbiol. 2011;2:117. doi: 10.3389/fmicb.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mickelsen PA, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mickelsen PA, Blackman E, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982;35:915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BC. Isolation of haemin-binding proteins of Neisseria gonorrhoeae. J Med Microbiol. 1992;36:121–127. doi: 10.1099/00222615-36-2-121. [DOI] [PubMed] [Google Scholar]
- 26.Schryvers AB, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 27.Perkins-Balding D, Ratliff-Griffin M, Stojiljkovic I. Iron transport systems in Neisseria meningitidis. Microbiol Mol Biol Rev. 2004;68:154–171. doi: 10.1128/MMBR.68.1.154-171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caza M, Kronstad JW. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol. 2013;3:80. doi: 10.3389/fcimb.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dlouhy AC, Outten CE. The iron metallome in eukaryotic organisms. Met Ions Life Sci. 2013;12:241–278. doi: 10.1007/978-94-007-5561-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drabkin DL. Metabolism of the hemin chromoproteins. Physiol Rev. 1951;31:345–431. doi: 10.1152/physrev.1951.31.4.345. [DOI] [PubMed] [Google Scholar]
- 33.Cabantchik ZI. Labile iron in cells and body fluids: physiology, pathology, and pharmacology. Front Pharmacol. 2014;5:45. doi: 10.3389/fphar.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992;67:657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 36.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 37.Schryvers AB, Gonzalez GC. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect Immun. 1989;57:2425–2429. doi: 10.1128/iai.57.8.2425-2429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarantonelli M-L, Szatanik M, Giorgini D, Hong E, Huerre M, Guillou F, Alonso J-M, Taha M-K. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun. 2007;75:5609–5614. doi: 10.1128/IAI.00781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornelissen CN, Kelley M, Hobbes MM, Anderson JE, Cannon JG, Cohen MS, Sparling PF. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JE, Hobbs MM, Biswas GD, Sparling PF. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol Microbiol. 2003;48:1325–1337. doi: 10.1046/j.1365-2958.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin L, Pantapalangkoor P, Tan B, Bruhn KW, Ho T, Nielsen T, Skaar EP, Zhang Y, Bai R, Wang A, Doherty TM, Spellberg B. Transferrin iron starvation therapy for lethal bacterial and fungal infections. J Infect Dis. 2014;210:254–264. doi: 10.1093/infdis/jiu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klausner RD, Ashwell G, van Renswoude J, Harford JB, Bridges KR. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983;80:2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wally J, Buchanan SK. A structural comparison of human serum transferrin and human lactoferrin. Biometals. 2007;20:249–262. doi: 10.1007/s10534-006-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert LA, Perri H, Halbrooks PJ, Mason AB. Evolution of the transferrin family: conservation of residues associated with iron and anion binding. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:129–141. doi: 10.1016/j.cbpb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 45.MacGillivray RTA, Moore SA, Chen J, Anderson BF, Baker H, Luo Y, Bewley M, Smith CA, Murphy MEP, Wang Y, Mason AB, Woodworth RC, Brayer GD, Baker EN. Two high resolution structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry. 1998;37:7919–7928. doi: 10.1021/bi980355j. [DOI] [PubMed] [Google Scholar]
- 46.Wally J, Halbrooks PJ, Vonrhein C, Rould MA, Everse SJ, Mason AB, Buchanan SK. The crystal structure of iron-free human serum transferrin provides insight into inter-lobe communication and receptor binding. J Biol Chem. 2006;281:24934–24944. doi: 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aisen P, Leibman A, Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978;253:1930–1937. [PubMed] [Google Scholar]
