Abstract
Neurorestorative therapies for stroke aim to reverse disability by reparative mechanisms (rather than to thrombolyse or to neuroprotect). A substantial and persuasive body of pre-clinical evidence has come from the evaluation of antibodies against Nogo-A (a myelin-associated inhibitor of plasticity) in rat models of stroke. Particularly impressive is the benefit of this therapy in models of permanent middle cerebral artery occlusion (MCAO) when given to elderly animals after a one week delay, in adult rats with co-morbidities, and in adult rats when treatment is delayed by up to 9 weeks after stroke (although antibodies against Nogo-A did not reverse disability in mice after proximal MCAO with reperfusion). We predict that antibodies against Nogo-A will improve outcome when combined with suitable additional rehabilitation, and also that antibodies against Nogo-A will improve outcome in animal models of haemmorhagic stroke that affect the same brain regions as ischemic stroke caused by MCAO. Antibodies against Nogo-A have been shown to be safe in Phase I clinical trials for acute spinal cord injury, and this may eventually facilitate a trial in stroke.
Keywords: Neurorestoration, stroke, cerebral ischemia, Nogo, antibodies, plasticity
Introduction
The World Health Organization defines stroke as "rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin," (1); a definition which encompasses both ischaemic and haemorrhagic types of stroke.
Stroke is one of the most common causes of death worldwide, accounting for approximately 9% of all deaths on earth (2). In the western world 10-12% of deaths occur due to stroke. The vast majority of strokes occur in people aged more than 65 years old (3, 4). As of 2002, stroke-related disability is considered as the sixth most common cause of reduced disability-adjusted life-years (5). Due to the accumulating elderly population in western societies, stroke-related disability in the west is projected to become the fourth most important cause of reduced disability-adjusted life-years in 2030 (6). Stroke also brings with it a massive financial burden. Globally, stroke is responsible for using 2–4% of total health-care costs, and for over 4% of direct health-care costs in industrialized countries: as at 2009 the total cost in England alone was estimated to be £8.9 billion per annum (7, 8). Despite stroke being such a disabling and costly disease, the amount of money injected into stroke research is dishearteningly small relative to other diseases of similar burden (9).
Thrombolysis, secondary prevention, risk factor reduction and rehabilitation are the core principles of current ischemic stroke management and have improved the outcome of stroke. A particular challenge for implementation of thrombolytic therapies is the timeframe in which treatment needs to be initiated. The clot-busting drug tissue plasminogen activator (tPA) needs to be given to ischemic stroke patients within 3 hours (10) from the onset of symptoms, although there can be benefits up to 4.5 hours. Intraarterial thrombolysis is also used up to six hours after stroke. The time to admission for people with stroke is highly variable and is dependent on a large number of different factors. Studies have revealed average times to stroke admission and brain imaging ranging anywhere from 90 minutes to 680 minutes (11–15), and while these figures vary substantially, late arrival continues to be one of the most common reasons for failing to qualify for treatment with tPA. Public awareness, availability and efficiency of emergency medical services and distance to the nearest treatment centre are just a few variables of many that affect the time to stroke admission and subsequent treatment (16).
Development of novel therapeutic agents is difficult due to the nature of the pathology of stroke. Neurons lay claim to a large portion of the body’s blood flow, making them extremely susceptible to hypoxia. An interrupted blood supply will lead to irreversible neuronal damage within the local area in minutes and the surrounding penumbral areas are compromised if normoxia is not restored. The mechanisms of cell death in stroke are extremely complex, involving numerous signalling molecules and pathways. Neuroprotection was previously seen as a potentially brilliant new strategy to counter neuronal damage in cerebral ischaemia but clinical trials of neuroprotective drugs such as glutamate antagonists, ion channel blockers, anti-inflammatories, and free-radical scavengers have not led to clinical therapies. This was a major setback both pharmacologically and financially as many millions of dollars had been invested in pushing these drugs as far as Phase III clinical trials (17–23).
One source of hope is that where translation of acute neuroprotective therapies has failed, translation of neurorestorative therapies may succeed, by targeting regenerative mechanisms via modulation of CNS plasticity in subacute or chronic phases of stroke (24): these phases represent more stable baselines from which to assess recovery of function, a larger number of patients might be treated, rehabilitation time might be reduced, with potential benefits for patients, carers and the healthcare system as a whole. A neurorestorative therapy could be used in conjunction with current therapies to improve the overall outcome after stroke. The following is a critical review of the available literature involving Nogo-A in experimental stroke models and the potential for therapeutic benefit by modulating this inhibitory pathway.
Expression of Nogo-A and Nogo receptors in animal models of stroke
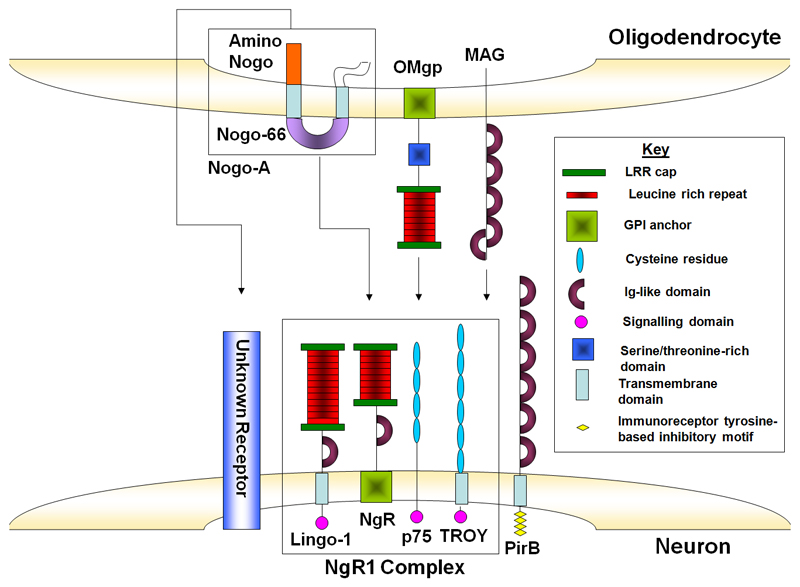
Nogo-A is a membrane protein that is predominantly expressed in oligodendrocytes of the adult mammalian CNS (25–28) and has a central role in constraining axonal regeneration and collateral sprouting when the mammalian CNS is damaged (29). More than two decades of work show that Nogo-A restricts spontaneous fibre regeneration and repair in the adult CNS (29) by retarding neurite outgrowth and triggering collapse of neuronal growth cones by binding to the Nogo receptors (NgR1 and 2) (30) and another, elusive receptor (31, 32) (Figure 1). Signaling via Rho to the cytoskeleton is an important mechanism occurring further along the NgR activation pathway (33).
