Abstract
Currently, cold temperatures are one of the main factors threatening rapeseed production worldwide; thus, it is imperative to identify cold-resistant germplasm and to cultivate cold-resistant rapeseed varieties. In this study, the cold resistance of four Brassica rapa varieties was analyzed. The cold resistance of Longyou6 and Longyou7 was better than that of Tianyou2 and Tianyou4. Thus, an F2 population derived from Longyou6 and Tianyou4 was used to study the correlation of cold resistance and physiological indexes. Our results showed that the degree of frost damage was related to the relative conductivity and MDA content (r1 = 0.558 and r2 = 0.447, respectively). In order to identify the markers related to cold resistance, 504 pairs of SSR (simple sequence repeats) primers were used to screen the two parents and F2 population. Four and five SSR markers had highly significant positive correlation to relative conductivity and MDA, respectively. In addition, three of these SSR markers had a highly significant positive correlation to both of these two indexes. These three SSR markers were subsequently confirmed to be used to distinguish between cold-resistant and non-cold-resistant varieties. The results of this study will lay a solid foundation for the mapping of cold-resistant genes and molecular markers assisted selection for the cold-resistance.
Keywords: cold resistance, Brassica rapa, correlation, SSR markers
Introduction
In recent years, damage due to cold temperatures in China has caused great harm to the agricultural industry. Chinese rapeseed suffered severe cold damage because of continuous low temperatures in winter, leading to heavy losses and aggravating China’s edible oil gap in 2008 (Zhang et al. 2008). Therefore it is of importance to cultivate cold-resistant rapeseed varieties. Brassica rapa has a long history of domestication in China, and some varieties have a robust cold hardiness, attracting the attention of researchers. Recently, B. rapa cultivation for cold resistance achieved a major breakthrough, and some cold-tolerant B. rapa varieties have been approved for agricultural use by the Chinese government, including ‘Longyou6’ and ‘Longyou7’. These varieties have been widely grown near 48° latitude; for example, in the Xinjiang province, where these varieties grow well and there is little loss of yield, even when the temperatures reach −32°C (Sun 2013). Thus, these B. rapa varieties are good sources from which to develop cold resistant cultivars in China.
To date, a few research studies on the cold resistance of B. rapa have been published (Liu et al. 2014, Sun et al. 2007, Zhu et al. 2007). However, most of these studies were focused primarily on the characteristics of ground shoots under artificial control conditions. Studies on the physiological and biochemical characteristics of the underground roots have not been widely reported. Numerous studies have shown that cold resistance is closely related to plant cell membranes, enzymes, and a physiologically active defense system (Deng and Chen 2001, Jia and Guan 2012, Pu and Sun 2010). The unsaturated fatty acid content of the plasma membrane is associated with cold resistance in plants; varieties with a higher unsaturated fatty acid content in the membrane have better cold resistance (Roughan 1985). Low temperatures promote the synthesis of membrane phospholipids (Sun et al. 2009). Cold-resistant varieties are particularly adept at increasing the synthesis of membrane phospholipids in response to low temperatures (Willemot 1975). Under normal temperature conditions, the reactive oxygen content in plants is at a low level that does not hinder growth and development. However, when subjected to low temperature, the plants produce an increased amount of reactive oxygen contents, resulting in peroxidation of membrane phosphor lipid, structural rearrangements of plasma membrane enzymes, and subsequent changes in the catalytic function of the membrane proteins (Boyer and Westgate 2004).
Since cold resistance in plants is controlled by numerous complex factors, it is difficult to determine the contributing factors under normal weather conditions. Studies have shown that morphological and physiological indicators can be used to evaluate cold resistance in plants. For cowpea and other plants subjected to low temperature stress, the activities of catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) initially increase and then decrease (Peng et al. 1994). When the plants are subjected to cold stress, they have the ability to remove reactive oxygen by regulating the activity of protective enzymes while also regulating the concentration of intracellular proline, soluble sugars, and soluble proteins to maintain the stability of the intracellular environment, and to reduce stress and injury to plants due to low temperatures (Bais et al. 2003). Some studies have also shown that the cold resistance of plants is correlated with proline accumulation, which participates in the maintenance of osmotic balance between protoplasts and the external environment, as well as stabilizes the structure of biological macromolecules and maintains the structural integrity of the membrane (Hou and Tang 1999). The soluble sugar content is positively correlated with cold resistance in most plants. With the decrease of temperature, the soluble sugar content increases gradually, and the soluble sugar content increases more in the cold-resistant varieties than in the non-cold resistant varieties. However, in some plants, there is no correlation between the soluble sugar content and cold resistance (Lindow and Arny 1978). Soluble protein has been proven to enhance the cold hardiness of plants, and increasing the soluble protein concentration can enhance the cells ability to retain moisture, improving cold resistance capability (Jiang et al. 2002). Several studies have shown that varieties with high malondialdehyde (MDA) content also have higher levels of membrane lipid peroxidation, resulting in lower cold resistance (Fechner et al. 1986, Lin et al. 2012). The relative conductivity is also considered to be an important indicator, having a significantly negative correlation with cold resistance in B. napus (Huang et al. 2014). Therefore, these physiological indicators are often used to indirectly evaluate cold resistance in plants.
