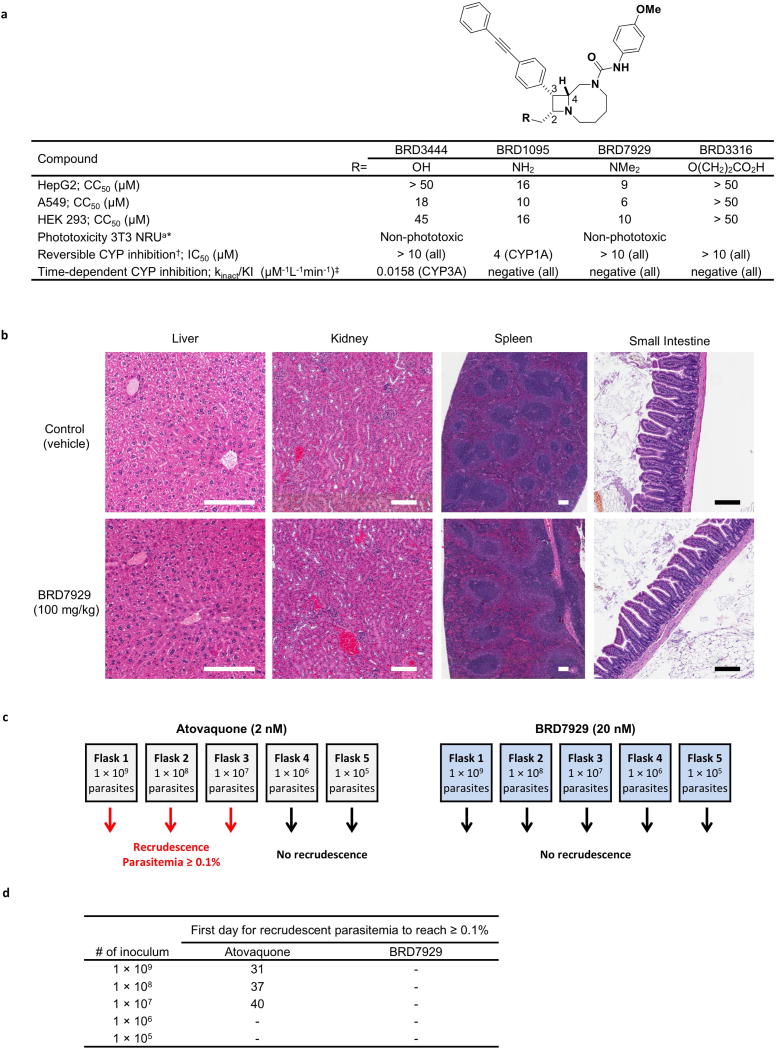
Extended Data Figure 7. Safety and resistance propensity profiling of the bicyclic azetidine series.
a, Results of in vitro cytotoxicity, phototoxicity and CYP inhibition assays. * Phototoxicity was assessed using the NIH 3T3 neutral red assay at Cyprotec; †CYP1A, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A; ‡CYP1A, CYP2C9, CYP2D6, CYP3A. b, Histopathology analysis of mice treated with a high dose (100 mg kg−1) of BRD7929. CD-1 mice were orally treated with 100 mg kg−1 BRD7929 and organs were collected 10 days after treatment. No significant tissue damage was detected. Representative images are shown here. Scale bars, 200 µm. c, d, Measurement of the minimal inoculum for resistance of BRD7929. Cultures containing various numbers of inoculum (1 × 105–1 × 109) were exposed to a constant level of drug pressure (EC90). Parasites developed resistance to atovaquone at the lowest inoculum of 1 × 107 but not to BRD7929.