Abstract
The expression of small intestinal cytochromes P450 (P450s) has not been systematically measured in cynomolgus monkeys, which are widely used in preclinical drug studies to predict pharmacokinetics and toxicity in humans: therefore, P450 content of small intestine was quantified in 35 cynomolgus monkeys by immunoblotting using 11 selective antibodies.
CYP2D, CYP2J2, CYP3A4, and CYP3A5 were detected in all 35 animals, while CYP1A and CYP2C9/19 were detected in 31 and 17 animals, respectively. CYP2C9 and CYP2C19 were detected with the same antibody. CYP1D, CYP2A, CYP2B6, CYP2C76, and CYP2E1 were not detected in any of the 35 animals examined.
On analysis of pooled microsomes (35 animals), CYP3A (3A4 + 3A5) was most abundant (79% of total immunoquantified CYP1-3 proteins), followed by CYP2J2 (13%), CYP2C9/19 (4%), CYP1A (3%), and CYP2D (0.4%). On analysis of individual microsome samples, each P450 content varied 2- to 6-fold between animals, and no sex differences were observed in any P450 content.
These findings should help to increase the understanding of drug metabolism, especially the first-pass effect, in cynomolgus monkey small intestines.
Introduction
Cytochromes P450 (P450s) are a gene superfamily consisting of a large number of genes, 57 functional genes and 58 pseudogenes in human (Nelson et al. 2004). Many P450s, especially those of the CYP1-3 families, play critical roles in metabolism of drugs and other xenobiotic chemicals and are responsible for approximately 80% of oxidative metabolism in humans (Wilkinson 2005). As shown by immunoblotting, CYP3A is the most abundant, in terms of total hepatic P450 content, in human liver, followed by CYP2C9, CYP1A2, CYP2E1, and CYP2D6 (Shimada et al. 1994; Guengerich 2003). In addition to the liver, the small intestine is also an important site for first-pass metabolism of orally ingested drugs. For example, CYP3A4 in the small intestine contributes significantly to first-pass metabolism of midazolam (Paine et al. 1996). CYP3A is most abundant in total P450 content of human small intestine, followed by CYP2C, CYP1A2, CYP2E1, CYP2A6, CYP2D6, and CYP2B6 (Paine et al. 2006).
Cynomolgus monkey (Macaca fascicularis) is a primate species widely used in drug metabolism studies. More than 20 cynomolgus P450s have been identified and their sequences are highly similar (>90%) to human P450s, except for CYP2C76, which is not orthologous to any human P450 and is responsible for species differences in drug metabolism between cynomolgus monkeys and humans (Uno et al. 2010a; Uno et al. 2011a). Previously we showed that CYP3A was most abundant in total hepatic P450 content of the cynomolgus monkey liver, followed by CYP2A, CYP2B, CYP2C, CYP2E, and CYP2D (Uehara et al. 2011). In cynomolgus monkey small intestine, gene expression of several P450s has been detected, including CYP1A1, CYP1D1, CYP2C9, CYP2C19, CYP2C76, CYP2D17, CYP2J2, CYP3A4, and CYP3A5 (Nakanishi et al. 2010; Uno et al. 2011b). However, protein content of these P450s has not been systematically measured in cynomolgus monkey small intestine. In this paper, cynomolgus CYP2C20, CYP2C43, CYP2C75, and CYP3A8 are designated as CYP2C8, CYP2C9, CYP2C19, and CYP3A4, respectively, as recommended by the P450 Nomenclature Committee (http://drnelson.uthsc.edu/cytochromeP450.html) (Uno et al. 2011a).
In this study, expression levels were analyzed using selective antibodies for CYP1-3 proteins, including CYP1A, CYP1D1, CYP2A, CYP2B6, CYP2C9/19, CYP2C76, CYP2D, CYP2E1, CYP2J2, CYP3A4, and CYP3A5 in the small intestines of 35 cynomolgus monkeys. Cynomolgus CYP2C9 and CYP2C19 were quantified together because the antibody used did not distinguish these two CYP2C isoforms. The data were used to calculate specific content of each P450 and presented as mean values and inter-animal variations.