In recent years, molecular biology techniques have been used to determine the genetic components of cold resistance, and some cold resistance genes have been cloned and studied. RCI3, a POD-activity related cold-inducible gene, was isolated from rice, which not only enhances low temperature tolerance in plants, but also enhances tolerance of moisture and salt stress (Llorente et al. 2002). At present, cold regulated (COR) genes have been isolated and identified from Arabidopsis thaliana, canola, wheat and other plants (Cui et al. 2003, Zhang et al. 2016, Zhong et al. 2006). The COR genes encode various functional proteins to resist cold stress and improve cold resistance (Li et al. 2004). In addition, studies have found that LEA (Late embryogenesis abundant) genes may also contribute to cold hardiness in plants (Battaglia et al. 2008, Hundertmark and Hincha 2008). In addition, transcription factors related to cold resistance have been identified, including APETALA2 (AP2), NAC, MYB, bZIP and WRKY (Liao et al. 2008, Ishiguro and Nakamura 1994, Jofuku et al. 1994, Paz-Ares et al. 1987, Souer et al. 1996). APETALA2 (AP2) was first cloned from Arabidopsis, encoding a protein containing two AP2/ERF domains. Expression of AP2/EREBP transcription factor gene may increase the cold resistance of plants (Jofuku et al. 1994). Subsequently, this gene was isolated from maize, wheat, tomato, soybean, rice and other plants (Chen et al. 2009, Egawa et al. 2006, Gu et al. 2002, Matsukura et al. 2010, Moose and Sisco 1996).
In contrast, few cold resistance genes have been isolated from rapeseed, and there are very few reports on genetics and gene mapping. One reason is that cold resistance is a very complex issue, and it is difficult to determine the extent of cold damage directly; thus, we must use other indexes to measure cold resistance indirectly. In addition, results from different studies are sometimes inconclusive; therefore we set out to determine which specific indexes are related to cold resistance. In this study, we measured morphological and physiological indexes in various temperature conditions, in order to study correlation between these indicators and cold resistance in B. rapa rapeseed. We also constructed a segregating population and developed molecular markers for cold resistance gene mapping and marker assisted selection (MAS).
Materials and Methods
Two cold resistant B. rapa varieties (‘Longyou6’ and ‘Longyou7’) and two cold susceptible B. rapa varieties (‘Tianyou2’ and ‘Tianyou4’), were used in this study, kindly supplied by Gansu Agricultural University. In order to identify the markers linked to cold resistance genes, an F2 population consisting of 136 individuals was derived by hybridizing Longyou6 with Tianyou4.
Hydroponic growth was used to cultivate the four parental varieties and the F2 population outdoors until the temperature reached 5°C. At least 120 plants for each variety were then transferred to V9 matrix (Peat:soil:perlite = 2:1:1, pH 4.5–7, TianFeng Horticulture, Shandong, China), and outdoor cultivation was continued. For the next several weeks, the local low temperature was observed and recorded, and the plants with low vigor were eliminated. Whenever the low temperature was below 5°C for 3 consecutive days, the leaves and roots of 15 individuals from each variety were selected on the morning of the third day to measure physiological indexes, including malondialdehyde (MDA), peroxidase (POD), catalase (CAT), and relative conductivity (for leaves only). In total, three replicates were assessed. The MDA content was measured by the 2-thiobarbituric acid (TBA) assay. The protocol was as follows: the samples were ground in 10% trichloroacetic acid and centrifuged at 12000 rpm for 10 min; the supernatant was boiled for 15 min; the absorbance was measured at 450 nm, 532 nm and 600 nm, respectively. The POD activity was determined by the guaiacol colorimetric assay. The protocol was as follows: the samples were ground in 0.05 mol/L phosphoric acid (pH 5.5), and centrifuged at 3000 rpm for 15 min; the mixed solution was added with the 2.9 ml 0.05 mol/L phosphoric acid, 1 ml 0.05 mol/L guaiacol (pH 5.5) and 1 ml 2% H2O2; the mixed solution was incubated at 37°C for 5 min, and then for absorbance was measured at 470 nm. The CAT activity was measured by monitoring the UV absorbance. The brief method was as follows: the samples were ground in phosphoric acid (pH 7.0), and centrifuged at 4000 rpm for 15 min; the reaction solution was prepared by mixing 0.2 ml supernatant in 1.5 ml phosphoric acid (pH 7.0) and 1 ml distilled water; the mixed solution was incubated at 25°C for 3 min. The absorbance at 240 nm was measured after 300 μl 0.1 mol/L H2O2 was added. The membrane permeability (RPP) was determined by the conductance method. First, the samples were cut into small pieces, and then soaked in distilled water for 4 h at room temperature, the solution conductivity was measured. After being boiled for 15 min, the conductivity of the solution was measured again. The relative conductivity was calculated according to the equation: relative conductivity = extravasation conductivity/boil conductivity. The detailed protocols for calculating these four indicators were described by Li et al. (Li 2000). The days on which the above tests were completed were Oct 25, 2012; Dec 18, 2012; Dec 27, 2012; Jan 31, 2013; and Mar 12, 2013, with recorded low temperatures of 5°C, 0°C, −5°C, 0°C, and 5°C, respectively.
On the first day of measurement (Oct 25, 2012; 5°C low temperature), 20 individuals for each variety were used to measure morphological indexes, including leaf number, leaf length, root length, root diameter, and leaf area size, and three replicates were assessed. The wet weight and dry weight of leaf and root mass were measured, and the ratio of wet and dry weight of leaf and root mass, respectively, were also determined. The formula was as follows: dry and wet mass ratio (%) = dry weight/fresh weight × 100%. After preparing the original data as an excel format, the SPSS11.0 software was used for statistical analyses for these data (SPSS Inc., Chicago, IL). LSR (Least significant range) test (Gai 2000) was used to analyze whether there were significant differences among the four varieties. According to the results of multiple comparisons, the cold resistance of these varieties can be determined.