- 48.Cornelissen CN, Biswas GD, Tsai J, Paruchuri DK, Thompson SA, Sparling PF. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray-Owen SD, Schryvers AB. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 50.Morgenthau A, Pogoutse A, Adamiak P, Moraes TF, Schryvers AB. Bacterial receptors for host transferrin and lactoferrin: molecular mechanisms and role in host-microbe interactions. Future Microbiol. 2013;8:1575–1585. doi: 10.2217/fmb.13.125. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeRocco AJ, Yost-Daljev MK, Kenney CD, Cornelissen CN. Kinetic analysis of ligand interaction with the gonococcal transferrin-iron acquisition system. Biometals. 2009;22:439–451. doi: 10.1007/s10534-008-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornelissen CN, Sparling PF. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boulton IC, Gorringe AR, Allison N, Robinson A, Gorinsky B, Joannou CL, Evans RW. Transferrin-binding protein B isolated from Neisseria meningitidis discriminates between apo and diferric human transferrin. Biochem J. 1998;334:269–273. doi: 10.1042/bj3340269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noinaj N, Easley NC, Oke M, Mizuno N, Gumbart J, Boura E, Steere AN, Zak O, Aisen P, Tajkhorshid E, Evans RW, Gorringe AR, Mason AB, Steven AC, Buchanan SK. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012;483:53–58. doi: 10.1038/nature10823. Volume: We recommend the movie provided with the supplementary information, depicting the proposed mechanism of iron extraction from transferrin and transport of the ion across the OM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calmettes C, Alcantara J, Yu R-H, Schryvers AB, Moraes TF. The structural basis of transferrin sequestration by transferrin-binding protein B. Nat Struct Mol Biol. 2012;19:358–360. doi: 10.1038/nsmb.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noinaj N, Buchanan SK, Cornelissen CN. The transferrin–iron import system from pathogenic Neisseria species. Mol Microbiol. 2012;86:246–257. doi: 10.1111/mmi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siburt CJ, Roulhac PL, Weaver KD, Noto JM, Mietzner TA, Cornelissen CN, Fitzgerald MC, Crumbliss AL. Hijacking transferrin bound iron: protein-receptor interactions involved in iron transport in N. gonorrhoeae. Metallomics. 2009;1:249–255. doi: 10.1039/b902860a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adhikari P, Berish SA, Nowalk AJ, Veraldi KL, Morse SA, Mietzner TA. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J Bacteriol. 1996;178:2145–2149. doi: 10.1128/jb.178.7.2145-2149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker Siburt CJ, Mietzner TA, Crumbliss AL. FbpA – a bacterial transferrin with more to offer. Biochim Biophys Acta. 2012;1820:379–392. doi: 10.1016/j.bbagen.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Khun HH, Kirby SD, Lee BC. A Neisseria meningitides fbpABC mutant is incapable of using nonheme iron for growth. Infect Immun. 1998;66:2330–2336. doi: 10.1128/iai.66.5.2330-2336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ling JM, Schryvers AB. Perspectives on interactions between lactoferrin and bacteria. Biochem Cell Biol. 2006;84:275–281. doi: 10.1139/o06-044. [DOI] [PubMed] [Google Scholar]
- 63.Sun XL, Baker HM, Shewry SC, Jameson GB, Baker EN. Structure of recombinant human lactoferrin expressed in Aspergillus awamori. Acta Crystallogr D Biol Crystallogr. 1999;55:403–407. doi: 10.1107/s0907444998011226. [DOI] [PubMed] [Google Scholar]
- 64.Baker HM, Baker EN. Lactoferrin and iron: structural and dynamic aspects of binding and release. BioMetals. 2004;17:209–216. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 65.Aisen P, Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972;257:314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- 66.Brooks CL, Arutyunova E, Lemieux MJ. The structure of lactoferrin-binding protein B from Neisseria meningitidis suggests roles in iron acquisition and neutralization of host defences. Acta Crystallogr F Struct Biol Commun. 2014;70:1312–1317. doi: 10.1107/S2053230X14019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noinaj N, Cornelissen CN, Buchanan SK. Structural insight into the lactoferrin receptors from pathogenic Neisseria. J Struct Biol. 2013;184:83–92. doi: 10.1016/j.jsb.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgenthau A, Livingstone M, Adamiak P, Schryvers AB. The role of lactoferrin binding protein B in mediating protection against human lactoferricin. Biochem Cell Biol. 2012;90:417–423. doi: 10.1139/o11-074. [DOI] [PubMed] [Google Scholar]