Materials and Methods
Materials
Polyclonal antibodies used in this study were commercially available; anti-human CYP1A1 antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), anti-human CYP2A6, anti-human CYP2C9, anti-human CYP2D6, and anti-human CYP3A4 antibodies from Nosan Corporation (Yokohama, Japan), anti-human CYP2B6 and anti-human CYP3A5 antibodies from BD Gentest (Woburn, MA), and anti-human CYP2E1 antibody from BIOMOL Research Laboratories (Plymouth Meeting, PA). Anti- cynomolgus CYP2C76 and anti-human CYP2J2 antibodies were prepared as described previously (King et al. 2002; Uno et al. 2006; Uno et al. 2011b). The secondary antibodies (goat anti-mouse, donkey anti-goat, and goat anti-rabbit horseradish peroxidase-conjugated IgGs) were purchased from Santa Cruz Biotechnology, Inc. Chemicals and reagents for the polyacrylamide gels, including sodium dodecyl sulfate (SDS), bis/acrylamide (37.5:1), ammonium persulfate, and TEMED were purchased from Bio-Rad Laboratories (Hercules, CA). Polyvinylidene difluoride membranes (Hybond-P) and an enhanced chemiluminescence Western blotting detection reagent were purchased from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). All other chemicals and reagents were purchased from Sigma (St. Louis, MO) or Wako Pure Chemical Industries (Osaka, Japan) unless otherwise specified.
Tissue samples and microsomal preparation
Small intestine samples (jejunum) were collected from 35 purpose-bred cynomolgus monkeys (18 males and 17 females, 3–4 years of age, weighing 3–5 kg) of Cambodian origin (Shin Nippon Biomedical Laboratories, Ltd., Kagoshima, Japan). All cynomolgus monkeys were housed in a temperature and humidity-controlled room with a 12-h light/dark cycle, and were fed ad libitum with a standard diet, Teklad Global Certified 25% Protein Primate Diet (Harlan Sprague-Dawley, Indianapolis, IN, USA). This study was reviewed and approved by the Institutional Animal Care and Use Committee at Shin Nippon Biomedical Laboratories, Ltd. (Kainan, Japan). Intestinal microsomes were prepared as described previously (Nakanishi et al. 2011). Microsomal protein concentrations were measured by the Bradford method using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories) with bovine serum albumin as the standard.
Preparation of recombinant P450 proteins
The recombinant proteins were expressed in Escherichia Coli and cell membranes were prepared as described previously for CYP1A1, CYP1A2, CYP1D1, CYP2A23, CYP2A24, CYP2A26, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2C76, CYP2D17, CYP2D44, CYP2E1, CYP2J2, CYP3A4, CYP3A5, and CYP3A43 (Uno et al. 2007; Uehara et al. 2010; Uno et al. 2011b). The content of each P450 protein in the cell membrane preparation was determined by Fe2+ · CO vs. Fe2+ difference spectra as described previously (Omura and Sato 1964).
Immunoblotting
To measure P450 expression in the small intestines of 35 cynomolgus monkeys, immunoblotting was performed as described previously using the antibodies, selectivity of which have been confirmed against each recombinant P450 of cynomolgus monkey as described below (Uehara et al. 2011). Anti-human CYP2B6, anti-cynomolgus CYP2C76, anti-human CYP2E1, anti-human CYP2J2, anti-human CYP3A4, and anti-human CYP3A5 antibodies selectively react with cynomolgus CYP2B6, CYP2C76, CYP2E1, CYP2J2, CYP3A4, and CYP3A5, respectively. Anti-human CYP1A1, anti-human CYP2A6, anti-human CYP2C9, anti-human CYP2D6 antibodies react with 2–3 isoforms of highly homologous P450s together, cynomolgus CYP1A1/2, CYP2A23/24/26, CYP2C9/19, and CYP2D17/44, respectively. Anti-human CYP1A1 also reacts with cynomolgus CYP1D1, but size difference enables detection of cynomolgus CYP1D1 and cynomolgus CYP1A1/2 separately. Microsomal proteins or recombinant cynomolgus P450 proteins were separated on 10% SDS polyacrilamide gels and transferred to Hybond-P filters. The filters were incubated with the primary antibody (1:200 – 1:100000), including anti-human CYP1A1, anti-human CYP2A6, anti-human CYP2B6, anti-human CYP2C9, anti-cynomolgus CYP2C76, anti-human CYP2D6, anti-human CYP2E1, anti-human CYP2J2, anti-human CYP3A4, and anti-human CYP3A5 antibodies. The filters were then incubated with the secondary antibody (1:5000). The proteins of interest were visualized using an ECL Western blotting detection reagent with the Chemi-Doc imaging system (Bio-Rad Laboratories). The optical density of the bands was quantified using Image J software (National Institutes of Health, Bethesda, MD). Standard curves for quantification were generated using recombinant P450 of cynomolgus monkey, and the amount of each P450/well was calculated relative to the standard curve. For cynomolgus CYP1A, CYP2A, CYP2C9/19, and CYP2D, the recombinant CYP1A1, CYP2A23, CYP2C9, and CYP2D17 proteins of cynomolgus monkey were used as standard for quantification, respectively. The amount of each P450 protein/lane was divided by the amount of total protein loaded to determine specific content.