Figure 1. Structure of the principle myelin associated inhibitors and their receptors.
Schematic showing the structure of Nogo-A, MAG and OMgp, the three major myelin associated inhibitors of neurite outgrowth expressed by oligodendrocytes. Nogo-66, OMgp and MAG can bind to PirB or the NgR1 complex expressed by neurons. The structure of PirB and the NgR1 complex and its components: NgR1, Lingo-1 and P75 or TROY is shown. The amino terminal of Nogo-A may exert its inhibitory action through binding an undetermined neuronal receptor.
A role for Nogo-A’s anti-regenerative properties have been seen in experiments involving either antibody-mediated targeting of Nogo-A, Nogo-A gene deletions, NgR blockade or blockade of the downstream messengers Rho-A and Rho-kinase in primate, rat and mouse models of spinal cord injury, and although in some of these paradigms conflicting data has been obtained, there is now a large and persuasive body of evidence from multiple laboratories showing that antibodies against Nogo-A improve outcome after spinal cord injury and enhance collateral sprouting and long-distance axon regeneration (e.g., of the corticospinal tract).
Nogo-A and NgR expression has been investigated in neonatal rats with hypoxia-ischaemia. These rats displayed up-regulation of both Nogo-A and NgR in the ischaemic cortex after hypoxia-ischaemia (34). Expression of Nogo-A mRNA was enhanced at 6 hours and reached a peak at 12 hours; Nogo-A protein expression was increased at 12 hours, peaking at around 24 hours. The authors also found a similar change in mRNA and protein levels of NgR. They also reported that Nogo-A and NgR colocalised to neurons 24 hours after injury.
A larger number of groups has investigated the expression of Nogo-A and Nogo receptors in the adult ischaemic brain to evaluate their potential roles in the pathophysiology of stroke. In an adult rat model of transient global ischaemia, expression of Nogo-A, NgR and RhoA proteins increased transiently over a 48-hour period, but this returned to normal levels by 7 days. Nogo-A was highly expressed in the myelin sheath whereas NgR expression was increased in axons and dendrites (35). In a rat model of transient focal ischemia, Nogo-A, NgR, and RhoA protein levels were all increased by 24 hours but returned to normal levels by 96 hours. Again, Nogo-A was mainly located in the myelin sheath, whereas RhoA was expressed predominantly in the cytoplasm and NgR was mainly axonally and dendritically expressed (36). In contrast, another group has reported that Nogo-A is expressed in cortical neurons in the brains of normal rats and rats after focal cortical ischemia, and was transiently enhanced in the external capsule after stroke and expression eventually returned to baseline levels. However, Nogo-A expression was neither increased nor decreased in any other white matter area that was studied up to 28 days after stroke (37).
With respect to the ageing brain, one study has indicated that normal, elderly rats did not exhibit changes in levels of mRNA for inhibitory ligands such as myelin associated glycoprotein (MAG) or oligodendrocyte myelin glycoprotein (Omgp), or NgR or other members of the Nogo-A receptor complex (Lingo-1, Troy; see Figure 1) in the cortex or hippocampus (38), whereas Nogo-A mRNA was shown to be reduced in the hippocampus (38). In contrast, a different study showed 6-fold higher expression of Nogo-A mRNA in aged rats relative to young adult rats (39) although the level of Nogo-A protein was the same in young and aged adult rats showing that there may be a mismatch between gene expression and protein expression (39). More work remains to be done to reconcile these two papers. In one of these studies, upon inducing stroke by distal middle cerebral artery occlusion (MCAO), Nogo-A mRNA levels in young adult rats increased by more than 4 fold and peaked at 2 weeks post stroke (39). However in aged rats, Nogo-A mRNA levels declined post stroke, reaching a low of 0.14 fold 7 days after stroke. Surprisingly, Nogo-A- protein was reduced in both old and young adult rats one and two weeks after stroke.
A study making use of archival fixed tissue from marmosets that had received permanent occlusion of the middle cerebral artery revealed a transient increase in the number of Nogo-A positive cells within some areas of white matter surrounding the lesion at 2 months post stroke; this returned to baseline by 3 and 4 months. White matter located distally from the lesion, and peri-lesional grey matter expressed normal levels of Nogo-A (40). This study suggests that Nogo-A levels may be raised for longer periods after ischaemic injury in marmosets than in rodents (40).
A number of papers have examined Nogo-A and NgR expression in developing and adult humans (41–43). In the adult, Nogo-A mRNA expression was observed in oligodendrocytes and motor neurons (e.g., in the spinal cord) and in sensory ganglia neurons. In adult human tissues Nogo-R gene activity was found in neocortex, hippocampus and a subset of large and medium-sized neurons of the dorsal root ganglia. Nogo-R mRNA was not expressed in the adult human spinal cord at detectable levels. Nogo-A and NgR have also been examined acutely or chronically after cerebral ischemia in adult humans (44, 45): Nogo-A protein was upregulated in oligodendrocytes surrounding ischemic lesions whereas NgR protein was increased in reactive astrocytes and microglia/macrophages (44). Moreover, after cerebral infarction in humans, Nogo-A protein (and other myelin inhibitors) persisted in the spinal cords (in the degenerating corticospinal tract) for up to three years (45): this is particularly important information given that several therapies deliver therapies against Nogo-A to the spinal cord after stroke (see below).
In summary, a reasonable number of studies reveal the presence of Nogo-A (and other myelin-associated inhibitors), NgR (and other receptor components), and their downstream effectors (e.g., RhoA) after stroke, although the time-window for any increase is yet to be delineated and may vary according to the method of inducing stroke and the age and species of the subject. This increase is often reported as being localized to peri-lesional areas. Nogo-A expression persists in debris, in surviving oligodendrocytes, and in neurons. These data strongly support a conclusion that Nogo-A has a role to play in the pathology of stroke. Somewhat controversially, there is evidence to contradict an up-regulation of the Nogo-A pathway in stroke in a model that is clinically relevant; Nogo-A protein expression was decreased in aged rats subjected to distal MCAO. One reasonable conclusion is that residual Nogo-A in elderly rats, humans and other primates after stroke is still substantial enough to prevent complete spontaneous repair.