Subordinate functions were developed to analyze and evaluate the contribution of each factor to cold resistance according to three formulas (Zhu et al. 2011). The subordinate value of each indicator was calculated using the formula: U(Xj) = (Xj − Xmin)/(Xmax − Xmin), j = 1, 2, ... n. Where, Xj, Xmin and Xmax represent the mean value, minimum value and maximum value of indicator j, respectively. The contribution of each indicator was calculated according to the formula: , j = 1, 2, ... n. Where, Pj was the contribution of indicator j. The comprehensive evaluation value of the varieties was calculated according to the formula: , i = 1, 2, ... k. Where, k was the number of varieties. In order to compare the cold resistance among the four B. rapa varieties, the comprehensive evaluation value of the four varieties were analyzed using LSR test, and the multiple comparison was conducted (Gai 2000).
The frost damage of the F2 population derived from Tianyou4 and Longyou6 was investigated in February, 2013, using methods previously described (Liu 1985). 136 individual plants were assessed individually for frost damage using a 0–4 scale, where 0 = no frost damage; 1 = frost damage on several large leaves; 2 = frost damage in half of the leaves, with central leaves undamaged; 3 = frost damage on most of the leaves, with central leaves undamaged; 4 = frost damage on all leaves including central leaves. For statistical analysis, a frost index was calculated using the following formula: Frost index = (1 × S1 + 2 × S2 + 3 × S3 + 4 × S4)/(total number of plants × 4). S1, S2, S3 and S4 represent the number of plants classified into grades 1–4, respectively. The leaves of the F2 population were only measured for relative conductivity and MDA content in 0°C conditions (on Jan 31, 2013). These two indexes were measured by previously described methods (Li 2000). The frost damage rating of each individual (0–4) and the obtained physiological index data (the relative conductivity and MDA content) in F2 were prepared. The r test (Gai 2000) was used to analyze the correlation between cold resistance and the physiological indexes in F2 by the SAS8.1 software (SAS Inc., USA).
Genomic DNA was extracted from young leaves of F2 individuals at the seedling stage by the CTAB method (Doyle and Doyle 1990). Final DNA concentration was adjusted to 50 ng/ul, SSR technology was used to screen the F2 population and the two parental populations, and SSR amplification was performed as described by Lowe et al. (2002). Sequences of all of the SSR markers were obtained from public sources deposited at http://ukcrop.net/perl/ace/search/BrassicaDB (Lowe et al. 2004). 504 pairs of SSR primers distributed across the whole genome of B. napus were initially used to screen the parents, Longyou6 and Tianyou4, after which the polymorphic SSR primers were used to amplify the F2 population. The amplified bands were expressed as ‘1’ or ‘0’, and the r test (Gai 2000) was conducted to analyze the correlation between the SSR markers and the physiological indexes by the SAS8.1 software (SAS Inc., USA). The SSR markers that can show a highly significant positive correlation to both physiological indexes were used to screen strong cold-resistant (grade 0) and cold-susceptible (grade 4) plants in the F2 population and some cold-resistant B. rapa lines to validate the markers.
Results
Comparison of morphological characteristics before winter
On Oct 25, 2012, the low temperature was below 5°C, which essentially stopped the growth of rapeseed. By comparing the morphological characteristics of four B. rapa varieties, we found that the leaf number and leaf length of Tianyou2 and Tianyou4 were higher than those of Longyou6 and Longyou7, and the difference was significant (P < 0.05). However, in comparing other morphological characteristics, including root length, root diameter, dry to wet mass ratio of leaves, and dry to wet mass ratio of roots, Longyou6 and Longyou7 values were higher than Tianyou2 and Tianyou4, and the difference was significant (P < 0.05) or extremely significant (P < 0.01). These results demonstrated that the underground portions of the cold resistance varieties, (i.e. roots), grew more rapidly before winter compared to the non-cold resistant varieties. Dry matter accumulation in cold varieties was greater than in non-cold resistant varieties (Table 1).
Table 1.
Morphological indexes results and dry to wet mass ratio
| Testing indexes | Tianyou2 | Tianyou4 | Longyou6 | Longyou7 |
|---|---|---|---|---|
| Leaf number | 8 ± 1.00ab | 9 ± 1.00a | 7 ± 1.00b | 7 ± 0.00b |
| Leaf length (cm) | 16.30 ± 0.44a | 15.70 ± 0.75a | 13.15 ± 0.06b | 11.85 ± 0.04c |
| Root length (cm) | 13.90 ± 0.10c | 13.20 ± 0.30d | 15.70 ± 0.44b | 16.50 ± 0.40a |
| Root diameter (cm) | 1.50 ± 0.26b | 1.40 ± 0.17b | 2.40 ± 0.26a | 2.10 ± 0.35a |
| Dry to wet mass ratio of leaf (%) | 12.7 ± 0.35b | 12.3 ± 0.53b | 15.2 ± 0.36a | 14.7 ± 0.56a |
| Dry to wet mass ratio of root (%) | 17.1 ± 0.36c | 16.9 ± 0.53c | 20.7 ± 0.56a | 19.0 ± 0.26b |
Evaluation of cold resistance
In order to investigate the cold resistance of the four parental varieties, subordinate functions were developed to analyze and evaluate the contribution of individual factors to cold resistance. When subjected to the same low temperature stress, the difference in comprehensive evaluation values between varieties was very large. In addition, aboveground and underground comprehensive evaluation values were also different within the same variety. The comprehensive evaluation values were larger in the varieties with stronger cold resistance. The aboveground and underground comprehensive evaluation values of Longyou6 and Longyou7 were larger than those of Tianyou2 and Tianyou4, and the differences were significant (P < 0.05) or extremely significant (P < 0.01), indicating that Longyou6 and Longyou7 are more cold resistant than Tianyou2 and Tianyou4 (Table 2). Therefore, Longyou6 was selected to construct an F2 population with Tianyou4 in order to identify cold resistance genes in B. rapa.
Table 2.