Results
The expression of CYP1-3 proteins in the small intestines of 35 cynomolgus monkeys was quantified by immunoblotting using the selective antibodies that were evaluated for their specificities against each cynomolgus P450 protein in our previous study (Uehara et al. 2011). These antibodies enabled us to detect cynomolgus CYP1A1/2, CYP1D1, CYP2A23/24/26, CYP2B6, CYP2C9/19, CYP2C76, CYP2D17/44, CYP2E1, CYP2J2, CYP3A4, and CYP3A5 separately. Figure 1 shows the immunoblots of standards and five representative samples of small intestine microsomes. CYP2D, CYP2J2, CYP3A4, and CYP3A5 were detected in all 35 animals while CYP1A and CYP2C9/19 were detected in 31 and 17 animals, respectively. CYP1D1, CYP2A, CYP2B6, CYP2C76, and CYP2E1 were not detected in any of the 35 animals examined (Figure 1). Therefore, CYP1A, CYP2C9/19, CYP2D, CYP2J2, CYP3A4, and CYP3A5 were quantified in small intestine samples.
Figure 1.
Immunoblotting of cynomolgus monkey small intestine microsomes.
The amounts of recombinant proteins (standards), cynomolgus CYP1A1, CYP1D1, CYP2A23, CYP2B6, CYP2C9, CYP2C76, CYP2D17, CYP2E1, CYP2J2, CYP3A4, and CYP3A5, ranged from 0.01 to 0.1, 0.025 to 0.15, 0.025 to 0.4, 0.025 to 0.2, 0.01 to 0.15, 0.01 to 0.1, 0.0005 to 0.005, 0.01 to 0.2, 0.001 to 0.3, 0.005 to 0.2, and 0.01 to 0.2 pmol, respectively. Small intestine microsomes were analyzed for each animal at 30 μg of microsomal protein for CYP1A (1/2), CYP1D1, CYP2A(23/24/26), CYP2C9/19, CYP2C76, CYP2D (17/44), CYP2E1, and CYP2J2, 10 μg of microsomal protein for CYP3A4, or 5 μg of microsomal protein for CYP3A5. The results are shown for five representative animals, and for the pooled sample (prepared from 17 males and 18 female microsomes) in triplicate.
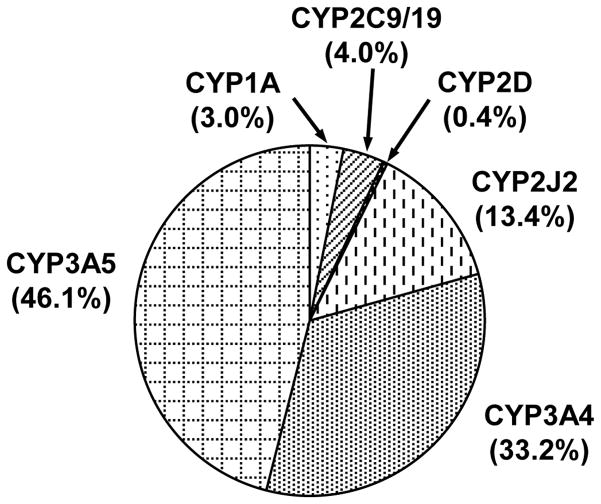
Among the CYP1-3 proteins analyzed, CYP3A5 was the most abundant, and CYP3A5 content averaged 12.6 pmol/mg protein, with a range of 9.05 to 17.3 pmol/mg protein (Table 1). The second most abundant P450 was CYP3A4 with an average content of 8.53 pmol/mg protein, ranging from 4.43 to 11.9 pmol/mg protein (Table 1). The content of total CYP3A (CYP3A4 + CYP3A5) averaged 21.1 pmol/mg protein, ranging from 16.4 to 30.0 pmol/mg protein (Table 1), making it the most abundant P450 of the CYP1-3 proteins in cynomolgus monkey small intestine, followed by CYP2J2, CYP2C9/19, CYP1A, and CYP2D, which averaged 4.23, 1.14, 1.04, and 0.09 pmol/mg protein, respectively. Analysis of pooled samples (containing 17 males and 18 females) showed the same trend (Table 1).
