Abstract
Introduction
Recurrent atrial fibrillation (AF) after ablation is associated with reconnection of initially isolated pulmonary vein (PV) trigger sites. Substrates are often targeted in addition to PVI, but it is unclear how substrates progress over time. We studied if substrates in recurrent AF are conserved or have developed de novo from pre-ablation AF.
Methods and Results
Of 137 patients undergoing Focal Impulse and Rotor Mapping (FIRM) at their index procedure for AF, 29 consecutive patients (60 ± 8 years, 79% persistent) recurred and were also mapped at repeat procedure (21 ± 20 months later) using carefully placed 64-pole baskets and RhythmView™ (Topera, Menlo Park, CA, USA) to identify AF sources and disorganized zones. Compared to index AF, recurrent AF had a longer cycle length (177 ± 21 vs. 167 ± 19 milliseconds, P = 0.01). All patients (100%) had 1 or more conserved AF rotors between procedures with surrounding disorganization. The number of sources was similar for recurrent AF post-PVI versus index AF (3.2 ±1.4 vs. 3.1 ± 1.0, P = 0.79), but was lower for recurrent AF after FIRM+PVI versus index AF (4.4 ± 1.4 vs. 2.9 ± 1.7, P = 0.03). Overall, 81% (61/75) of AF sources lay in conserved regions, while 19% (14/75) were detected de novo.
Conclusion
Electrical propagation patterns for recurrent AF after unsuccessful ablation are similar in individual patients to their index AF. These data support temporospatial stability of AF substrates over 1–2 years. Trials should determine the relative benefit of adding substrate mapping and ablation to PVI for recurrent AF.
Keywords: atrium, fibrillation, FIRM ablation, focal sources, human, rotors
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia world-wide,1 for which ablation is increasingly performed. If AF recurs, it is common to see reconnection of initially isolated trigger sites near pulmonary veins (PV).2 However, while ablation for AF often includes empirical substrate ablation,2–4 it is unstudied if electrical propagation is similar or differs between post-ablation recurrent AF and initial AF. This hinders the design of optimal lesion sets for recurrent paroxysmal or persistent AF.
We hypothesized that biatrial substrates for AF may be conserved in patients in whom AF recurs post-ablation. This hypothesis was motivated by dynamic linking of electrograms5 and ECG reproducibility of human AF for prolonged time periods.6 Optical mapping reveals localized rotors in sheep,7 canine,8 and human9 AF that should also conserve electrical propagation patterns in AF over time. It is unclear why rotors are undetected by traditional mapping of human longstanding persistent AF,10 goats,11 and other models, but this may reflect uncertainties of discerning local from far field activity in AF from traditional electrogram analysis.12 Some studies that fail to show human AF rotors are difficult to interpret, showing atrial cycle lengths of 250–500 milliseconds (dominant frequency 2–4 Hz) in AF, likely due to analytic error,13 or map small regions that could miss localized sources.10 Focal Impulse and Rotor Modulation (FIRM)14,15 reveals stable rotors in human AF that are similar to those revealed by optical mapping in human hearts,9 where ablation may produce good clinical success.16–19
We studied whether electrical substrates (organized sources and disorganized zones) are conserved if AF recurs after failed prior ablation in individual patients, referenced to pre-ablation AF. We performed detailed analysis of FIRM maps from carefully placed basket catheters in index and repeat procedures separated by months to years, in patients with paroxysmal and persistent AF.
Methods
Summary of Study Design and Enrollment
Of 137 patients who underwent FIRM mapping to identify AF rotor and focal sources15 at the University of California/VA San Diego Medical Centers, we identified 29 consecutive patients with recurrent atrial arrhythmias also undergoing FIRM mapping at repeat procedure. Patients were aged ≥21 years, with AF refractory to or intolerant of ≥ 1 class I or III antiarrhythmic medication2 who provided written informed consent for this study. Patients undergoing FIRM-only ablation without PVI at index procedure20 are described elsewhere.
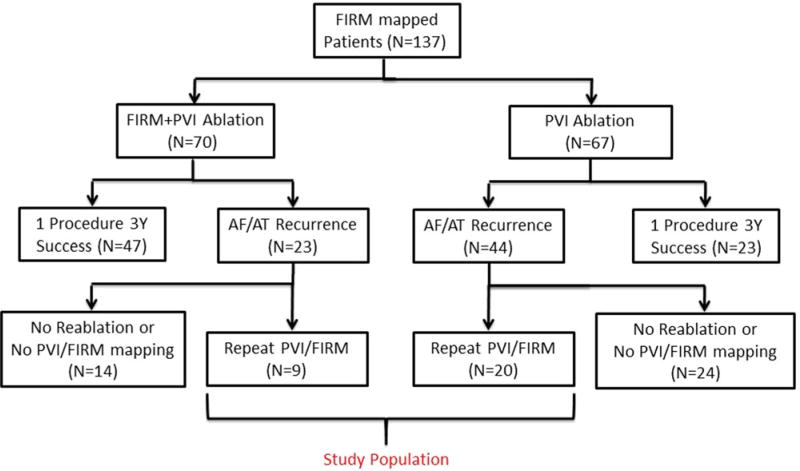
After FIRM mapping, index ablation comprised PVI (plus a left atrial roof line for patients with persistent AF), or FIRM-guided ablation plus PVI. Follow-up monitoring met guidelines.2 Patients with recurrent arrhythmias after a 3-month blanking period were offered repeat ablation. We report the N = 29 patients undergoing FIRM mapping at index and repeat procedure, for a total of 58 paired procedures in 3 years of follow-up. Figure 1 shows the patient flow.