Comprehensive analysis of cold resistance using physiological indexes
| Temperature | Cultivars | Leaf | Mean | Root | Mean | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Relative conductivity | MDA content | POD activity | CAT activity | MDA content | POD activity | CAT activity | ||||
| 5°C | Tianyou2 | 0.26 ± 0.01b | 0.49 ± 0.01b | 1.00 ± 0.17a | 0.20 ± 0.1b | 0.49 ± 0.03b | 0.00 ± 0.00d | 1.00 ± 0.10a | 0.19 ± 0.03b | 0.40 ± 0.04b |
| Tianyou4 | 0.00 ± 0.00b | 0.00 ± 0.00c | 0.43 ± 0.06b | 0.00 ± 0.00c | 0.11 ± 0.02c | 0.35 ± 0.07c | 0.73 ± 0.07b | 0.00 ± 0.00b | 0.36 ± 0.04b | |
| Longyou6 | 1.00 ± 0.26a | 0.82 ± 0.07a | 0.00 ± 0.00c | 1.00 ± 0.10a | 0.70 ± 0.07a | 1.00 ± 0.17a | 0.22 ± 0.17c | 0.74 ± 0.09a | 0.65 ± 0.07a | |
| Longyou7 | 0.90 ± 0.20a | 1.00 ± 0.26a | 0.00 ± 0.00c | 0.89 ± 0.12a | 0.70 ± 0.07a | 0.65 ± 0.09b | 0.00 ± 0.00d | 1.00 ± 0.26a | 0.55 ± 0.12a | |
| 0°C | Tianyou2 | 0.00 ± 0.00c | 0.24 ± 0.03b | 0.00 ± 0.00d | 0.14 ± 0.03c | 0.09 ± 0.01c | 0.36 ± 0.01c | 0.36 ± 0.02c | 0.23 ± 0.05c | 0.32 ± 0.03b |
| Tianyou4 | 0.37 ± 0.02b | 0.00 ± 0.00c | 0.18 ± 0.02c | 0.00 ± 0.00d | 0.14 ± 0.01c | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00c | |
| Longyou6 | 1.00 ± 0.26a | 1.00 ± 0.10a | 0.61 ± 0.05b | 0.76 ± 0.05b | 0.84 ± 0.06b | 1.00 ± 0.17a | 0.76 ± 0.03b | 1.00 ± 0.19a | 0.92 ± 0.06a | |
| Longyou7 | 0.88 ± 0.05a | 0.99 ± 0.02a | 1.00 ± 0.17a | 1.00 ± 0.10a | 0.97 ± 0.08a | 0.78 ± 0.03b | 1.00 ± 0.12a | 0.78 ± 0.06b | 0.85 ± 0.04a | |
| −5°C | Tianyou2 | 0.13 ± 0.02d | 0.47 ± 0.02c | 0.09 ± 0.01d | 0.08 ± 0.01c | 0.19 ± 0.01c | 0.00 ± 0.17c | 0.13 ± 0.01c | 0.14 ± 0.05c | 0.09 ± 0.01c |
| Tianyou4 | 0.37 ± 0.03c | 0.00 ± 0.00d | 0.18 ± 0.03c | 0.00 ± 0.00d | 0.14 ± 0.01d | 0.30 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.10 ± 0.02c | |
| Longyou6 | 0.89 ± 0.02b | 1.00 ± 0.11a | 0.55 ± 0.04b | 0.85 ± 0.03b | 0.82 ± 0.05b | 0.92 ± 0.05b | 0.86 ± 0.08a | 1.00 ± 0.19a | 0.93 ± 0.03a | |
| Longyou7 | 1.00 ± 0.11a | 0.80 ± 0.05b | 1.00 ± 0.04a | 1.00 ± 0.06a | 0.95 ± 0.02a | 1.00 ± 0.05a | 1.00 ± 0.03b | 0.65 ± 0.06b | 0.88 ± 0.02b | |
| 0°C | Tianyou2 | 0.10 ± 0.02c | 0.47 ± 0.04c | 0.01 ± 0.02c | 0.27 ± 0.03c | 0.21 ± 0.00c | 0.00 ± 0.00d | 0.04 ± 0.01c | 0.08 ± 0.02c | 0.04 ± 0.01d |
| Tianyou4 | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.00 ± 0.00c | 0.00 ± 0.00d | 0.00 ± 0.00d | 0.39 ± 0.04c | 0.00 ± 0.00c | 0.00 ± 0.00d | 0.13 ± 001c | |
| Longyou6 | 0.76 ± 0.03b | 1.00 ± 0.07a | 0.38 ± 0.03b | 1.00 ± 0.06a | 0.79 ± 0.02b | 1.00 ± 0.09a | 0.59 ± 0.02b | 1.00 ± 0.03a | 0.86 ± 0.04a | |
| Longyou7 | 1.00 ± 0.04a | 0.73 ± 0.04b | 1.00 ± 0.10a | 0.93 ± 0.02b | 0.92 ± 0.03a | 0.77 ± 0.04b | 1.00 ± 0.04a | 0.67 ± 0.04b | 0.82 ± 0.03b | |
| 5°C | Tianyou2 | 0.00 ± 0.00d | 0.45 ± 0.03b | 0.00 ± 0.00d | 0.09 ± 0.03c | 0.14 ± 0.01c | 0.26 ± 0.04b | 1.00 ± 0.01a | 0.00 ± 0.00c | 0.42 ± 0.02b |
| Tianyou4 | 0.23 ± 0.03c | 0.00 ± 0.00c | 0.30 ± 0.03c | 0.00 ± 0.00d | 0.13 ± 0.01c | 0.00 ± 0.00d | 0.42 ± 0.03b | 0.01 ± 0.00c | 0.14 ± 0.01c | |
| Longyou6 | 0.69 ± 0.02b | 0.97 ± 0.02a | 0.54 ± 0.03b | 1.00 ± 0.09a | 0.80 ± 0.01b | 0.17 ± 0.01c | 0.00 ± 0.00d | 1.00 ± 0.10a | 0.39 ± 0.04b | |
| Longyou7 | 1.00 ± 0.05a | 1.00 ± 0.03a | 1.00 ± 0.07a | 0.85 ± 0.03b | 0.96 ± 0.01a | 1.00 ± 0.08a | 0.35 ± 0.04c | 0.61 ± 0.04b | 0.65 ± 0.04a | |
The data for Relative conductivity, MDA, POD, and CAT are subordinate value of each indicator. Mean is comprehensive evaluation value of each cultivar.