Antibodies against Nogo-A promote functional recovery after stroke
Many reports from spinal cord injury models have confirmed that interference with Nogo-A signaling induces axonal sprouting, dendritic sprouting and functional recovery (46). These promising findings led to research into whether neutralization of Nogo-A can induce axonal sprouting and functional recovery in stroke. To explore the consequence of Nogo-A inhibition on the plasticity of cortical efferents and functional recovery in focal ischaemic stroke, IN-1, an anti-Nogo-A monoclonal IgM antibody, was administered to adult rats following MCAO (47). Functional outcome was measured with a skilled pellet-reaching task for which forelimb dexterity is essential, mediated by corticospinal tracts (CST), rubrospinal tracts and reticulospinal tracts (48–51). Control rats (MCAO only) that received no treatment or a control antibody (IgG, anti-horseradish peroxidase) were permanently impaired, as shown by their forelimb test and they improved modestly until plateauing at around 50% of the baseline function, whereas IN-1 treated rats progressively recovered to a mean of 80% of their baseline level. Previous reports from spinal cord models support these findings; IN-1 has induced recovery of skilled forelimb movements after a unilateral CST lesion in the medullary pyramids (52, 53).
It is impressive that the effects of antibodies against Nogo-A have also been examined in animals with co-morbidities typically seen in human stroke victims. For example, the Nogo-A–specific antibody, 7B12 (IgG), has been demonstrated to improve long-term neurological outcome when given intraventricularly to normotensive and Spontaneously Hypertensive rats (SHR), when administered after 24 hours after stroke (54). (SHRs are prone to vascular abnormalities, have reduced collateral cerebral blood flow, suffer spontaneous strokes throughout the brain and often perish prematurely (55)). The authors induced cerebral ischaemia by permanent MCAO or using photothrombotic methods and used Montoya’s staircase test to evaluate grasping ability of the forepaw contralateral to the infarct. Magnetic Resonance Imaging showed that there were no differences in mean lesion volumes between groups at 24 hours or 9 weeks after stroke. Grasping performance in controls returned to a mean of ~55% of the baseline where it plateaued, until the end of the study. In rats treated with 7B12, grasping ability greatly improved to reach ~70% of baseline levels (54). 7B12 improved outcome in the both healthy normotensive rats and SHRs. These results indicate that Anti-Nogo-A antibodies could be an effective treatment for stroke at least up until a 24-hour time frame.
In a subsequent experiment, IN-1 treatment was delayed until 1 week after stroke in adult rats (56). This resulted in recovery of skilled forelimb function, which was again quantified with a forelimb test that required dexterity. In this study, rats treated with IN-1, 1-week post stroke, managed to recover up to 75% of their baseline function in a skilled forelimb task (56). Encouragingly, animals given IN-1 a week after stroke showed significant recovery at 5 weeks from the time of administration, while animals administered IN-1 during the stroke (47), took only 6 weeks to show significant improvement (a week longer). There was no difference between the level of functionality recovered and the delay in treatment, suggesting that there may be no advantage to early administration of antibodies against Nogo-A and that there may be no deleterious role for Nogo-A in the early pathology of stroke.
Most impressively, antibodies against Nogo-A (11C7, IgG) were infused intracerebroventricularly 9 weeks after ischemic stroke in adult rat (57), when spontaneous behavioural recovery had reached plateau. Encouragingly, they displayed improvement in skilled forelimb movements in this model of chronic stroke. Even when given 9 weeks after stroke, the treated animals showed significant improvement 5 weeks after commencing treatment and achieved a mean of 78% of their baseline ability, when assessed at the end of the study (57). These results are supported by previous studies mentioned above (47, 54, 56) where administration of Anti-Nogo-A antibodies immediately, after 24 hours or after 1 week resulted in similar levels of functional recovery. This study has confirmed that even when delayed for long periods, anti-Nogo-A treatment could rehabilitate sensorimotor function to at least 75% in rats with experimental stroke (57). This study is of particular importance because the data give hope that anti-Nogo-A therapy can be used not just in early stages of treatment for stroke, but also in the chronic stages of such diseases, making it available in principle to a larger proportion of stroke survivors.
This body of work is also distinguished by its demonstration that anti-Nogo-A therapy (7B12, IgG) causes improvements in skilled paw function when initiated in very elderly rats (25 months old) one week after permanent distal MCAO (58).
Another major outcome of stroke is cognitive impairment. An investigation into the effect of anti-Nogo-A antibodies on stroke in aged rats revealed superior performance in a spatial memory task (the Morris water maze nagivational task), in rats that were administered anti-Nogo-A therapy 1 week after stroke (59). Gross motor deficits after stroke have the potential to distort the results of the Morris water maze test, but the authors measured swim velocity in the test subjects and noted no difference between stroke groups. Their observations of the rats also confirmed the lack of gross motor defects as they were seen to ambulate, feed, groom, and swim comparably (59). The study is important as it was performed in aged rats and there was a 1-week delay in treatment, making it highly clinically relevant, as it is a realistic timeframe in which human stroke patients might be treated.
Neglect is another common and debilitating consequence of stroke in humans. It is a multifaceted spatial-attentional disorder where the individual fails to respond to a stimulus on the side of the body contralateral to the brain injury (60). In one study, rats underwent frontal (medial agranular cortex) lesions which induce neglect and then were immediately administered anti-Nogo-A antibodies (IN-1, 7B12 or 11C7) by implanting hybridoma cells intraventricularly. The authors used an orientation test in which they measured each rat’s response towards or away from a transient stimulus to assess the level of neglect and recovery from neglect. They found that all three anti-Nogo groups recovered to a significantly greater extent than the control antibody group; this difference was present when considering total neglect or individual modalities of neglect separately (60). Although the model of injury was aspirative rather than ischemic stroke, this study is important because neglect is a major factor in stroke. For example, patients with neglect do not do benefit maximally from rehabilitation due to disregard of their affected limb: accordingly, a treatment that enhanced recovery from neglect could improve rehabilitation and thus the overall patient outcome.
It should be noted that in many of these studies, the antibodies were given intraventricularly through the cortex on the intact side (by infusion or by implanting hybridoma cells), which is not a clinically straightforward route, and involves additional disturbance of the blood-brain barrier. However, the authors have also evaluated the intrathecal route after a one week delay in adult rats using 11C7 antibodies against Nogo-A with good CNS penetrance (61); recovery was also obtained using this clinically-feasible route of administration. In total, this body of work is impressive in its scope.