Figure 1.
Patient flow into the study. For a high quality, full color version of this figure, please see Journal of Cardiovascular Electrophysiology’s website: www.wileyonlinelibrary.com/journal/jce
Substrate Localization—Index Procedure
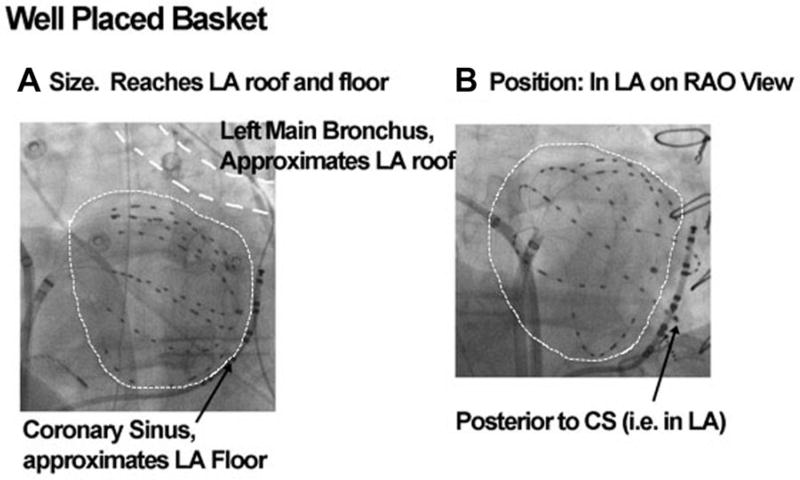
Antiarrhythmic medications were discontinued for ≥5 half-lives, amiodarone for ≥60 days (median 365 days) prior to each procedure. Heparin was infused to maintain activated clotting time >350 seconds. A 64-pole basket catheter (Constellation™, Boston Scientific, Natick, MA, USA) was positioned within the right atrium (RA), then advanced transseptally to the left atrium (LA). To prevent erroneous analysis, we ensured good basket placement (Fig. 2), avoiding undersized or malpositioned baskets in recent studies.13 The 4–6 mm interelectrode and <1 cm interspline spacing of baskets near their poles is sufficient to resolve predicted human AF sources, which are often larger than the theoretical minimum as we have described,21,22 and as shown optically.9 We mapped spontaneous and/or induced AF, which is similar to spontaneous AF23,24 (Table 1) and recorded pulmonary vein signals. Electrograms were filtered at 0.05–500 Hz and digitized at 1 kHz. Only electrodes with discernible atrial signals are used to create FIRM maps,15 with other sites marked black.
Figure 2.
Careful basket placement. Left atrium outlined by dashed white lines, with well-placed basket (A) sized to approximate roof (marked by left main bronchus) and floor (CS). (B) Positioning within LA, confirmed as posterior to the coronary sinus.
TABLE 1.
Clinical Characteristics of Population
| Characteristic | Overall | FIRM+ Conventional Index Case |
Conventional Index Case |
P |
|---|---|---|---|---|
| No. FIRM mapped | 29 | 9 | 20 | - |
| Age (years) | 60 ± 8 | 61 ± 6 | 60 ± 9 | 0.81 |
| AF type | 0.63 | |||
| Paroxysmal | 1 | 5 | ||
| Persistent | 8 | 15 | ||
| Presenting rhythm | 0.31 | |||
| AF | 19 | 7 | 12 | |
| Sinus | 10 | 2 | 8 | |
| LA diameter (mm) | 49 ± 8 | 55 ± 8 | 45 ± 6 | <0.01 |
| Left ventricular | 53 ± 12 | 45 ± 14 | 57 ± 8 | <0.01 |
| Comorbid conditions | ||||
| Hypertension | 21 | 7 | 14 | 1.00 |
| Diabetes | 11 | 5 | 6 | 0.24 |
| BMI (mean) | 33 | 33 | 33 | 0.93 |
| Obstructive sleep apnea | 17 | 7 | 10 | 0.23 |
| Congestive heart failure | 15 | 7 | 8 | 0.11 |
| GFR, mL/min (mean) | 78 | 81 | 76 | 0.50 |
| Magnesium, mg/dL (mean) | 2 | 2 | 2 | 0.20 |
Statistical comparison between groups was done using Mann-Whitney U for continuous variables and Fisher exact test for contingency tables.
P < 0.05 compared to initial case.
Briefly, FIRM-mapping algorithms identify electrical rotors as phase singularities with surrounding fibrillatory conduction (disorganization). Rotors were considered AF sources if present at the same location in several epochs over 5 minutes, typically within areas of ≈3 cm2),25 excluding transient rotational and focal activity.10,26
Index Ablation and Follow-Up
At index procedure, all patients received conventional ablation with or without FIRM (which, if done, was performed first). Radiofrequency energy was delivered with a 3.5 mm tip irrigated (Thermocool, Biosense-Webster, Diamond Bar, CA, USA) or, in heart failure subjects, 8 mm tip (Blazer, Boston Scientific) catheter. AF cycle length (CL) was measured as the mean of 10 cycles in the coronary sinus.27
In FIRM-guided subjects, ablation commenced with lesions to cover the AF source precession area.25 Ablation lasted < 10 minutes (typically 5 minutes) per source.15 Conventional ablation2 was standardized and comprised wide area isolation of left and right PV pairs verified with a circular mapping catheter (Lasso™, Biosense-Webster). In persistent AF, an LA roof line was also performed. Clinically relevant atrial tachycardias, including focal and macroreentrant atrial tachycardias in the left atrial roof, mitral isthmus or CTI, were appropriately ablated. No other ablation was performed. Table 2 summarizes the ablation performed in each case.