Table 1.
Individual P450 content immunoquantified in cynomolgus monkey small intestine.
| P450 | P450 content (Mean ± S.D.)
|
n | Range | Detection limit | |||
|---|---|---|---|---|---|---|---|
| Pooled | Individuals | Males | Females | ||||
| pmol/mg protein | pmol/mg protein | ||||||
| CYP1A | 0.80 ± 0.04 | 1.04 ± 0.39 | 1.03 ± 0.40 | 1.05 ± 0.41 | 31 | 0.35 – 1.79 | 0.33 |
| CYP1D | BDL | BDL | BDL | BDL | N.A. | BDL | 0.83 |
| CYP2A | BDL | BDL | BDL | BDL | N.A. | BDL | 0.83 |
| CYP2B6 | BDL | BDL | BDL | BDL | N.A. | BDL | 0.83 |
| CYP2C9/19 | 1.04 ± 0.05 | 1.14 ± 0.56 | 1.21 ± 0.69 | 1.04 ± 0.31 | 17 | 0.52 – 3.02 | 0.33 |
| CYP2C76 | BDL | BDL | BDL | BDL | N.A. | BDL | 0.33 |
| CYP2D | 0.11 ± 0.01 | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.09 ± 0.03 | 35 | 0.03 – 0.15 | 0.02 |
| CYP2E1 | BDL | BDL | BDL | BDL | N.A. | BDL | 0.33 |
| CYP2J2 | 3.51 ± 0.11 | 4.23 ± 2.01 | 3.86 ± 1.62 | 4.59 ± 2.31 | 35 | 1.40 – 8.07 | 0.03 |
| CYP3A4 | 8.71 ± 0.74 | 8.53 ± 1.84 | 7.40 ± 1.58 | 9.59 ± 1.41 | 35 | 4.43 – 11.9 | 0.50 |
| CYP3A5 | 12.1 ± 0.4 | 12.6 ± 2.2 | 12.1 ± 2.3 | 13.1 ± 2.0 | 35 | 9.05 – 17.3 | 2.00 |
| CYP3A (3A4+3A5) | 20.8 | 21.1 ± 3.2 | 19.5 ± 2.9 | 22.7 ± 2.8 | 35 | 16.4 – 30.0 | N.A. |
| Total | 26.3 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
N.A., not applicable
BDL, below detection limit
Each P450 of cynomolgus monkey was immunoquantified using selective antibodies as described in Materials and Methods.
Pooled microsomes were prepared from 17 male and 18 female microsomes.