To our knowledge, there is only one paper which reports a negative outcome when using antibodies against Nogo-A after stroke (62), and the fact that it is in the public domain is a credit to the authors, given a widespread failure of stroke researchers to report negative outcomes in the literature (63). This study used mice (not rats) and different model of stroke, involving 30 minutes of transient, proximal MCAO and subsequent reperfusion, rather than permanent MCAO using a ligature (47, 57, 61) or MCAO by electrocoagulation (54) or by photothrombosis of the cortex (64) in rats. Treatment of mice (intracerebroventricularly or intrastriatally) 30 minutes after transient, proximal MCAO (i.e., during the period of reperfusion) with 11C7 antibodies against Nogo-A led to no measurable improvements in grip strength measured three days after stroke (62): however, treatment of mice prior to proximal MCAO (and reperfusion) with antibodies against Nogo-A led to a worsening of outcomes, measured using the rotarod and grip strength tests at seven (but not three) days after stroke (62). Nogo-A knockout mice also exhibited worsening of grip strength after transient proximal MCAO. In this small study there was also some evidence for increased mortality in Nogo-A knockout mice. Accordingly, additional work is required (using models of stroke that involve transient occlusion and reperfusion) to determine whether antibodies against Nogo-A can be used safely and effectively, because in humans reperfusion may be occurring either spontaneously or after thrombolysis.
Another study in rats targeted NgR, which is a common receptor for the Nogo-66 inhibitory domain of Nogo-A, Omgp, and MAG (64, 65) and reported functional recovery and corticofugal plasticity when an NgR antagonist was given 7 days post stroke for a duration of 2 months. Another group has confirmed that a function-blocking NgR fragment causes forelimb sensorimotor recovery, even when given 1 week after stroke (64). Knockout mice devoid of NgR or Nogo-A and Nogo B also show enhanced sensorimotor function relative to heterozygous controls (64) after photothrombotic cortical ischemia. However, in another study, after proximal MCAO and reperfusion, Nogo-A knockout mice showed a slight worsening of grip strength 7 days after stroke (p. 7 in (62)).
Researchers have explored whether adenovirus-mediated gene knockdown of NgR (AdNgR) in rats decreased cortical and hippocampal expression of NgR mRNA and protein after cerebral ischemia and subsequent reperfusion (66). It was found that intracerebral infusion of AdNgR prevented MCAO induced enhancement of NgR and resulted in functional recovery in skilled forelimb performance, which was assessed by a Montoya staircase test (66). The AdNgR rats showed significantly improved performance from 3-9 weeks when compared to controls. Adenovirus was shown to transfect the ischaemic brain, including hippocampal neurons. After 24 hours of AdNgR treatment cortical and hippocampal levels of NgR mRNA and protein in AdNgR rats were reduced by 60% when compared to control MCAO rats; this inhibition dropped to 40% after 2 weeks (66). They also saw a reduction in NgR mRNA and protein levels 9 weeks after stroke, which was also accompanied by functional recovery. NgR knockdown did not have any effect on infarct volume. This observation is consistent with a previous study (64) in which antagonists that blocked Nogo-A/NgR signaling did not induce neuroprotection. Thus gene knockdown of NgR may overcome inhibition of axon growth signaling and allow for greater axon regeneration, which may aid in sensorimotor recovery in stroke (see below).
In summary, antibodies against Nogo-A induce significant functional recovery in many experimental stroke models. Sensorimotor function after treatment with anti-Nogo-A antibodies has reached as much as 78% of the baseline ability in rodents with MCAO. The time window for initiating treatment that promotes successful recovery of function is currently up to 9 weeks from the time of stroke but future experiments may show this extends even further. Moreover, comparing across experiments, treatment even as late as 9 weeks is as effective as treatment given immediately after stroke. As well as sensorimotor function, anti-Nogo-A therapy has shown some efficacy in improving neglect in rats with medial agranular cortex lesions. These results are important as stroke affects a wide range of brain functions, which makes a drug that targets multiple aspects of stroke symptoms an exciting prospect. Anti-Nogo-A treatment has been effective in experimental models of MCAO in aged rats and hypertensive rats. Collectively, this data is very promising due to the clinical relevance of many of the models.
Nogo-A induces neuroanatomical plasticity
There are several mechanisms behind the observed functional improvements. Blockade of Nogo-A reduces growth cone collapse and overcomes inhibition of neurite outgrowth (25, 26, 67), thereby providing a more suitable environment for new neurite growth (47). Functional recovery in stroke is accompanied by plasticity of cortical efferents from the unlesioned hemisphere to the striatum, red nucleus, pons and cervical spinal cord. In vivo and in vitro studies have shown enhanced expression of growth promoting genes including c-Jun and GAP-43 in presence of IN-1 (68, 69) and these probably occur in sprouting neurons in response to released inhibition of growth.
Normally, the corticorubral tract descends mainly ipsilaterally with only a small contralateral component (52, 70–72). Anti-Nogo-A antibodies enhance corticorubral sprouting, originating from the undamaged hemisphere and extending over the midbrain’s midline to the parvocellular region of the red nucleus whether treatment was started immediately after stroke (47) or one or nine weeks after stroke (56, 57). This results in increased bilateral supply to the denervated red nucleus (47) and then, if appropriate synapses are made, rubrospinal axons may relay cortical sensorimotor commands to the spinal cord. The authors compared the corticorubral crossing indices in these studies and found similarities in the crossing index between their 1 week-delayed treatment (527±74 SEM) and that of the immediate treatment study (469±81 SEM) for treated animals and for control rats (263±60 SEM and 232±37 SEM, respectively) (57). This data suggests that treatment with anti-Nogo-A therapy is just as efficacious at inducing corticorubral axonal plasticity when given after 9 weeks as it is when given immediately. IN-1 treatment has also shown increased plasticity of corticostriatal (73) corticopontine, and corticorubral fibres (74) following unilateral aspiration lesions to the cortex. Corticostriatal fibre outgrowth has also been reported after cortical ablation (73) or cortical thermocoagulation injury (75)
The 7B12 anti-Nogo antibody was shown to induce midline crossing in the cervical spinal cord of the CST fibres from the less-affected sensorimotor cortex when administered to normotensive and spontaneously hypertensive rats following MCAO (54). Some spontaneous CST crossing in the spinal cord occurs in rats after stroke, but CST crossing was significantly higher in 7B12-treated animals. However, 7B12 did not induce sprouting of all pathways indiscriminately: corticopontine midline crossing in control antibody treated rats was similar to that in 7B12 treated rats. Regardless of treatment, there was a correlation between fibre outgrowth and improvement in forepaw function in both normotensive and SHRs. This implies that after photothrombotic cortical injury and MCAO in SHRs, behavioural outcome can be improved by lesion-induced fibre outgrowth in the cervical spinal cord. Although there was a correlation between corticospinal midline crossing and behavioural improvement, other brain regions and systems are also likely to be involved in this sensorimotor task and that they may be influenced by 7B12. Indeed, in elderly rats after stroke and anti-Nogo antibody treatment, functional brain imaging indicated increased probability of activation in the thalamus during electrical stimulation of the affected paw, 8 weeks after stroke (58).