TABLE 2.
AF Triggers and Substrates Between Index and Repeat Procedures
| Characteristic | All Patients (n = 29) |
FIRM+Conventional Index Case (n = 9) |
Conventional Index Case (n = 20) |
P |
|---|---|---|---|---|
| Index case | ||||
| Number of concurrent sources | 3.2 ± 1.4 | 4.4 ± 1.0 | 3.2 ± 1.4 | 0.02 |
| No. of left atrial AF sources | 2.3 ± 0.7 | 2.3 ± 0.7 | 1.9 ± 0.6 | 0.10 |
| Total left atrial sources | 63 | 24 | 39 | |
| No. of right atrial AF sources | 1.9 ± 0.6 | 2.0 ± 0.5 | 1.8 ± 0.6 | 0.49 |
| Total right atrial sources | 34 | 16 | 18 | |
| No. PV connected | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.95 |
| AF cycle length, milliseconds | 167 ± 19 | 165 ± 18 | 168 ± 19 | 0.67 |
| Ablation | ||||
| No. PV isolated | 3.9 ± 0.4 | 3.9 ± 0.3 | 3.9 ± 0.4 | 0.95 |
| Linear ablation | 26 | 7 | 19 | 0.45 |
| Roof | 24 | 7 | 17 | |
| Mitral isthmus | 1 | 0 | 1 | |
| Cavotricuspid isthmus | 9 | 0 | 9 | |
| Repeat case† | (n = 26) | (n = 8) | (n = 18) | |
| Time since index case/months | 21.4 ± 19.9 | 11.9 ± 4.8 | 25.7 ± 22.6 | 0.08 |
| No. of concurrent sources | 3.0 ± 1.2* | 2.9 ± 1.7* | 3.1 ± 1.0 | 0.73 |
| No. of left atrial AF sources | 2.0 ± 0.8* | 1.9 ± 0.9* | 1.9 ± 0.7 | 0.80 |
| Conserved versus index | 37/46 | 10/13 | 27/33 | 0.70 |
| New versus index | 9/46 | 3/13 | 6/33 | |
| No. of right atrial AF sources | 1.8 ± 0.7 | 1.7 ± 0.5 | 1.9 ± 0.7 | 0.51 |
| Conserved versus index | 24/29 | 9/10 | 15/19 | 0.63 |
| New versus index | 5/29 | 1/10 | 4/19 | |
| No. PV connected | 2.7 ± 1.7* | 3.0 ± 1.5* | 2.5 ± 1.7* | 0.46 |
| AF cycle length, milliseconds | 177 ± 21* | 180 ± 19* | 176 ± 23 | 0.62 |
Statistical analyses were done using Mann–Whitney U for unmatched groups, Wilcoxon signed rank test for matched pairs, and Fisher exact test for contingency tables.
P < 0.05 compared to initial case.
3/29 patients had atrial tachycardias and were non-inducible for AF.
Repeat ablation was not permitted within the 3-month blanking period, after which recurrences were evaluated using implanted ECG monitors when possible or external event monitors, quarterly and also for symptoms.
This report focuses on patients with recurrent arrhythmias after the blanking period, defined as > 1% burden on implanted ECG monitors or >30 seconds on intermittent monitors.2 Patients who refused or were refractory to antiarrhythmic treatment were offered repeat ablation with FIRM mapping. The N = 29 patients with FIRM mapping at index and repeat procedures are included in this report.
Substrate Localization at Repeat Procedure
Substrate localization comprised FIRM mapping in patients with recurrent AF (n = 26) and patients with organized atrial tachycardia (n = 3), included to assess their potential mechanistic relationship to AF sources and/or prior ablation. The strategy for repeat ablation in this series was discussed case-by-case with each patient, and included conventional ablation alone, FIRM plus conventional ablation, or FIRM ablation alone.
Determination if AF Sources are Conserved or De Novo
We compared numbers and locations of AF sources for initial/repeat procedures in blinded fashion and random sequence. For each case, anatomic regions harboring each AF source were determined from FIRM-movies relative to electrode locations on electroanatomic images (NavX, St. Jude Medical, Sylmar, CA, USA; Carto, Biosense-Webster), lesion markers and structures such as pulmonary and caval veins and atrioventricular valve annuli.
AF source locations were then compared for initial/repeat procedures. Anatomic regions harboring AF sources were defined as spatially conserved if they lay within ≈ 1 cm on electroanatomic shells between procedures (see Fig. 3A, B), assigned by majority vote of 5 investigators (GGL, TB, AAS, DEK, SMN). The interobserver correlation, Kappa was 0.89. There was no attempt to resolve disagreements. Sources not meeting these criteria were considered “de novo.”