Investigation of frost damage in F2
In order to investigate frost damage in the F2 population, 136 individual plants were analyzed. Among these, 14 plants were free of frost with a percentage of 10.30%. The percentages of grade 1–3 were 13.25%, 25.7% and 37.5%, respectively. The remaining plants were classified as grade 4 with a percentage of 13.25%, though most of these plants were unviable at the time of analysis. The average frost index of the F2 population was 2.30.
Analysis of relative conductivity and MDA content
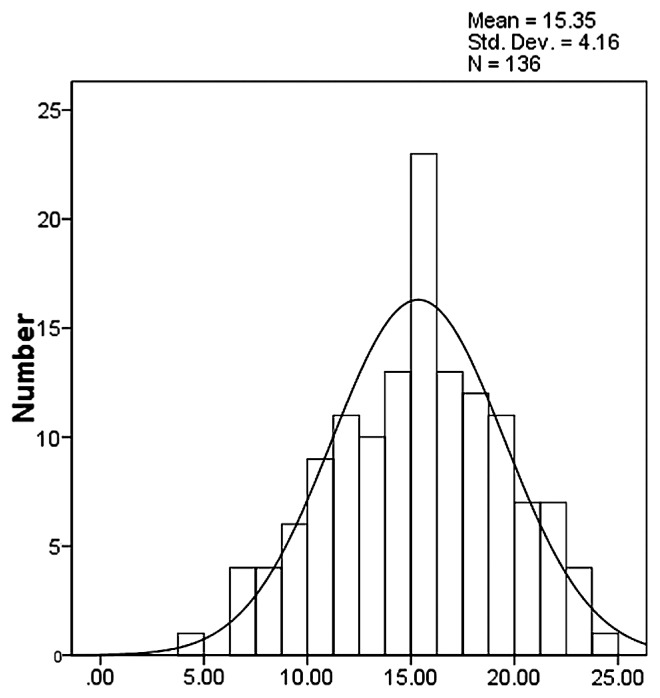
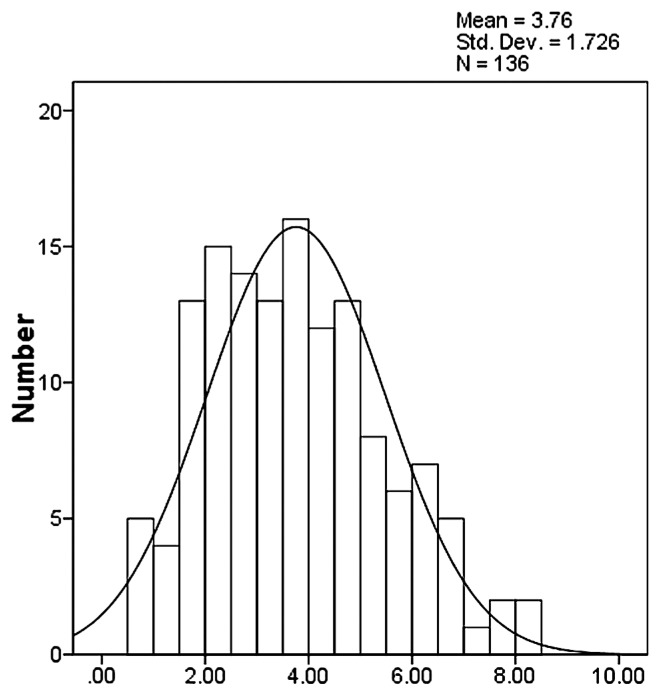
The relative conductivity and the MDA content of each individual plant in the F2 population was measured. The maximum value of relative conductivity was 24.84%, and the minimum was 4.76% (average = 15.35%). The maximum MDA content was 8.52 μmol/gFW, the minimum was 0.75 μmol/gFW, and the average was 3.76 μmol/gFW (Table 3). These two indexes displayed a normal distribution, respectively (Figs. 1, 2), showing that they were quantitative traits.
Table 3.
The relative conductity and MDA content in F2 population and two parents
| Relative conductivity (%) | MDA content (μmol·g−1·FW) | ||
|---|---|---|---|
| Parents | Longyou 6 | 10.92 ± 2.20 | 2.39 ± 0.94 |
| Tianyou 4 | 21.20 ± 2.50 | 4.73 ± 0.33 | |
| F2 | Maximum | 24.84 | 9.02 |
| Minimum | 5.37 | 0.95 | |
| Mean | 15.34 | 3.75 |
Fig. 1.
Distribution of relative conductivity in F2 population.
Fig. 2.
Distribution of MDA content in F2 population.
Correlation analysis of cold resistance and physiological indexes
SAS8.1 software was used to analyze the correlation between cold resistance and physiological indexes in F2. Our results showed that the degree of frost damage was significantly correlated with relative conductivity and MDA content, with correlation coefficients of 0.558 and 0.447 (r0.01,150 = 0.208), respectively. The relative conductivity and MDA content were also significantly correlated with a correlation coefficient of 0.833 (Table 4). Therefore, we determined that relative conductivity and MDA content can be used to analyze the cold resistance of plants in our study.
Table 4.