The hippocampus has a central role in spatial memory tasks (76), spurring investigation into the effect of anti-Nogo-A treatment on neuroanatomical plasticity in the hippocampus after MCAO (59). Functional improvements were seen after administering anti-Nogo-A antibodies to aged rats after MCAO: however, although there was a decrease in dendritic complexity in CA3, CA1 and the dentate gyrus neurons of the ipsilesional hippocampus after stroke, there were no differences between rats treated with anti-Nogo-A antibodies and rats treated with control antibodies. To explain their results the authors proposed that structural plastic changes might be occurring in other areas such as the medial entorhinal cortex, lateral septum, striatum and subiculum (77). Indeed, septohippocampal axonal growth has previously been seen with anti-Nogo-A treatment (78). Another possible explanation is that biochemical and electrophysiological changes might be occurring in the hippocampus or other areas that are important for spatial memory tasks (59).
In the study investigating recovery from neglect the authors chose animals that had recovered after anti-Nogo-A antibody treatment, and they made a knife cut in the corpus callosum on the ipsilesional side either medial to the dorsocentral striatum (DCS) or lateral to the DCS (because the DCS is implicated in recovery from neglect (79)). Only the rats subjected to medial cuts re-exhibited neglect suggesting that the mechanism behind recovery involves transcallosal inputs from the less-affected hemisphere to areas medial to the cingulum bundle, such as the ipsilesional DCS (60).
Adenoviral-mediated NgR knockdown after MCAO in rats resulted in greater axon sprouting of corticorubral and corticostriatal pathways from the unlesioned hemisphere (66). Knockout mice devoid of NgR or Nogo-A and Nogo B also show enhanced sensorimotor function when compared to heterozygous controls. The authors of this study also found that the axonal sprouting of intact pathways to the denervated red nucleus and spinal cord was responsible for the functional recovery (64).
A large number of studies have hypothesized that sprouting of cortical efferent pathways is responsible for functional motor improvement after interference with Nogo-A signalling (47, 52, 73, 80). This is in line with several studies of motor corticofugal pathways in newborn rats with unilateral sensorimotor cortical lesions, where the less-affected, contralesional hemisphere sprouted bilateral projections to the striatum (73), thalamus (81), red nucleus (70), tectum (82), basilar pontine gray (83), and spinal cord (66, 84).
In summary, anti-Nogo-A treatment induces axonal sprouting in rats that received treatment after stroke, axonal sprouting that has more often than not (56) been correlated with functional sensorimotor recovery. Sprouting of axons from contralesional cortex regions into subcortical structures of the damaged hemisphere is seen in rats treated with anti-Nogo-A antibodies. This new sprouting of corticoefferent axons has been observed to originate from cortical areas contralateral to the lesion and has extended to the red nucleus, pontine nuclei, and spinal cord of rats that were subjected to stroke and were subsequently treated with anti-Nogo-A antibodies. These changes have occurred regardless of whether there was a delay in administration of anti-Nogo-A antibodies. We look forward to additional experiments examining whether these collateral sprouts form functional synapses after stroke (85): advances are being made in this area in models of spinal cord injury (86, 87). Brain imaging indicates that Nogo-A antibodies also alter activity in the thalamus. Altogether, these changes likely cause the improvement of sensorimotor movement, although formal proof of this relationship remains to be shown for most types of plasticity, for example by re-lesioning (60).
Future directions and translation
It will be important to determine whether, after stroke, treatment with Nogo-A antibodies delivers improvements which are not seen with rehabilitation alone. This is important because other groups have shown that a similar magnitude of recovery of forelimb grasping occurs on the Montoya’s staircase pellet-reaching task with rehabilitation alone, even when rehabilitation is only started 15 days after stroke (88). In the case of chronic stroke, antibodies against Nogo-A do appear to provide a more beneficial outcome than delayed rehabilitation, because antibodies against Nogo-A can provide a benefit even when treatment is initiated after 9 weeks (57) whereas rehabilitation declines in efficacy considerably by 4 weeks (88). However, those rehabilitation studies were done with a different model of (smaller) focal stroke and it will be important to compare rehabilitation plus antibodies to Nogo-A in a single study. One careful study in a model of spinal cord injury show that this issue does need to be examined empirically because in some circumstances, rehabilitation does not combine additively or synergistically with treatment with antibodies against Nogo-A but can actually worsen outcome (86). In mitigation, a large number of studies now show that the efficacy of rehabilitation depends on the type, specificity, intensity, and timing of rehabilitation, and it seems likely that an effective combination of rehabilitation and antibodies against Nogo-A can be found.
It will also be important to ensure the highest methodological quality of preclinical studies going forward (89, 90), to avoid bias and to estimate effect sizes correctly (63). The vast majority of papers relating to anti-Nogo therapeutics do not mention whether or not treatment allocations were concealed, how randomization was performed (e.g., randomized block design) and at what stages blinding was in effect. In stroke studies (especially those with elderly rodents), drop-out due to mortality or treatment side effects are a particular concern, and it will be important to report these incidences as they have signal importance (and have been reported in a small number of Nogo-A knockouts (62)). Inclusion and exclusion criteria, and numbers of animals omitted from analyses should also be reported. These and other criteria are laid out in the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments), which have been endorsed by many journals 1, including stroke specialty journals (89, 91).
It will also be important to determine whether antibodies against Nogo-A improve outcome in haemmorhagic stroke because this might increase the number of stroke patients that can be included in any clinical trial. It seems reasonable to predict that antibodies against Nogo-A will improve outcome in haemmorhagic strokes that affect similar areas of the brain as MCAO ischemic strokes (with all other things being equal) because the mechanism of repair induced by Nogo-A antibodies does not appear to involve neuroprotection and, accordingly, the cause whereby stroke originally occurs may well be irrelevant.