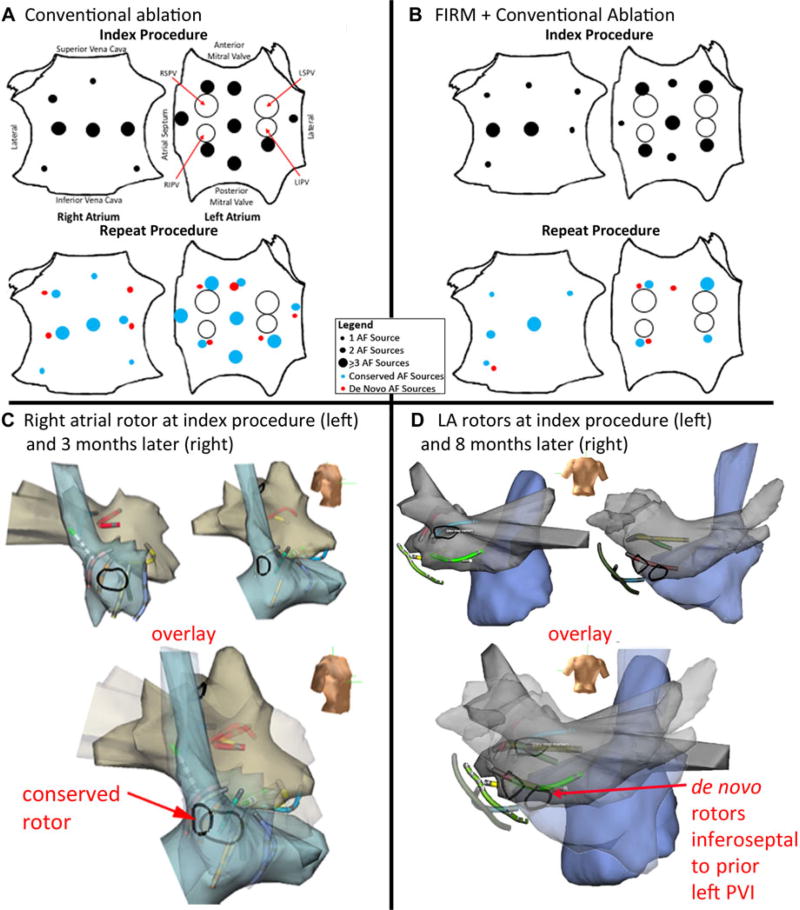
Figure 3.
Electroanatomical shells of conserved and de novo rotor sites, with summary of rotor site distributions for index/repeat procedures. A. Right atrial shells for index/repeat procedures (3 months apart, aligned by basket splines) show rotor at conserved lateral site. B. Left atrial shells for index/repeat procedures (8 months apart) show de novo rotor in posterior left atrium. C. Biatrial schematics show distribution of AF sources at index (top) and repeat (bottom) procedures for conventional ablation, with similar number of sources. D. Sources in the FIRM + conventional ablation group (top: index; bottom: repeat procedures); number of sources is lower at repeat procedure.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile-range (IQR) as appropriate. Comparisons between 2 groups were made with Wilcoxon signed ranks test (matched pairs) and Mann–Whitney U-test (unmatched) and summarized with means and standard deviations. To estimate the significance of source conservation, electrode coordinates of each AF source were marked on the electrode grid, and considered conserved if coordinates at index/repeat procedures overlapped or were immediately adjacent. A non-parametric binomial probability test was applied. Nominal values are expressed as n (%) and compared with Fisher exact tests (e.g., numbers with PV potentials present).
Results
This study describes a total of 58 matched index/repeat PV and substrate mapping procedures in N = 29 patients (demographics in Table 1).
Index AF
Table 2 summarizes the results of mapping. At index procedure, each patient showed AF sources and PV potentials. Sources were 65% left and 35% right atrial (100% rotors). Thirty-two of 97 (33%) of rotors localized to the region of wide area PVI (Fig. 3C,D).
Recurrent AF or Atrial Tachycardia
Each patient with recurrent AF (26/29 patients) showed sources and 25/26 showed at least 1 reconnected PV. AF CL was longer in recurrent AF than index AF (176 ± 21 vs. 167 ± 19 milliseconds, P = 0.01) (excluding 3 patients with recurrent AT).
As expected, there were fewer connected PVs in recurrent than index AF (2.7 ± 1.7 vs. 3.9 ± 0.4, P < 0.01). In patients after PVI, the number of AF rotors was similar between procedures (3.2 ± 1.4 vs. 3.1 ± 1.0, P = 0.80) consistent with lack of rotor-targeted ablation (Fig. 3). In patients after FIRM plus PVI, recurrent AF had fewer sources than index AF (4.4 ± 1.0 vs. 2.9 ± 1.7, P = 0.01; Table 2).
At repeat procedure, AF sources were 61% left atrial and 39% right atrial (P = 0.63 vs. index). Notably, each of the 3 cases of recurrent organized atrial tachycardia arose at sites of prior AF ablation (e.g., focal tachycardia at a prior roof line in Figure 3C of reference28) but not at index AF rotors.
Spatial Conservation of AF Rotors
Each patient with recurrent AF had at least 1 conserved rotor region between index and repeat procedures. For all AF sources, 81% (61/75) of source regions were conserved (P < 0.01, Table 2). Figure 3 shows examples of conserved (Fig. 3A) and de novo (Fig. 3B) rotors on anatomic shells registered to splines. Figure 3C,D show the biatrial distribution of rotors.