Correlation between degree of frost damage and physiological indexes
| Degree of frost damage | Relative conductivity | MDA content | |
|---|---|---|---|
| Degree of frost damage | – | 0.558** | 0.447** |
| Relative conductivity | 0.558** | – | 0.833** |
| MDA content | 0.447** | 0.833** | – |
stand for correlation at 0.01 levels. r0.05(150) = 0.159, r0.01(150) = 0.208.
SSR markers related to cold resistance
A total of 504 pairs of primers were used to screen the two parental populations, Longyou6 and Tianyou4. Of these, 97 primers showed polymorphism between the two parental lines, with a percentage of 9%. These polymorphic primers were then used to screen the F2 population, from which 48 polymorphic loci were identified. In order to study the relationship between these loci and relative conductivity and MDA content, we analyzed the correlation of the SSR markers with relative conductivity and MDA content, respectively. The results showed that 10 SSR markers were correlated with relative conductivity and 11 SSR markers were correlated with MDA content. Four SSR markers (Na10-C03, BrGMS4511, BrGMS397, and BnGMS164) had a highly significant positive correlation to relative conductivity. Five SSR markers (Na10-C03, BrGMS4511, BrGMS397, BRAS011 and BnGMS67) also had a highly significant positive association with MDA content. Three SSR markers (Na10-C03, BrGMS4511 and BrGMS397) had a highly significant positive correlation to both of these two indexes (Table 5). These three markers were then used to screen the outlying plants in the F2 population (14 plants at grade 0 and 18 plants at grade 4) and 9 B. rapa lines (5 plants at grade 0 and 4 plants at grade 4). These markers were able to effectively distinguish between the cold-resistant and non-cold resistant plants (Fig. 3); thus confirming that these three markers are correlated with cold resistance in our study.
Table 5.
Correlation between SSR markers and physiological indexes
| Primers name | Relative conductivity | MDA content | Primers number | Relative conductivity | MDA content |
|---|---|---|---|---|---|
| Na10-C03 | 0.236** | 0.307** | BrGMS397 | 0.251** | 0.226** |
| 0.149 | 0.192* | BrGMS3120 | 0.174* | 0.181* | |
| BRAS011 | 0.162* | 0.271** | BrGMS171 | 0.205* | 0.163* |
| BrGMS102 | 0.205* | 0.193* | BnGMS67 | 0.091 | 0.316** |
| Ol13-G05 | 0.196* | 0.152 | BnGMS164 | 0.302** | 0.172* |
| BrGMS4511 | 0.336** | 0.307** | BrGMS579 | 0.340* | 0.348* |
stand for correlation at 0.05 and 0.01 levels, respectively. r0.05(150) = 0.159, r0.01(150) = 0.208.
Fig. 3.
Analysis of the PCR products obtained using BrGMS4511 on part of F2 plants and B.rapa lines. R and S represented the resistant and susceptible individuals, respectively. M: 100 bp DNA ladder.
Discussion
Frost frequently occurs throughout the world, and particularly in 2008, serious frost damage was experienced in most areas of China, leading to heavy losses in rapeseed production (Zhang et al. 2008). It is imperative to improve the cold resistance of rapeseed, and the most effective method is to develop cold-resistant varieties for rapeseed breeding. Most researchers agree that B. rapa has better cold resistance than other Brassica species (Li 2011, Yu and Park 2014), as this species includes many cold resistance genes. Longyou6 and Longyou7 are two B. rapa varieties cultivated in the northwest of China, where they thrive despite low temperatures that often reach −20°C, often lasting for 3 months. In order to evaluate the cold resistance of these two varieties, a variety of indicators were used. In addition, the subordinate function values of each physiological index were determined to evaluate the cold resistance of these plants and the results showed these two varieties were more cold resistant than other varieties, which was in consistent with previous studies (Gai et al. 2005, Pu and Sun 2010).
The cold resistance of crops is due to complex interacting factors, some of which are only activated in freezing conditions (Ma et al. 2016). Thus, it is difficult to evaluate these factors in normal growing conditions. An effective way to evaluate cold resistance is by using the physiological indexes of plants. In our research, the degree of frost damage of the F2 population was found to be significantly correlated with relative conductivity and MDA content, respectively; therefore, these two indexes can be used to evaluate the cold resistance of plants indirectly. More interestingly, the Mendelian ratios and the Chi-square test of the frost damage rating showed that the plants with grade 0 and grade 1 accounted for nearly one-fourth of the individuals in the F2. Therefore, the cold resistance of B. rapa in our study was likely to be controlled by a major gene, which needs further validation, because this result was based on the classification of the grade 1 plants as cold-resistant ones.
With the development of molecular biological techniques, it is possibility that identifying the molecular markers linked to relative conductivity or MDA content, which could be used for screening the cold resistant plants. Five and six SSR markers associated with relative conductivity and MDA content, respectively, were identified in our research. These markers will be helpful in the selection of cold resistant plants in the seedling stage or in normal (no frost) growing conditions. In this study, three SSR markers (Na10-C03, BrGMS4511 and BrGMS397) showed high accuracy in distinguishing between cold-resistant and non-cold resistant plants; thus, these markers can be used in MAS and gene mapping. In addition, it was found that these three markers were correlated with both relative conductivity and MDA content; therefore we propose that a single QTL locus on one chromosome controls these two traits (Na10-C03: A01; BrGMS397: A02; BrGMS4511: A03). So far, many cold-related genes have been annotated in B. rapa, including 1 on the chromosome A01, 2 on the chromosome A02, 6 on the chromosome A03 (https://www.ncbi.nlm.nih.gov/gene/). The SSR marker BrGMS397 (A02: 8.97 Mb) is located near a gene encoding a cold-inducible RNA-binding protein (A02: 4.97 Mb). The marker BrGMS4511 (A03:16.17 Mb) is also close to two cold-related genes (A03: 20.56 Mb and 21.74 Mb, respectively). Additionally, the results in the present study showed that the relative conductivity and MDA content were inherited in quantitative traits. Therefore, it was inferred that the candidate genes of cold resistance in our study resist in these three regions. For other SSR markers identified in this study, they were found to be located on several chromosomes, indicating that several QTLs were responsible for these two traits. Future studies will be directed toward isolating the single QTL locus that controls both relative conductivity and MDA content. To achieve this goal, we plan to increase the density of molecular markers, and to use the QTL mapping method to identify the genes related to cold resistance found on chromosomes A01, A02, and A03.