To date, we do not know whether the human anti-human Nogo-A antibodies have yet been evaluated in animal models of stroke, and we know of no studies in non-human primates after stroke using antibodies against Nogo-A, although several studies have reported benefits of antibodies against Nogo-A in monkey models of spinal cord injury (92–97). However, we do know of two Phase I clinical trials using antibodies against Nogo-A: Novartis’ ATI355 human anti-human Nogo-A antibody (46) and GlaxoSmithKline’s 1223249 antibody (NCT00406016 and NCT01435993, respectively; www.clinicaltrials.gov). The latter Phase I study is recruiting patients with multiple sclerosis (MS). The Novartis Phase I study was carried out in SCI patients and so far results have been positive with regards to safety and adverse effects (46). Any resulting Phase II trials should test the efficacy of the drug and if results are also positive, it will be a major breakthrough in the treatment of CNS injury, particularly against backdrop of such an alarming failure rate of the pharmaceutical industry to produce successful therapeutic agents for stroke.
Conclusion
In recent times experimental research has greatly enhanced the understanding of functional recovery and reorganization after injury to the CNS. Neuroplastic changes such as axonal sprouting, synapse formation and reorganization of activity patterns occurs at several different levels in the CNS after a lesion. These mechanisms help to restore function after cortical and spinal injuries. Interference with pathways that restrict neuronal regeneration and plasticity have been successful in aiding recovery after SCI and stroke, with one major pre-clinical success being the inhibition of Nogo-A or NgR signaling by antibodies, receptor blockers and genetic knockdown/knockout techniques (46). Successful clinical trials in SCI or MS may follow through to future clinical trials in stroke, which could potentially revolutionise the treatment of this debilitating disorder (46).
Acknowledgements
Thanks to Dr Thomas Hutson for providing Figure 1 from his PhD thesis. Thanks also to two anonymous reviewers whose comments strengthened this manuscript.
Abbreviations
- ARRIVE guidelines
Animal Research: Reporting of In Vivo Experiments guidelines
- MAG
Myelin-associated glycoprotein
- MCAO
Middle cerebral artery occlusion
- NgR
Nogo receptor
- Omgp
Oligodendrocyte myelin glycoprotein
- SHR
Spontaneously hypertensive rat
- tPA
Tissue type plasminogen activator
Footnotes
Conflict of interest statement: The authors have no conflicts of interest.
References
- 1.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bulletin of the World Health Organization. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 3.Bonita R. Epidemiology of stroke. Lancet. 1992;339:342–344. doi: 10.1016/0140-6736(92)91658-u. [DOI] [PubMed] [Google Scholar]
- 4.Truelsen T, Piechowski-Jozwiak B, Bonita R, Mathers C, Bogousslavsky J, Boysen G. Stroke incidence and prevalence in Europe: a review of available data. Eur J Neurol. 2006;13:581–598. doi: 10.1111/j.1468-1331.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 6.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 7.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age and ageing. 2009;38:27–32. doi: 10.1093/ageing/afn281. [DOI] [PubMed] [Google Scholar]
- 8.Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RA, McNeil JJ, Donnan GA. Cost of stroke in Australia from a societal perspective: results from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke a journal of cerebral circulation. 2001;32:2409–2416. doi: 10.1161/hs1001.097222. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM. The high cost of not funding stroke research: a comparison with heart disease and cancer. Lancet. 2001;357:1612–1616. doi: 10.1016/s0140-6736(00)04730-9. [DOI] [PubMed] [Google Scholar]
- 10.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 11.Barsan WG, Brott TG, Broderick JP, Haley EC, Levy DE, Marler JR. Time of hospital presentation in patients with acute stroke. Archives of internal medicine. 1993;153:2558–2561. [PubMed] [Google Scholar]
- 12.Kothari R, Jauch E, Broderick J, Brott T, Sauerbeck L, Khoury J, Liu T. Acute stroke: delays to presentation and emergency department evaluation. Annals of emergency medicine. 1999;33:3–8. doi: 10.1016/s0196-0644(99)70431-2. [DOI] [PubMed] [Google Scholar]
- 13.Fang J, Yan W, Jiang GX, Li W, Cheng Q. Time interval between stroke onset and hospital arrival in acute ischemic stroke patients in Shanghai, China. Clinical neurology and neurosurgery. 2011;113:85–88. doi: 10.1016/j.clineuro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Agyeman O, Nedeltchev K, Arnold M, Fischer U, Remonda L, Isenegger J, Schroth G, Mattle HP. Time to admission in acute ischemic stroke and transient ischemic attack. Stroke a journal of cerebral circulation. 2006;37:963–966. doi: 10.1161/01.STR.0000206546.76860.6b. [DOI] [PubMed] [Google Scholar]
- 15.Evenson KR, Foraker RE, Morris DL, Rosamond WD. A comprehensive review of prehospital and in-hospital delay times in acute stroke care. Int J Stroke. 2009;4:187–199. doi: 10.1111/j.1747-4949.2009.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzimondi G, Bassein L, Fiorani L, Nonino F, Montaguti U, Celin D, Re G, D'Alessandro R. Variables associated with hospital arrival time after stroke: effect of delay on the clinical efficiency of early treatment. Stroke a journal of cerebral circulation. 1997;28:537–542. doi: 10.1161/01.str.28.3.537. [DOI] [PubMed] [Google Scholar]
- 17.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Ginsberg MD. Life after cerovive: a personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke a journal of cerebral circulation. 2007;38:1967–1972. doi: 10.1161/STROKEAHA.106.479170. [DOI] [PubMed] [Google Scholar]
- 20.Faden AI, Stoica B. Neuroprotection: challenges and opportunities. Archives of neurology. 2007;64:794–800. doi: 10.1001/archneur.64.6.794. [DOI] [PubMed] [Google Scholar]
- 21.Young AR, Ali C, Duretete A, Vivien D. Neuroprotection and stroke: time for a compromise. Journal of neurochemistry. 2007;103:1302–1309. doi: 10.1111/j.1471-4159.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- 22.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 23.The bitterest pill. Nature. 2006;444:532–533. doi: 10.1038/444532a. [DOI] [PubMed] [Google Scholar]