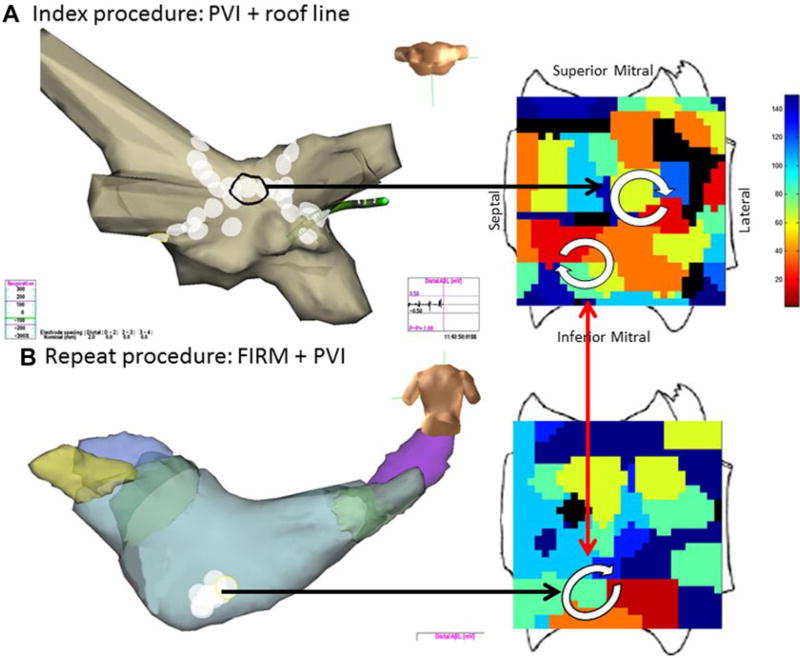
Figure 4A shows a case of PVI and roof line ablation at index procedure. FIRM maps, collected prospectively but analyzed after the case, revealed 1 LA rotor that was coincidentally ablated by the roof line, and another rotor in low posterior left atrium outside PVI lesion sets that was not ablated. AF recurred and repeat FIRM mapping at 5 months (panel 4B) showed elimination of the ablated roof rotor but continued rotational activation in the unablated low posterior region.
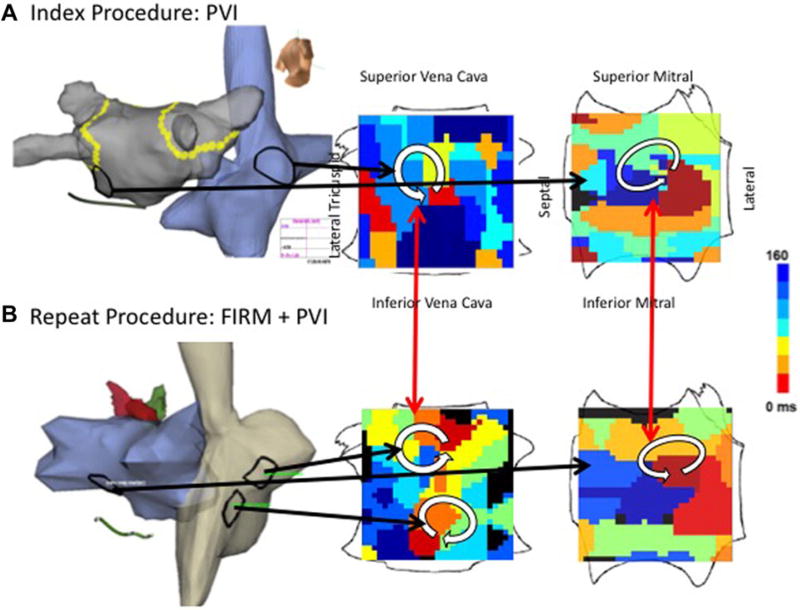
Figure 4.
Temporospatial conservation of rotational AF sites in inferior left atrium in a man with longstanding persistent AF. A. At index procedure, anatomic roof line bisected a roof rotor; wide area PVI was also performed. The inferior LA rotor was not prospectively treated by FIRM ablation. B. During recurrent AF 5 months later, the previously ablated roof rotor was absent but the inferior LA rotor was still present.
Figure 5A shows FIRM+PVI ablation in a man with longstanding persistent AF. Biatrial FIRM maps demonstrated 2 RA and 1 LA rotors that were targeted for elimination, followed by PVI. AF recurred at 10 months and at repeat study 13 months later, a solitary rotor remained in the high RA region.
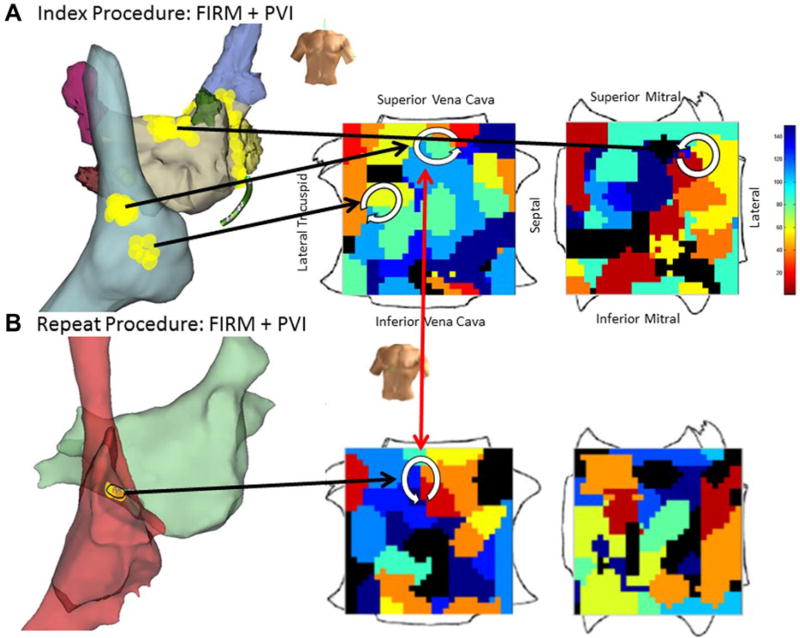
Figure 5.