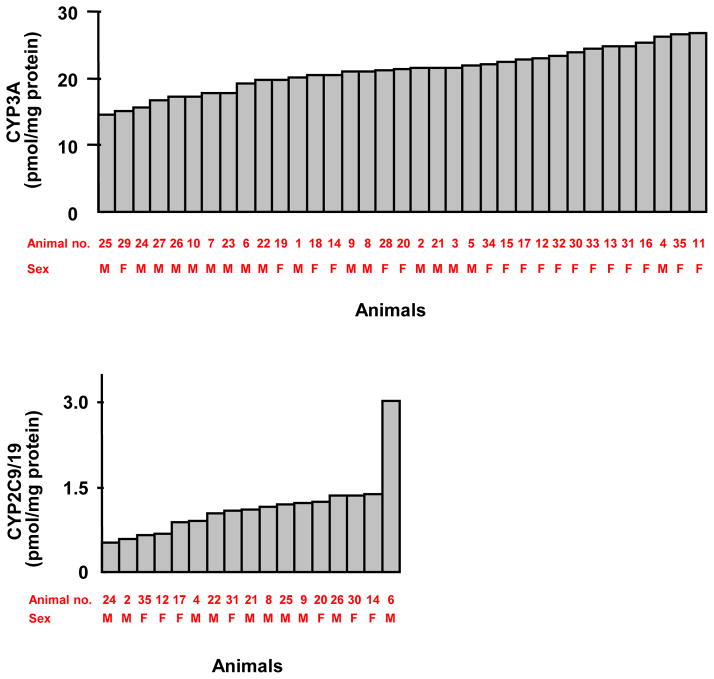
Due to the limited quantities of microsomal samples which were too dilute, total P450 content of each microsomal sample could not be measured by difference spectra. Hence, the content of each P450 was expressed as a percentage of total immunoquantified CYP1-3 proteins; 79.3% (CYP3A), 13.4% (CYP2J2), 4.0% (CYP2C9/19), 3.0% (CYP1A), and 0.4% (CYP2D) (Figure 2). P450 content varied 2- to 6-fold in the animals in which expression was detectable. CYP3A content was the least variable (1.8-fold) of the CYP1-3 proteins measured, followed by CYP3A5 (1.9-fold) and CYP3A4 (2.7-fold) (Table 1). CYP2C9/19 content was the most variable (5.8-fold) of the CYP1-3 proteins (Table 1); however, the variability decreased (2.8-fold) when the animal with the greatest expression (3.02 pmol/mg protein) was excluded from the analysis (Fig. 3). Individual CYP3A and CYP2C9/19 data are shown in Figure 3. There were no significant sex differences in P450 content (Table 1).
Figure 2.
Pie graph of P450 in cynomolgus monkey small intestine. Mean expression of each P450 is indicated as a percentage of total immunoquantified CYP1-3 protein.
Figure 3.
Variations in P450 content in cynomolgus monkeys. The amounts of CYP3A (CYP3A4 + CYP3A5) and CYP2C9/19 immunoquantified in 35 cynomolgus monkey small intestines are shown. The x-axis represents individual jejunum samples, for which animal numbers and sex are also indicated. Of 35 animals analyzed, the CYP3A amount varied the least. In contrast, the CYP2C9/19 amount varied the most among 17 animals with quantifiable CYP2C9/19, but the variations were greatly smaller when the animal with the greatest expression was excluded from the analysis.
Discussion
Cynomolgus monkey is an important primate species for drug metabolism studies. Although the small intestine is an important site for first-pass metabolism of numerous drugs and toxic xenobiotics, the P450 content of cynomolgus monkey small intestine has not been determined. Such information could increase our understanding of first-pass metabolism of drugs in the small intestine. Therefore, individual P450 expression levels, in small intestine microsomes from 35 cynomolgus monkeys, were measured by immunoblotting using selective P450 antibodies.
Among the CYP1-3 proteins, CYP3A (CYP3A4 + CYP3A5) was expressed most abundantly (79.3% of total immunoquantified CYP1-3 proteins) in cynomolgus monkey small intestine (Figure 2), similar to that of human (80%) (Paine et al. 2006). In all 35 animals, CYP3A5 was most abundant of the CYP1-3 proteins in cynomolgus monkey small intestine, followed by CYP3A4 (Table 1), indicating that CYP3A4 and CYP3A5 might play important roles for drug metabolism in small intestine. Because cynomolgus CYP3A is also most abundant in liver (Uehara et al. 2011), CYP3A is important for overall first-pass metabolism of drugs in cynomolgus monkeys. CYP3A content in small intestine was lower in cynomolgus monkey (21 pmol/mg protein) (Table 1) than in human (74 pmol/mg protein) (Paine et al. 2006). A previous study reported that testosterone 6β-hydroxylation in small intestine (jejunum) is comparable in cynomolgus monkeys and humans (Nakanishi et al. 2011). This could be attributable to the higher metabolic activity of CYP3A enzymes in cynomolgus monkeys compared to that in human (Iwasaki et al. 2010). The inter-individual variations of cynomolgus CYP3A content were 2.7-fold (CYP3A4) and 1.9-fold (CYP3A5) (Table 1), which were smaller than the inter-individual variations of human CYP3A content, 17-fold (CYP3A4) and 5.1-fold (CYP3A5) (Paine et al. 2006). The smaller inter-individual variations of cynomolgus CYP3A content is possibly due to the similarities in their genetic background, considering that cynomolgus monkeys are bred in colonies. The abundant expression of CYP3A in small intestine and liver of cynomolgus monkey, similar to human, suggests that cynomolgus monkey might be an ideal animal species in studies of CYP3A-mediated drug metabolism.