Acknowledgements
The research was supported by National key research and development program (2016YFD0100200; 2016YFD0101300), Zhongying youth scholar program of Northwest A&F University and Shaanxi science and technology innovation program (2016KTCQ02-03).
Literature Cited
- Bais, H.P., Vepachedu, R., Gilroy, S., Callaway, R.M. and Vivanco, J.M. (2003) Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science 301: 1377–1380. [DOI] [PubMed] [Google Scholar]
- Battaglia, M., Olvera-Carrillo, Y., Garciarrubio, A., Campos, F. and Covarrubias, A.A. (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 148: 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer, J. and Westgate, M. (2004) Grain yields with limited water. J. Exp. Bot. 55: 2385–2394. [DOI] [PubMed] [Google Scholar]
- Chen, M., Xu, Z., Xia, L., Li, L.C., Cheng, X.G., Dong, J.H., Wang, Q.Y. and Ma, Y.Z. (2009) Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.). J. Exp. Bot. 60: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, B.M., Li, Y.X., Le, J.H., Zheng, M.G. and Bao, H.F. (2003) Cloning and sequence analysis of COR15a gene from Arabidopsis thaliana. Journal of Shihezi University 7: 87–89. [Google Scholar]
- Deng, J.M. and Chen, J.L. (2001) Advances of studies on plant freezing-tolerance mechanism: freezing tolerance gene expression and its function. Chinese Bulletin of Botany 18: 521–530. [Google Scholar]
- Doyle, J.J. and Doyle, J.L. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Egawa, C., Kobayashi, F., Ishibashi, M., Nakamura, T., Nakamura, C. and Takumi, S. (2006) Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes Genet. Syst. 81: 77–91. [DOI] [PubMed] [Google Scholar]
- Fechner, J., Goy, J., Artigou, J.Y., Bedu, O., Loeper, J., Emerit, J. and Grosgogeat, Y. (1986) Membrane lipid peroxidation in coronary insufficiency. Presse Med. 15: 1077–1080. [PubMed] [Google Scholar]
- Gai, J.Y. (2000) The test statistical method. Beijing: China Agricultural University press; 230–231. [Google Scholar]
- Gai, Y., Niu, J.Y., Sun, W.C., Wang, H.L. and Zhu, T.T. (2005) Effect of lower temperature treatment on some cold resisting physiological indexes of winter turnip rape. Journal of Gansu Agricultural University 40: 182–185. [Google Scholar]
- Gu, Y.Q., Wildermuth, M.C., Chakravarthy, S., Loh, Y.T., Yang, C.M., He, X.H., Han, Y. and Martin, G.B. (2002) Tomato transcription factors Pti4, Pti5 and Pti6 activate defense responses when expressed in Arabidopsis. Plant Cell 14: 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, C.X. and Tang, Z.C. (1999) Function and mechanism of compatible solutes. Chinese Bulletin of Botany 35: 1–7. [Google Scholar]
- Huang, H.L., Cao, Z.Y., Tang, B., Ning, Z.L., Cui, Z.B. and Zhou, Y.P. (2014) Electrical conductivity analysis of 17 rapeseed (Brassica napus L.) varieties’ cold resistance. Hunan Agricultural Sciences 21: 1–3. [Google Scholar]
- Hundertmark, M. and Hincha, D.K. (2008) LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genomics 9: 118–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, S. and Nakamura, K. (1994) Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5′ upstream regions of genes coding for sporamin and beta-amylase from sweet potato. Mol. Gen. Genet. 244: 563–571. [DOI] [PubMed] [Google Scholar]
- Jia, M.Z. and Guan, C.Y. (2012) Review of physiology, biochemistry and gene engineering on cold resistance of rapeseed. Chinese Journal of Oil Crop Sciences 34: 556–561. [Google Scholar]
- Jiang, F.Y., Li, A. and Wang, B.Q. (2002) Review on physiology of chilling stress and chilling resistance of plants. Fujian Journal of Agricultural Sciences 17: 190–195. [Google Scholar]
- Jofuku, K.D., den Boer, B.G.W., Van Montagu, M. and Okamuro, J.K. (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. (2000) The experiment principle and the technology of plant physiology. Higher Education Press, Beijing, pp. 167–261. [Google Scholar]
- Li, L., Wang, X.J. and Zhao, M.A. (2004) Plant cold resistance genes. Plant Physiology Communications 40: 643–650. [Google Scholar]
- Li, Q. (2011) Study on cold resistance of different winter rape varieties. Xinjiang Agricultural Sciences 48: 804–809. [Google Scholar]
- Liao, Y., Zou, H.F., Wei, W., Hao, Y.J., Tian, A.G., Huang, J., Liu, Y.F., Zhang, J.S. and Chen, S.Y. (2008) Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 228: 225–240. [DOI] [PubMed] [Google Scholar]
- Lin, Y., Guo, W., Xu, Z. and Jia, Z. (2012) Cold resistance and changes on MDA and soluble sugar of leaves of ligustrunlucidum ait in winter. Chinese Agricultural Science Bulletin 28: 68–72. [Google Scholar]
- Lindow, S. and Arny, D. (1978) Plant cold hardiness and freezing stress: mechanisms and crop implications 1: 249. [Google Scholar]
- Liu, H.L. (1985) Genetics and breeding in rapeseed. Shanghai Scientific & Technical Publisher Press, Shanghai, pp. 90–94. [Google Scholar]