- 24.Soleman S, Yip PK, Duricki DA, Moon LD. Delayed treatment with chondroitinase ABC promotes sensorimotor recovery and plasticity after stroke in aged rats. Brain. 2012;135:1210–1223. doi: 10.1093/brain/aws027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 26.GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 27.Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22:5505–5515. doi: 10.1523/JNEUROSCI.22-13-05505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Barrette B, Vallieres N, Dube M, Lacroix S. Expression profile of receptors for myelin-associated inhibitors of axonal regeneration in the intact and injured mouse central nervous system. Mol Cell Neurosci. 2007 doi: 10.1016/j.mcn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, Kaupmann K, et al. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5393–5406. doi: 10.1523/JNEUROSCI.23-13-05393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 33.Schweigreiter R, Walmsley AR, Niederost B, Zimmermann DR, Oertle T, Casademunt E, Frentzel S, Dechant G, Mir A, Bandtlow CE. Versican V2 and the central inhibitory domain of Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways that converge at RhoA. Molecular and cellular neurosciences. 2004;27:163–174. doi: 10.1016/j.mcn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Yao Y, Jiang X, Chen D, Xiong Y, Mu D. Expression of Nogo-A and NgR in the developing rat brain after hypoxia-ischemia. Brain research. 2006;1114:212–220. doi: 10.1016/j.brainres.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 35.Zhou C, Li Y, Nanda A, Zhang JH. HBO suppresses Nogo-A, Ng-R, or RhoA expression in the cerebral cortex after global ischemia. Biochemical and biophysical research communications. 2003;309:368–376. doi: 10.1016/j.bbrc.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Xia F, Han J, Wang J. Patterns of Nogo-A, NgR, and RhoA expression in the brain tissues of rats with focal cerebral infarction. Translational research: the journal of laboratory and clinical medicine. 2009;154:40–48. doi: 10.1016/j.trsl.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Cheatwood JL, Emerick AJ, Schwab ME, Kartje GL. Nogo-A expression after focal ischemic stroke in the adult rat. Stroke a journal of cerebral circulation. 2008;39:2091–2098. doi: 10.1161/STROKEAHA.107.507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trifunovski A, Josephson A, Bickford PC, Olson L, Brene S. Selective decline of Nogo mRNA in the aging brain. Neuroreport. 2006;17:913–916. doi: 10.1097/01.wnr.0000221831.95598.a3. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiology of disease. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Eslamboli A, Grundy RI, Irving EA. Time-dependent increase in Nogo-A expression after focal cerebral ischemia in marmoset monkeys. Neuroscience letters. 2006;408:89–93. doi: 10.1016/j.neulet.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 41.Josephson A, Trifunovski A, Widmer HR, Widenfalk J, Olson L, Spenger C. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol. 2002;453:292–304. doi: 10.1002/cne.10408. [DOI] [PubMed] [Google Scholar]
- 42.Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol. 2001;169:319–328. doi: 10.1006/exnr.2001.7659. [DOI] [PubMed] [Google Scholar]
- 43.O'Neill P, Whalley K, Ferretti P. Nogo and Nogo-66 receptor in human and chick: implications for development and regeneration. Dev Dyn. 2004;231:109–121. doi: 10.1002/dvdy.20116. [DOI] [PubMed] [Google Scholar]
- 44.Satoh J, Onoue H, Arima K, Yamamura T. Nogo-A and nogo receptor expression in demyelinating lesions of multiple sclerosis. J Neuropathol Exp Neurol. 2005;64:129–138. doi: 10.1093/jnen/64.2.129. [DOI] [PubMed] [Google Scholar]
- 45.Buss A, Pech K, Merkler D, Kakulas BA, Martin D, Schoenen J, Noth J, Schwab ME, Brook GA. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. Brain. 2005;128:356–364. doi: 10.1093/brain/awh355. [DOI] [PubMed] [Google Scholar]
- 46.Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci. 2010;1198(Suppl 1):E22–34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
- 47.Papadopoulos CM, Tsai SY, Alsbiei T, O'Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 48.Whishaw IQ, Gorny B, Sarna J. Paw and limb use in skilled and spontaneous reaching after pyramidal tract, red nucleus and combined lesions in the rat: behavioral and anatomical dissociations. Behavioural brain research. 1998;93:167–183. doi: 10.1016/s0166-4328(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 49.Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. Journal of neurophysiology. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- 51.Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T. Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. Journal of neurophysiology. 2011;106:122–126. doi: 10.1152/jn.00089.2011. [DOI] [PubMed] [Google Scholar]
- 52.Z'Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional recovery and enhanced corticofugal plasticity after unilateral pyramidal tract lesion and blockade of myelin-associated neurite growth inhibitors in adult rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thallmair M, Metz GAS, Z'Graggen WJ, Raineteau O, Kaitje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nature Neuroscience. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 54.Wiessner C, Bareyre FM, Allegrini PR, Mir AK, Frentzel S, Zurini M, Schnell L, Oertle T, Schwab ME. Anti-Nogo-A antibody infusion 24 hours after experimental stroke improved behavioral outcome and corticospinal plasticity in normotensive and spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2003;23:154–165. doi: 10.1097/01.WCB.0000040400.30600.AF. [DOI] [PubMed] [Google Scholar]
- 55.Barone FC, Clark RK, Price WJ, White RF, Feuerstein GZ, Storer BL, Ohlstein EH. Neuron-specific enolase increases in cerebral and systemic circulation following focal ischemia. Brain research. 1993;623:77–82. doi: 10.1016/0006-8993(93)90012-c. [DOI] [PubMed] [Google Scholar]
- 56.Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 57.Tsai SY, Papadopoulos CM, Schwab ME, Kartje GL. Delayed anti-nogo-a therapy improves function after chronic stroke in adult rats. Stroke. 2011;42:186–190. doi: 10.1161/STROKEAHA.110.590083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markus TM, Tsai SY, Bollnow MR, Farrer RG, O'Brien TE, Kindler-Baumann DR, Rausch M, Rudin M, Wiessner C, Mir AK, Schwab ME, et al. Recovery and brain reorganization after stroke in adult and aged rats. Ann Neurol. 2005;58:950–953. doi: 10.1002/ana.20676. [DOI] [PubMed] [Google Scholar]