CYP2J2 was the second most abundant P450 among the CYP1-3 proteins in cynomolgus monkey small intestine, representing 13.4% of total immunoquantified CYP1-3 protein (Figure 2). In human small intestine, CYP2J2 is involved in first-pass metabolism of ebastine (Hashizume et al. 2002) and astemizole (Matsumoto et al. 2002), suggesting that CYP2J2 plays roles for drug metabolism in small intestine. CYP2J2 content in small intestine measured in this study was more abundant in cynomolgus monkey (3.51 pmol/mg protein) (Table 1) than in human (~1 pmol/mg protein) (Paine et al. 2006; Wang et al. 2007). Moreover, the inter-individual CYP2J2 variation was smaller in cynomolgus monkey (5.8-fold) in this study (Table 1), than in human CYP2J2 (15.5-fold) (Paine et al. 2006). Therefore, CYP2J2 appears to be expressed more abundantly and more stably in cynomolgus monkey small intestine than in human small intestine. In human, CYP2J2 metabolizes a number of CYP3A4 substrates, due to a large active site volume, similar to CYP3A4 (Lee et al. 2010), raising the possibility that CYP3A4 substrates are more extensively metabolized in cynomolgus monkey small intestine, by CYP2J2 and CYP3A4. Indeed, cynomolgus monkeys show poor oral bioavailability for some CYP3A4 substrates, such as nifedipine, verapamil, simvastatin, and methotrexate (Takahashi et al. 2008; Ogasawara et al. 2009; Nishimuta et al. 2011). It is of great importance to investigate drug-metabolizing profiles of cynomolgus CYP2J2 using CYP3A substrates.
CYP2C was the third most abundant P450 among the CYP1-3 proteins in cynomolgus monkey small intestine; CYP2C9/19 represented 4.0% of total immunoquantified CYP1-3 protein (Figure 2). Although cynomolgus CYP2C9 and CYP2C19 could not be quantified separately in this study, CYP2C19 is expressed much more abundantly (> 20-fold) in small intestine than CYP2C9 (Nakanishi et al. 2010). Therefore, CYP2C19 most likely accounts for the bulk of the CYP2C9/19 content measured in this study, in contrast to human small intestine, where CYP2C9 is more abundant (approximately 7-fold) than CYP2C19 (Paine et al. 2006).
In cynomolgus monkeys, CYP2C9/19 content (1.04 pmol/mg protein) in small intestine (Table 1) was one-tenth of the CYP2C9/19 content (11 pmol/mg protein) in liver (Uehara et al. 2011). Catalytic activities mediated by cynomolgus CYP2C9/19, such as tolbutamide methylhydroxylation, diclofenac 4′-hydroxylation, and S-mephenytoin 4′-hydroxylation, are much lower in small intestine than in liver (Nakanishi et al. 2011). These results suggest that small intestine would make a small contribution to first-pass metabolism of CYP2C9/19-metabolized drugs.
CYP1A (1/2) represented 3.0% of total immunoquantified CYP1-3 protein in cynomolgus monkey small intestine (Figure 2). CYP1A content varied 5.1-fold among 31 animals quantifiable in this study (Table 1), partly accounting for the inter-individual variations of 7-ethoxyresorufin O-deethylation in cynomolgus monkey small intestine (Nakanishi et al. 2013). 7-Ethoxyresorufin O-deethylation is mediated by CYP1D1 as well as CYP1A1/2 (Uno et al. 2011b), but CYP1D1 protein was not detected in small intestine (Figure 1). Similarly, in human small intestine, a large inter-individual variation (88-fold) is observed in 7-ethoxyresorufin O-deethylation (Paine et al. 1999), which might be attributable to variations in CYP1A protein content (Paine et al. 2006). Cynomolgus CYP1A protein was not detected in liver in our previous study (Uehara et al. 2011), but was detected in the small intestine of most animals in this study. If this implies more abundant CYP1A content in small intestine than in liver, intestine might play important roles in first-pass metabolism of CYP1A substrates.