Diminished substrate in post-ablation recurrent AF. A. At index procedure, FIRM-guided ablation was performed at rotors at the left atrial roof, high and low right atrium, then PVI. B. Repeat FIRM maps (13 months later) showed a single rotor in high right atrium, conserved from the index case.
De novo AF Sources
At repeat procedure, previously undetected (de novo) AF rotor regions were detected in 11/29 patients, comprising 19% (14/75) of sources. Half (7/14) were unrelated to prior ablation, but 50% lay at ablation sites including WACA, roof, and mitral lines. There was no difference between patients with de novo versus conserved sources in the duration of recurrent AF prior to repeat procedure (270 ± 320 vs. 445 ± 552 days; P = 0.34) or LA diameter (53 ± 11 vs. 53 ± 12 mm, P = 0.39).
Figure 6A shows electroanatomic maps in a 52-year-old gentleman with persistent AF, obesity (BMI 44.7 kg/m2) and obstructive sleep apnea who initially received wide-area PVI. FIRM maps (computed after the case) showed a LA and RA rotor (located at black outlines) unablated by PVI. AF recurred at 143 days. Repeat FIRM maps at 238 days showed conservation of the unablated left atrial AF rotor, with increased complexity of the right atrial map including a rotor near the previously unablated high RA rotor and a de novo rotor in the low lateral RA.
Figure 6.
De Novo AF Substrate Progression over 238 days in a man with persistent AF, obesity and obstructive sleep apnea. A. Index ablation comprised anatomical wide area PVI, that missed AF rotors at the mitral isthmus and lateral right atrium on subsequently analyzed FIRM maps. B. At repeat procedure (238 days), FIRM maps showed conservation of the unablated rotors at the mitral isthmus and lateral right atrium, and a new, third inferior right atrial rotor as part of increased RA complexity.
There were no major complications (cardiac perforation, phrenic nerve injury, stroke or deaths) in this series.
Discussion
This study provides novel insights into the spatial conservation or variability of electrical propagation in post-ablation recurrent AF compared to pre-ablation AF. First, using careful basket positioning and analysis, we found that all patients with recurrent AF showed at least 1 conserved electrical rotor region from the index procedure 21 ± 20 months earlier. Second, each patient had multiple rotors, of which 81% were conserved, either not targeted or recurrent after ablation, yet 19% were de novo. Third, these results were true for both paroxysmal and persistent AF. These data further support temporospatial stability of AF propagation patterns (electrical substrates), and provide insights into the natural history of substrate progression over 1–2 years. Further studies are needed to define the significance of such electrical features to recurrent AF.
Atrial Fibrillation Is Trigger and Substrate-Based
After AF is triggered from PV and non-PV sites, it can be sustained for days, weeks, or longer by substrates. Indeed, multicenter ablation trials for paroxysmal AF including the SMART-Touch AF trial added empirical substrate ablation to PVI.3,4,29 Structural substrates may take the form of low voltage30 and fibrosis on late gadolinium magnetic resonance imaging,31 that may produce conduction slowing, rotor anchoring, or fibrillatory conduction.32
Electrically, there is increasing evidence7 that AF substrates comprise rotors, recently demonstrated to be stable on the human endocardium, where they can be eliminated by targeted ablation in the optical mapping studies of human AF9 and animal models.7,8 On-treatment analysis of the CONFIRM trial suggests that elimination of sources directly or coincidentally (e.g., the roof line in Fig. 5A) may explain ablation success,33 including studies in which ablation is successful despite PV reconnection.34,35 Although the role of rotors in human AF is debated, this may be divided by mapping technique—optical mapping studies typically show rotors in most studies7,8 including human atria,9 while studies using traditional electrogram rules do not. Some studies disputing rotors are difficult to interpret, since in 1 work13 nearly half of recorded sites had atrial cycle lengths ≈250–500 milliseconds (dominant frequency 2–4 Hz) suggesting incorrect analysis, or prolapse of basket into the ventricle (compared to reference24),while other studies mapped small regions.10 Additional work must resolve discrepancies and validate the results of different mapping systems against the gold standard of optical mapping, as recently performed for FIRM.36
Conservation of AF Electrical Substrates
Mechanisms for the temporospatial stability of human AF sources remain to be defined. We have shown that rotational circuits that initiate human AF develop at sites of steep conduction restitution,37 and it is likely (although as yet unproven) that such sites may be stabilized electrically by gradients of repolarization14,38,39 or structurally by microscopic9 or macroscopic31 fibrosis. It is also unclear why AF sources were not eliminated by ablation in the 9 patients with prior FIRM ablation. Possible explanations include poor lesion durability, which may be improved by contact force sensing catheters, or rotors unablated to avoid phrenic nerve or esophageal damage.15 On the other hand, these patients represent the minority with AF recurrence and rotors numbers were reduced from the index procedure (Table 2). Alternatively, it is possible that ablation may anchor AF rotors,40 or that circuits found in this study are unrelated to the mechanisms of recurrent AF. These are questions that will be addressed by ongoing randomized trials that do and do not target such regions.
Natural History of AF: Substrate Progression
In some patients, AF may not progress over time and may regress.41 Nevertheless, few studies have mapped the natural history of AF substrates. In the present study, most AF source regions were conserved over ≈1–2 years, but 38% of patients (11/29) had a de novo region harboring AF sources. De novo regions were= mostly related to ablation at a non-rotor site (rotors at previously ablated FIRM sites were conserved, by definition). MRI may help to address if these represent sites of progressive fibrosis,31 or ablation lesions. Finally, it is possible that rotors at de novo sites were in fact present initially but undetected due to technical factors.13 In general, studies are needed to define the factors influencing AF substrate formation.