- 59.Gillani RL, Tsai SY, Wallace DG, O'Brien TE, Arhebamen E, Tole M, Schwab ME, Kartje GL. Cognitive recovery in the aged rat after stroke and anti-Nogo-A immunotherapy. Behav Brain Res. 2010;208:415–424. doi: 10.1016/j.bbr.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenneman MM, Wagner SJ, Cheatwood JL, Heldt SA, Corwin JV, Reep RL, Kartje GL, Mir AK, Schwab ME. Nogo-A inhibition induces recovery from neglect in rats. Behavioural brain research. 2008;187:262–272. doi: 10.1016/j.bbr.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai SY, Markus TM, Andrews EM, Cheatwood JL, Emerick AJ, Mir AK, Schwab ME, Kartje GL. Intrathecal treatment with anti-Nogo-A antibody improves functional recovery in adult rats after stroke. Exp Brain Res. 2007;182:261–266. doi: 10.1007/s00221-007-1067-0. [DOI] [PubMed] [Google Scholar]
- 62.Kilic E, ElAli A, Kilic U, Guo Z, Ugur M, Uslu U, Bassetti CL, Schwab ME, Hermann DM. Role of Nogo-A in neuronal survival in the reperfused ischemic brain. J Cereb Blood Flow Metab. 2010;30:969–984. doi: 10.1038/jcbfm.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nature reviews Neuroscience. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 66.Wang T, Wang J, Yin C, Liu R, Zhang JH, Qin X. Down-regulation of Nogo receptor promotes functional recovery by enhancing axonal connectivity after experimental stroke in rats. Brain research. 2010;1360:147–158. doi: 10.1016/j.brainres.2010.08.101. [DOI] [PubMed] [Google Scholar]
- 67.Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 68.Zagrebelsky M, Buffo A, Skerra A, Schwab ME, Strata P, Rossi F. Retrograde regulation of growth-associated gene expression in adult rat Purkinje cells by myelin-associated neurite growth inhibitory proteins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:7912–7929. doi: 10.1523/JNEUROSCI.18-19-07912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biological chemistry. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- 70.Naus CG, Flumerfelt BA, Hrycyshyn AW. An HRP-TMB ultrastructural study of rubral afferents in the rat. The Journal of comparative neurology. 1985;239:453–465. doi: 10.1002/cne.902390411. [DOI] [PubMed] [Google Scholar]
- 71.Kuchler M, Fouad K, Weinmann O, Schwab ME, Raineteau O. Red nucleus projections to distinct motor neuron pools in the rat spinal cord. J Comp Neurol. 2002;448:349–359. doi: 10.1002/cne.10259. [DOI] [PubMed] [Google Scholar]
- 72.Raineteau O, Fouad K, Bareyre FM, Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- 73.Kartje GL, Schulz MK, Lopez-Yunez A, Schnell L, Schwab ME. Corticostriatal plasticity is restricted by myelin-associated neurite growth inhibitors in the adult rat. Annals of neurology. 1999;45:778–786. doi: 10.1002/1531-8249(199906)45:6<778::aid-ana12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 74.Wenk CA, Thallmair M, Kartje GL, Schwab ME. Increased corticofugal plasticity after unilateral cortical lesions combined with neutralization of the IN-1 antigen in adult rats. The Journal of comparative neurology. 1999;410:143–157. doi: 10.1002/(sici)1096-9861(19990719)410:1<143::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 75.Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. The Journal of comparative neurology. 1996;373:484–497. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 76.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain research Brain research reviews. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 77.Knierim JJ. Neural representations of location outside the hippocampus. Learn Mem. 2006;13:405–415. doi: 10.1101/lm.224606. [DOI] [PubMed] [Google Scholar]
- 78.Cadelli D, Schwab ME. Regeneration of Lesioned Septohippocampal Acetylcholinesterase-positive Axons is Improved by Antibodies Against the Myelin-associated Neurite Growth Inhibitors NI-35/250. The European journal of neuroscience. 1991;3:825–832. doi: 10.1111/j.1460-9568.1991.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 79.Reep RL, Corwin JV, Cheatwood JL, Van Vleet TM, Heilman KM, Watson RT. A rodent model for investigating the neurobiology of contralateral neglect. Cogn Behav Neurol. 2004;17:191–194. [PubMed] [Google Scholar]
- 80.Kolb B, Gibb R, van der Kooy D. Cortical and striatal structure and connectivity are altered by neonatal hemidecortication in rats. J Comp Neurol. 1992;322:311–324. doi: 10.1002/cne.903220303. [DOI] [PubMed] [Google Scholar]
- 81.Yu XH, Moret V, Rouiller EM. Re-examination of the plasticity of the corticothalamic projection after unilateral neonatal lesion of the sensorimotor cortex in the rat: a phaseolus vulgaris-leucoagglutinin tracing study. Journal fur Hirnforschung. 1995;36:123–133. [PubMed] [Google Scholar]
- 82.Leong SK, Lund RD. Anomalous bilateral corticofugal pathways in albino rats after neonatal lesions. Brain Res. 1973;62:218–221. doi: 10.1016/0006-8993(73)90630-6. [DOI] [PubMed] [Google Scholar]
- 83.Castro AJ, Mihailoff GA. Corticopontine remodelling after cortical and/or cerebellar lesions in newborn rats. The Journal of comparative neurology. 1983;219:112–123. doi: 10.1002/cne.902190111. [DOI] [PubMed] [Google Scholar]
- 84.Castro AJ. Ipsilateral corticospinal projections after large lesions of the cerebral hemisphere in neonatal rats. Experimental neurology. 1975;46:1–8. doi: 10.1016/0014-4886(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 85.Emerick AJ, Neafsey EJ, Schwab ME, Kartje GL. Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. J Neurosci. 2003;23:4826–4830. doi: 10.1523/JNEUROSCI.23-12-04826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maier IC, Ichiyama RM, Courtine G, Schnell L, Lavrov I, Edgerton VR, Schwab ME. Differential effects of anti-Nogo-A antibody treatment and treadmill training in rats with incomplete spinal cord injury. Brain. 2009;132:1426–1440. doi: 10.1093/brain/awp085. [DOI] [PubMed] [Google Scholar]
- 87.Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments-The ARRIVE Guidelines. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, Hutton J, Altman DG. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4:e7824. doi: 10.1371/journal.pone.0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the Marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-A antibody IN-1. Eur J Neurosci. 2004;20:2479–2482. doi: 10.1111/j.1460-9568.2004.03716.x. [DOI] [PubMed] [Google Scholar]
- 93.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates--re-examination and extension of behavioral data. Eur J Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Freund P, Wannier T, Schmidlin E, Bloch J, Mir A, Schwab ME, Rouiller EM. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- 95.Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 96.Tuszynski M. Challenges to the report of Nogo antibody effects in primates. Nat Med. 2006;12:1231–1232. doi: 10.1038/nm1106-1231c. author reply 1232-1233. [DOI] [PubMed] [Google Scholar]
- 97.Ho C, Tessier-Lavigne M. Challenges to the report of Nogo antibody effects in primates. Nat Med. 2006;12:1232. doi: 10.1038/nm1106-1232a. author reply 1232-1233. [DOI] [PubMed] [Google Scholar]