CYP2D (17/44), highly similar to human CYP2D6, represented 0.4% of total immunoquantified CYP1-3 protein in cynomolgus monkey small intestine (Figure 2). CYP2D content (0.11 pmol/mg protein) (Table 1) is much lower (> 30-fold) in small intestine than in liver (3.2 pmol/mg protein) (Uehara et al. 2011). The difference of CYP2D content might account for the slower rate of bufuralol 1′-hydroxylation in small intestine than in liver (Nakanishi et al. 2011), because bufuralol 1′-hydroxylation is catalyzed by cynomolgus CYP2D17/44 (Uno et al. 2010b). CYP2D content in cynomolgus monkey liver is only 3% (Uehara et al. 2011), together with low CYP2D content in the small intestine, suggest a minor contribution of CYP2D to overall first-pass metabolism in cynomolgus monkeys.
In conclusion, CYP1-3 proteins were analyzed, and CYP3A was found to be the most abundant (79.3% of total immunoquantified CYP1-3 proteins) in cynomolgus monkey small intestine, followed by CYP2J (13.4%), CYP2C9/19 (4.0%), CYP1A (3.0%), and CYP2D (0.4%). Inter-individual variation of P450 content was 2- to 6-fold in cynomolgus monkey, which is generally less than in human (5- to 17-fold). These findings should help deepen our understanding of drug metabolism, especially first-pass metabolism, in cynomolgus monkey small intestine.
Acknowledgments
Authors greatly thank Dr. Ryoichi Nagata, Dr. Koichiro Fukuzaki, and Mr. Masahiro Utoh for their support to this work, and Mr. Patrick Gray for reviewing the manuscript.
Footnotes
Declaration of interest
This work was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Imaoka S, Mise M, Terauchi Y, Fujii T, Miyazaki H, Kamataki T, Funae Y. Involvement of CYP2J2 and CYP4F12 in the metabolism of ebastine in human intestinal microsomes. J Pharmacol Exp Ther. 2002;300:298–304. doi: 10.1124/jpet.300.1.298. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Murayama N, Koizumi R, Uno Y, Yamazaki H. Comparison of cytochrome P450 3A enzymes in cynomolgus monkeys and humans. Drug Metab Pharmacokinet. 2010;25:388–91. doi: 10.2133/dmpk.dmpk-10-nt-022. [DOI] [PubMed] [Google Scholar]
- King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, Spiecker M, Liao JK, Mohrenweiser H, Zeldin DC. Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol. 2002;61:840–52. doi: 10.1124/mol.61.4.840. [DOI] [PubMed] [Google Scholar]
- Lee CA, Neul D, Clouser-Roche A, Dalvie D, Wester MR, Jiang Y, Jones JP, III, Freiwald S, Zientek M, Totah RA. Identification of novel substrates for human cytochrome P450 2J2. Drug Metab Dispos. 2010;38:347–56. doi: 10.1124/dmd.109.030270. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hirama T, Matsubara T, Nagata K, Yamazoe Y. Involvement of CYP2J2 on the intestinal first-pass metabolism of antihistamine drug, astemizole. Drug Metab Dispos. 2002;30:1240–5. doi: 10.1124/dmd.30.11.1240. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Matsushita A, Matsuno K, Iwasaki K, Utoh M, Nakamura C, Uno Y. Regional distribution of cytochrome P450 mRNA expression in the liver and small intestine of cynomolgus monkeys. Drug Metab Pharmacokinet. 2010;25:290–7. doi: 10.2133/dmpk.25.290. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Matsushita A, Matsuno K, Iwasaki K, Utoh M, Nakamura C, Uno Y. Regional distribution of drug-metabolizing enzyme activities in the liver and small intestine of cynomolgus monkey. Drug Metab Pharmacokinet. 2011;26:288–94. doi: 10.2133/dmpk.DMPK-10-NT-101. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Yamashita H, Yoshikawa T, Tominaga T, Nojiri K, Sunaga Y, Muneoka A, Iwasaki K, Utoh M, Nakamura C, Yamazaki H, Uno Y. Cytochrome P450 metabolic activities in the small intestine of cynomolgus macaques bred in Cambodia, China, and Indonesia. Drug Metab Pharmacokinet. 2013 doi: 10.2133/dmpk.dmpk-13-nt-031. (In Press) [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Nishimuta H, Sato K, Mizuki Y, Yabuki M, Komuro S. Species differences in intestinal metabolic activities of cytochrome P450 isoforms between cynomolgus monkeys and humans. Drug Metab Pharmacokinet. 2011;26:300–6. doi: 10.2133/dmpk.DMPK-10-SH-119. [DOI] [PubMed] [Google Scholar]