Clinical Implications
There is an urgent need for further mapping studies of human AF to clarify mechanisms and help guide ablation, building upon optical mapping studies in human atria9 showing stable endocardial rotors that could be eliminated by ablation. Our data are consistent with the hypothesis that failure to eliminate electrical rotors may leave substrates that sustain AF if PVs reconnect or non-PV triggers emerge. Trials are needed to test this hypothesis. Techniques should further improve PV isolation, so that recurrent AF no longer relates to PV reconnection, or eliminate AF rotors, so that recurrent AF cannot relate to persistence of these sources.
Limitations
This was not a clinical trial of ablation strategies, and thus cannot prove if AF rotors cause recurrent AF. This study focused on the role of substrate in AF recurrence, rather than the well-established role of PV reconnection and other triggers. Index procedures in this cohort were mostly performed before the current standard of FIRM remapping was possible, which may change definitions of source elimination in contemporary practice but should not alter analyses of AF source stability over time. We assigned sources separated by < 1 cm to be “stable” because that is the interspline resolution near the poles, because it is a clinically meaningful interpretation of adjacent (i.e., 2 radiofrequency lesions across), and because higher desired precision may not be meaningful if electroanatomical maps or atrial contours change in the years between index and repeat procedures. Notably, such effects or clinical differences such as in anesthesia would be expected to reduce conservation of AF source regions yet we observed the converse. We did not quantify signal voltage or complex fractionated atrial electrograms (CFAE), since we have reported this25 and because interpretation is difficult in ablated tissue. The lack of focal AF sources may reflect the fact that focal sources constitute a minority of AF sources, the possibility that no patient with focal AF sources recurred, or the play of chance. This patient population was mostly male, although studies from many centers16–19 show similar AF mapping and ablation results in women.
Conclusions
Spatial mapping between pre-ablation index AF and post-ablation recurrent AF provides evidence that AF electrical substrates show spatial conservation over 1– 2 years. All patients showed at least 1 conserved region harboring AF rotors compared to initial study, although some AF sources developed de novo. Future studies are needed to further define substrate progression and if rotor ablation may reduce recurrent AF.
Acknowledgments
We thank Antonio Moyeda, RCVT, Judy Hildreth, RN, Cherie Jaynes, RN, Kathleen Mills, BA and also Stephanie Yoakum, RNP, Elizabeth Greer, RN and Donna Cooper, RN, for helping to perform the clinical study and collecting follow-up data.
This work was supported in part by an ACC-Merck fellowship to AAS, a Heart Rhythm Society Fellowship to TB, a Fulbright-British-Heart Foundation award to JAB, and grants from the National Institutes of Health (HL70529, HL83359, HL103800) and Doris Duke Charitable Foundation to SMN.
Dr. Krummen reports consulting work with Topera Inc. Additionally, his Institution has received fellowship support from Medtronic, Boston Scientific, St. Jude Medical and Biotronik. Dr. Narayan reports being co-inventor on intellectual property owned by the University of California and licensed to Topera Medical, Inc; he holds equity in Topera and also reports having received consulting fees from the American College of Cardiology, Uptodate, St Jude Medical, Medtronic and Janssen Pharmaceuticals.
Footnotes
Other authors: No disclosures.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins CH. 2012 HRS/EHRA/ECAS Expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm. 2012;9:632–696. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 3.Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, Kantipudi C, Mansour MC, Melby DP, Packer DL, Nakagawa H, Zhang B, Stagg RB, Boo LM, Marchlinski FE. Paroxysmal AF catheter ablation with a contact force sensing catheter: Results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64:647–656. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 4.Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS, Natale A RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrialfibrillation (RAAFT-2):Arandomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 5.Gerstenfeld E, Sahakian A, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation. 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 6.Xi Q, Sahakian AV, Ng J, Swiryn S. Atrial fibrillatory wave characteristics onsurface electrogram: ECGtoECG repeatability over twenty-four hours in clinically stable patients. J Cardiovasc Electrophysiol. 2004;15:911–917. doi: 10.1046/j.1540-8167.2004.03577.x. [DOI] [PubMed] [Google Scholar]
- 7.Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res. 2013;112:849–862. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou CC, Chang PC, Wen MS, Lee HL, Chen TC, Yeh SJ, Wu D. Epicardial ablation of rotors suppresses inducibility of acetylcholine-induced atrial fibrillation in left pulmonary vein-left atrium preparations in a beagle heart failure model. J Am Coll Cardiol. 2011;58:158–166. doi: 10.1016/j.jacc.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 11.Hoekstra BP, Diks CG, Allessie MA, DeGoede J. Spatial correlation analysis of atrial activation patterns during sustained atrial fibrillation in conscious goats. Arch Physiol Biochem. 2000;108:313–331. doi: 10.1076/apab.108.4.313.4302. [DOI] [PubMed] [Google Scholar]