- Liu, Z.G., Zhang, C.S., Sun, W.C., Yang, N.N., Wang, Y., He, L., Zhao, C.X., Wu, J.X., Fang, Y. and Zeng, X.C. (2014) Comparison of winter rapeseed varieties (lines) with different cold resistance planted in the northern-extending regions in China under low temperature before winter. Acta Agronomica Sinica 40: 346–354. [Google Scholar]
- Llorente, F., López-Cobollo, R.M., Catalá, R., Martínez-Zapater, J.M. and Salinas, J. (2002) A novel cold-inducible gene from Arabidopsis, RCI3, encodes a peroxidase that constitutes a component for stress tolerance. Plant J. 32: 13–24. [DOI] [PubMed] [Google Scholar]
- Lowe, A.J., Jones, A.E., Raybould, A.F., Trick, M., Moule, C.J. and Edwards, K.J. (2002) Transferability and genome specificity of a new set of microsatellite primers among Brassica species of the U triangle. Mol. Ecol. Notes 2: 7–11. [Google Scholar]
- Lowe, A.J., Moule, C., Trick, M. and Edwards, K.J. (2004) Efficient large-scale development of microsatellites for marker and mapping applications in Brassica crop species. Theor. Appl. Genet. 108: 1103–1112. [DOI] [PubMed] [Google Scholar]
- Ma, L., Sun, W.C., Liu, Z.G., Fang, Y., Wu, J.Y., Li, X.C., Zhang, S.J., Yuan, J.H., Chen, Q., Wang, K.Y.et al. (2016) Expression of cold resistance genes from winter rapeseed of Brassica rapa and B. napus under chilling stress. Chinese Journal of Oil Crop Science 38: 135. [Google Scholar]
- Matsukura, S., Mizoi, J., Yoshida, T., Todaka, D., Ito, Y., Maruyama, K., Shinozaki, K. and Yamaguchi-Shinozaki, K. (2010) Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol. Genet. Genomics 283: 185–196. [DOI] [PubMed] [Google Scholar]
- Moose, S.P. and Sisco, P.H. (1996) Glossy15, an APETALA2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev. 10: 3018–3027. [DOI] [PubMed] [Google Scholar]
- Paz-Ares, J., Ghosal, D., Wienand, U., Peterson, P.A. and Saedler, H. (1987) The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 6: 3553–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y.K., Hao, S.C. and Wang, Z.Y. (1994) Effects of low temperature on seedling growth and POD, COD, ATPase isozymes in Vigna Sinensis (L) Savi. Acta Agriculturae Boreali-Sinica 9: 76–80. [Google Scholar]
- Pu, Y.Y. and Sun, W.C. (2010) The Relationship between cold resistance of winter turnip rape varieties and its physiological characteristics. Mol. Plant Breed. 8: 335–339. [Google Scholar]
- Roughan, P.G. (1985) Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 77: 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souer, E., van Houwelingen, A., Kloos, D., Mol, J. and Koes, R. (1996) The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85: 159–170. [DOI] [PubMed] [Google Scholar]
- Sun, L., Chen, G.X., Cheng, J.L. and Lü, C.G. (2009) Effects of chitosan treatment on thylakoid membrane characters characteristics in rice seedling under low temperature. Journal of Nanjing Normal University (Natural Science Edition) 32: 93–97. [Google Scholar]
- Sun, W.C. (2013) Cultivation techniques of winter oilseed rape in northern arid and cold areas. Chinese Agricultural Press, Beijing, pp. 1–5. [Google Scholar]
- Sun, W.C., Wu, J.Y., Zeng, J., Zhu, H.X., Liu, Y.L. and Zhang, Y.H. (2007) Primary evaluation of cold tolerance among eight winter Brassica rapa. Journal of Hunan Agricultural University 33: 151–155. [Google Scholar]
- Willemot, C. (1975) Stimulation of phospholipid biosynthesis during frost hardening of winter wheat. Plant Physiol. 55: 356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J.G. and Park, Y.D. (2014) Characterization of a cold tolerance-related gene, BrCSR, derived from Brassica rapa. Korean Journal of Horticultural Science and Technology 32: 91–99. [Google Scholar]
- Zhang, T.G., Mao, Y.S., Chen, Q.Q., Zhou, K. and Sun, W.C. (2016) Cloning and expression analysis of COR15-like gene in Brassica campestris ‘Longyou 6’. Acta Agriculturae Boreali-occidentalis Sinica 25: 707–714. [Google Scholar]
- Zhang, X.K., Zhang, C.L., Liao, X. and Wang, H.Z. (2008) Investigation on 2008 low temperature and freeze injure on winter rape along Yangtze River. Chinese Journal of Oil Crop Sciences 30: 122–126. [Google Scholar]
- Zhong, K.Y., Ye, M.S., Hu, X.W. and Guo, J.C. (2006) Role of the transcription factors CBF in plant cold tolerance. Yi Chuan 28: 249–254. [PubMed] [Google Scholar]
- Zhu, H.X., Sun, W.C., Deng, B., Yan, N., Wu, J.Y., Fan, H.L., Ye, J., Zeng, J., Liu, Y.L. and Zhang, Y.H. (2007) Study on cold hardiness and its physiological and biochemical characteristics of winter turnip rape (Brassica campetris). Acta Agriculturae Boreali-Occidentalis Sinica 16: 34–38. [Google Scholar]
- Zhu, Z.H., Zheng, W.Y. and Zhang, X.K. (2011) Principal component analysis and comprehensive evaluation on morphological and agronomic traits of drought tolerance in Rapeseed (Brassica napus L.). Scientia Agricultura Sinica 44: 1775–1787. [Google Scholar]