- 69.Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121:130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 70.Stojiljkovic I, Hwa V, de Saint Martin L, O’Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen CJ, Sparling PF, Lewis LA, Dyer DW, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis LA, Dyer DW. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stojiljkovic I, Larson J, Hwa V, Anic S, So M. HmbR outer membrane proteins of pathogenic Neisseriae: iron-regulated, hemoglobin binding proteins with high degree of primary structure conservation. J Bacteriol. 1996;178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu W, Hunt DJ, Richardson AR, Stojiljkovic I. Use of heme compounds as iron sources by pathogenic Neisseriae requires the product of the hemO gene. J Bacteriol. 2000;182:439–447. doi: 10.1128/jb.182.2.439-447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu W, Wilks A, Stojiljkovic I. Degradation of heme in Gram-negative bacteria: the product of the hemO gene of Neisseriae is a heme oxygenase. J Bacteriol. 2000;182:6783–6790. doi: 10.1128/jb.182.23.6783-6790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maret W. Zinc and the zinc proteome. Met Ions Life Sci. 2013;12:479–501. doi: 10.1007/978-94-007-5561-1_14. [DOI] [PubMed] [Google Scholar]
- 78.Krężl A, Maret W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subramanian Vignesh K, Deepe GS., Jr Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016;611:66–78. doi: 10.1016/j.abb.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stříž I, Trebichavský I. Calprotectin – a pleiotropic molecule in acute and chronic inflammation. Physiol Res (Prague, Czech Repub) 2004;53:245–253. [PubMed] [Google Scholar]
- 81.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 82.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hunter MJ, Chazin WJ. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J Biol Chem. 1998;273:12427–12435. doi: 10.1074/jbc.273.20.12427. [DOI] [PubMed] [Google Scholar]
- 84.Korndörfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370:887–898. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 85.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 86.Brophy MB, Hayden JA, Nolan EM. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc. 2012;134:18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayden JA, Brophy MB, Cunden LS, Nolan EM. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc. 2013;135:775–787. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11:765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brini M, Ottolini D, Calì T, Carafoli E. Calcium in health and disease. Met Ions Life Sci. 2013;13:81–137. doi: 10.1007/978-94-007-7500-8_4. [DOI] [PubMed] [Google Scholar]
- 90.Williams RJP. Calcium binding proteins in normal and transformed cells. Cell Calcium. 1996;20:87–93. doi: 10.1016/s0143-4160(96)90054-8. [DOI] [PubMed] [Google Scholar]
- 91.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, Garcia C, Munro KA, Chazin WJ, Skaar EP. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–164. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110:3841–3846. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gagnon DM, Brophy MB, Bowman SEJ, Stich TA, Drennan CL, Britt RD, Nolan EM. Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J Am Chem Soc. 2015;137:3004–3016. doi: 10.1021/ja512204s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakashige TG, Stephan JR, Cunden LS, Brophy MB, Wommack AJ, Keegan BC, Shearer JM, Nolan EM. The hexahistidine motif of host-defense protein human calprotectin contributes to zinc withholding and its functional versatility. J Am Chem Soc. 2016;138:12243–12251. doi: 10.1021/jacs.6b06845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larson JA, Howie HL, So M. Neisseria meningitidis accelerates ferritin degradation in host epithelial cells to yield an essential iron source. Mol Microbiol. 2004;53:807–820. doi: 10.1111/j.1365-2958.2004.04169.x. [DOI] [PubMed] [Google Scholar]
- 96.West SE, Sparling PF. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Biegel Carson SD, Klebba PE, Newton SMC, Sparling PF. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J Bacteriol. 1999;181:2895–2901. doi: 10.1128/jb.181.9.2895-2901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strange HR, Zola TA, Cornelissen CN. The fbpABC operon is required for Ton-independent utilization of xenosiderophores by Neisseria gonorrhoeae strain FA19. Infect Immun. 2011;79:267–278. doi: 10.1128/IAI.00807-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jordan PW, Saundersm NJ. Host iron binding proteins acting as niche indicators for Neisseria meningitidis. PLoS ONE. 2009;4:e5198. doi: 10.1371/journal.pone.0005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barber MF, Elde NC. Escape from bacterial iron piracy through rapid evolution of transferrin. Science. 2014;346:1362–1366. doi: 10.1126/science.1259329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Armitage AE, Drakesmith H. The battle for iron. Science. 2014;346:1299–1300. doi: 10.1126/science.aaa2468. [DOI] [PubMed] [Google Scholar]