- 12.Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, Miyazaki S, Sacher F, Bordachar P, Clementy J, Jais P, Haissaguerre M, Hocini M. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: Evidence for localized drivers, rate acceleration and non-local signal etiologies. Heart Rhythm. 2011;8:244–253. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalife J, Filgueiras Rama D, Berenfeld O. Letter by Jalife et al regarding article, “Quantitative analysis of localized sources identified by Focal Impulse and Rotor Modulation Mapping in atrial fibrillation”. Circ Arrhythm Electrophysiol. 2015;8:1296–1298. doi: 10.1161/CIRCEP.115.003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Re-polarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of atrial fibrillation by the ablation of localized sources: The conventional ablation for atrial fibrillation with or without Focal Impulse and Rotor Modulation: CONFIRM trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, El-lenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial independent outcomes from Focal Impulse and Rotor Modulation ablation for atrial fibrillation: Multicenter FIRM registry. J Cardiovasc Electrophysiol. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomassoni G, Duggal S, Muir M, Hutchins L, Turner K, McLoney AM, Hesselson A. Long-term follow-up of FIRM-guided ablation of atrial fibrillation: A single-center experience. J Innovat Cardiac Rhythm Manage. 2015:2145–2151. [Google Scholar]
- 18.Rashid H, Sweeney A. Approaches for Focal Impulse And Rotor Mapping in complex patients: A US private practice perspective. J Innovat Cardiac Rhythm Manage. 2015;6:2193–2198. [Google Scholar]
- 19.Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B, Richter S, Bollmann A, Hindricks G. Successful repeat catheter ablation of re-current longstanding persistent atrial fibrillation with rotor elimination as the procedural endpoint: A case series. J Cardiovasc Electrophysiol. 2016;27:274–280. doi: 10.1111/jce.12874. [DOI] [PubMed] [Google Scholar]
- 20.Narayan SM, Krummen DE, Donsky A, Swarup V, Miller JM. Precise rotor elimination without concomitant pulmonary vein isolation for the successful elimination of paroxysmal atrial fibrillation. PRECISE-PAF. Heart Rhythm. 2013;10 1414-LB1401-1405. [Google Scholar]
- 21.Rensma P, Allessie M, Lammers W, Bonke F, Schalij M. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 22.Rappel W-J, Narayan SM. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013;23:023113. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo D, Atienza F, Jalife J, Martinez-Alzamora N, Bravo L, Almendral J, Gonzalez-Torrecilla E, Arenal A, Bermejo J, Fernandez-Aviles F, Berenfeld O. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace. 2012;14:1560–1566. doi: 10.1093/europace/eus180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayan SM, Krummen DE, Rappel W-J. Clinical mapping approach to identify rotors and focal beats in human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic electrophysiological mapping but not individual electrogram morphology identifies sustaining sites for human atrial fibrillation (AF rotors and focal sources relate poorly to fractionated electrograms) Circ Arrhythm Electrophysiol. 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forclaz A, Narayan SM, Scherr D, Linton N, Jadidi AS, Nault I, Rivard L, Miyazaki S, Uldry L, Wright M, Shah AJ, Liu X, Xhaet O, Der-val N, Knecht S, Sacher F, Jais P, Hocini M, Haissaguerre M. Early temporal and spatial regularization of persistent atrial fibrillation predicts termination and arrhythmia-free outcome. Heart Rhythm. 2011;8:1374–1382. doi: 10.1016/j.hrthm.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani G, Krummen DE, Shivkumar K, Miller JM. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared to trigger ablation alone: Extended followup of the CONFIRM (CONventional ablation with or without Focal Impulse and Rotor Modulation) Trial. J Am Coll Cardiol. 2014;63:1761–1768. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calkins H. Demonstrating the value of contact force sensing: More difficult than meets the eye. Circulation. 2015;132:901–903. doi: 10.1161/CIRCULATIONAHA.115.018354. [DOI] [PubMed] [Google Scholar]
- 30.Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts-Thomson KC, Wilson L, De Sciscio P, Young GD, Sanders P. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: Characterizing the “second factor”. J Am Coll Cardiol. 2009;53:1182–1191. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 32.Lalani G, Schricker A, Gibson M, Rostamanian A, Krummen DE, Narayan SM. Atrial conduction slows immediately before the onset of human atrial fibrillation: A bi-atrial contact mapping study of transitions to atrial fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narayan SM, Clopton P, Krummen DE, Shivkumar K, Miller J. Direct or concidental ablation of localized sources may explain the success of atrial fibrillation ablation. On treatment analysis from the CONFIRM trial. J Am Coll Cardiol. 2013;62:138–147. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: Is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–143. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 35.Jiang RH, Po SS, Tung R, Liu Q, Sheng X, Zhang ZW, Sun YX, Yu L, Zhang P, Fu GS, Jiang CY. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: Mechanistic implications. Heart Rhythm. 2014;11:969–976. doi: 10.1016/j.hrthm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Hansen BJ, Briggs C, Moore BT, Csepe TA, Li N, Zhao J, Garikipati NV, Janssen PM, Mohler PJ, Hummel JD, Fedorov VV. Human atrial fibrillation drivers seen simultaneously by Focal Impulse And Rotor Mapping and high-resolution optical mapping [abstract] Circulation. 2015;132:A18402. [Google Scholar]
- 37.Schricker A, Rostamian A, Lalani G, Krummen DE, Narayan SM. Human atrial fibrillation initiates by organized not disorganized mechanisms. Circ Arrhythm Electrophysiol. 2014;7:816–824. doi: 10.1161/CIRCEP.113.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials as a precursor of atrial fibrillation. Circulation. 2002;106:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 39.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: A mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappel WJ, Zaman JA, Narayan SM. Mechanisms for the termination of atrial fibrillation by localized ablation: Computational and clinical studies. Circ Arrhythm Electrophysiol. 2015;8:1325–1333. doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jahangir A, Lee V, Friedman PA, Trusty J, Hodge D, Kopecky S, Packer DL, Hammill SC, Shen W, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]