- Ogasawara A, Utoh M, Nii K, Ueda A, Yoshikawa T, Kume T, Fukuzaki K. Effect of oral ketoconazole on oral and intravenous pharmacokinetics of simvastatin and its acid in cynomolgus monkeys. Drug Metab Dispos. 2009;37:122–8. doi: 10.1124/dmd.108.022574. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–8. [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–6. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine MF, Schmiedlin-Ren P, Watkins PB. Cytochrome P-450 1A1 expression in human small bowel: interindividual variation and inhibition by ketoconazole. Drug Metab Dispos. 1999;27:360–4. [PubMed] [Google Scholar]
- Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23. [PubMed] [Google Scholar]
- Takahashi M, Washio T, Suzuki N, Igeta K, Fujii Y, Hayashi M, Shirasaka Y, Yamashita S. Characterization of gastrointestinal drug absorption in cynomolgus monkeys. Mol Pharm. 2008;5:340–8. doi: 10.1021/mp700095p. [DOI] [PubMed] [Google Scholar]
- Uehara S, Murayama N, Nakanishi Y, Zeldin DC, Yamazaki H, Uno Y. Immunochemical detection of cytochrome P450 enzymes in liver microsomes of 27 cynomolgus monkeys. J Pharmacol Exp Ther. 2011;339:654–61. doi: 10.1124/jpet.111.185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara S, Murayama N, Yamazaki H, Uno Y. A novel CYP2A26 identified in cynomolgus monkey liver metabolizes coumarin. Xenobiotica. 2010;40:621–9. doi: 10.3109/00498254.2010.501118. [DOI] [PubMed] [Google Scholar]
- Uno Y, Fujino H, Iwasaki K, Utoh M. Macaque CYP2C76 encodes cytochrome P450 enzyme not orthologous to any human isozymes. Curr Drug Metab. 2010a;11:142–52. doi: 10.2174/138920010791110854. [DOI] [PubMed] [Google Scholar]
- Uno Y, Fujino H, Kito G, Kamataki T, Nagata R. CYP2C76, a novel cytochrome P450 in cynomolgus monkey, is a major CYP2C in liver, metabolizing tolbutamide and testosterone. Mol Pharmacol. 2006;70:477–86. doi: 10.1124/mol.106.022673. [DOI] [PubMed] [Google Scholar]
- Uno Y, Hosaka S, Matsuno K, Nakamura C, Kito G, Kamataki T, Nagata R. Characterization of cynomolgus monkey cytochrome P450 (CYP) cDNAs: Is CYP2C76 the only monkey-specific CYP gene responsible for species differences in drug metabolism? Arch Biochem Biophys. 2007;466:98–105. doi: 10.1016/j.abb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Uno Y, Iwasaki K, Yamazaki H, Nelson DR. Macaque cytochromes P450: nomenclature, transcript, gene, genomic structure, and function. Drug Metab Rev. 2011a;43:346–61. doi: 10.3109/03602532.2010.549492. [DOI] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Kohara S, Murayama N, Yamazaki H. Cynomolgus monkey CYP2D44 newly identified in liver, metabolizes bufuralol and dextromethorphan. Drug Metab Dispos. 2010b;38:1486–92. doi: 10.1124/dmd.110.033274. [DOI] [PubMed] [Google Scholar]
- Uno Y, Uehara S, Murayama N, Yamazaki H. CYP1D1, pseudogenized in human, is expressed and encodes a functional drug-metabolizing enzyme in cynomolgus monkey. Biochem Pharmacol. 2011b;81:442–50. doi: 10.1016/j.bcp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Wang MZ, Wu JQ, Bridges AS, Zeldin DC, Kornbluth S, Tidwell RR, Hall JE, Paine MF. Human enteric microsomal CYP4F enzymes O-demethylate the antiparasitic prodrug pafuramidine. Drug Metab Dispos. 2007;35:2067–75. doi: 10.1124/dmd.107.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–21. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]