Summary
Peripheral biomarkers have myriad potential uses for treatment, prediction, prognostication, and pharmacovigilance in epilepsy. To date, no single peripheral biomarker has demonstrated proven effectiveness, although multiple candidates are in development. In this review, we discuss the major areas of focus including inflammation, blood–brain barrier dysfunction, redox alterations, metabolism, hormones and growth factors.
Keywords: Inflammation, Blood–brain barrier, High mobility group box 1 (HMGB1), S100B, brain derived neurotrophic factor, Insulin-like growth factor
Epilepsy affects 50 million people worldwide. A third of those individuals never achieve freedom from seizures regardless of which antiepileptic drug (AED), or combination of drugs, they are prescribed. Administration of drugs with dissimilar mechanism of action does not usually mitigate pharmacoresistance to AEDs.
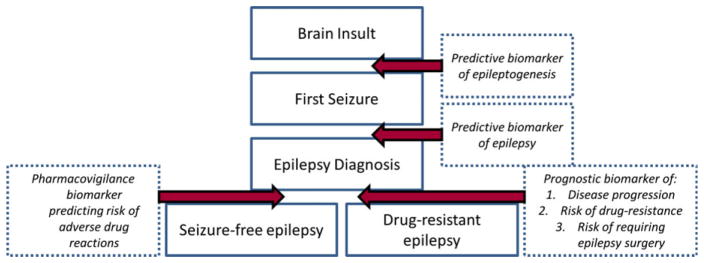
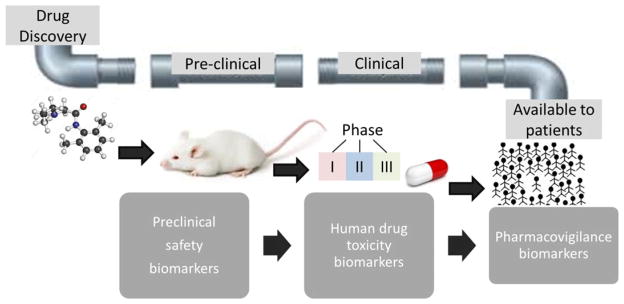
Currently the epilepsy field suffers from a lack of biomarkers to reliably identify at or near diagnosis patients who will develop drug-resistant epilepsy. Biomarkers have been defined as “cellular, biochemical or molecular alterations that are measurable in biological media such as human tissues, cells, or fluids.”1 More recently, a National Institutes of Health (NIH) working group broadened this definition to include “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to therapeutic intervention.”2 In the case of epilepsy, the spectrum of biomarkers ranges from brain imaging and electrophysiologic markers through to molecular and cellular markers in peripheral fluids and tissues. This review focuses on soluble biomarkers, namely blood and cerebrospinal fluid (CSF). Circulating biologic markers (“biomarkers”) in epilepsy have many potential uses including the ability to predict the development of epilepsy following a brain insult and/or following first seizure, prognosticate disease progression and pharmacoresistance to AEDs following first diagnosis, and improve pharmacovigilance by identifying susceptibility to adverse drug reactions (ADRs) (Fig. 1). They are relevant to the entire drug development process, from preclinical safety indications throughout early drug development trials in small populations to screening of large populations for safety signals postmarketing (Fig. 2).
Figure 1.
The field of epilepsy biomarker discovery is wide, covering prediction, prognostication, and pharmacovigilance. Candidate biomarkers may span multiple areas or have relevance to restricted processes only.
Figure 2.
Biomarkers are relevant to the whole developmental pipeline for new drug candidates.
Biomarkers of epileptogenesis are difficult and costly to discover. Even after severe epileptogenic brain insults such as a penetrating head injury, only a proportion of individuals will develop epilepsy. Furthermore, this process may take >10 years. As a result, few prospective pharmacologic studies to prevent seizure disorders after a brain insult have been undertaken. The results of the drug studies that have been done (on phenytoin, phenobarbital, their combination, carbamazepine, valproate, or magnesium) have been disappointing, possibly due to a lack of highly predictive biomarkers to enrich trial populations by including only those most likely to develop epilepsy. The ideal situation would be the identification of a panel of biomarkers charting the entire epileptogenic process covering the immediate post-insult epileptogenic period (epilepsy risk prediction) through to preictal (seizure prediction), postictal (seizure/non-epileptic seizure determination), and interictal phases (prediction of drug resistance and ADRs) once epilepsy is established. This will involve a combination of pre-clinical models and human patient studies. The advent of large-scale imaging technologies and clinical neurophysiology—along with genomics, proteomics, and metabolomics—raises the chances of discovery of predictive biomarkers, and their validation would help in construction of useful clinical trials at reasonable costs.
Molecular and cellular biomarkers should ideally be present in an accessible compartment such as blood, tissue, CSF, sputum, or urine. They should demonstrate low baseline variability in health with a large dynamic range of quantification such that changes in levels are easily detectable and measurable by a high throughput, simple technical analysis that should be cost-effective. In the specific case of drug development, translational biomarkers are highly sought after, and the marker should work in humans as well as in at least two different species and in humans.
Epilepsy Biomarkers in Development
To date, no validated molecular or cellular biomarker for epilepsy exists. Indeed, there are few human studies to date that have examined candidate peripheral biomarkers (Table 1). The ideal situation would be the identification of a panel of biomarkers that would assess the entire epileptogenic process covering the immediate post-insult epileptogenic period through ictal and interictal phases. Peripheral biomarkers are particularly useful in brain disorders such as epilepsy, as they are minimally invasive. Biomarkers for brain inflammation, growth factors, microRNAs, oxidative stress, and metabolic dysfunction may advance the early diagnosis of epilepsy as well as the identification of patients that would benefit from anti-inflammatory treatment. The state-of-the-art knowledge on peripheral epilepsy biomarkers in the areas of inflammation, blood–brain barrier (BBB) dysfunction, redox, metabolism, hormones, and growth has been examined during the XIII Workshop on Neurobiology of Epilepsy (XIII WONOEP) organized by the Neurobiology Commission of the International League Against Epilepsy (ILAE) and an extended summary of the discussed issues is reported herein. Other areas of biomarker discovery look promising but are beyond the scope of this article and have been highlighted in Table 1.
Table 1.
Clinical studies examining peripheral biomarkers in epilepsy
| Author | N | Patients | Specimen | Biomarker | Outcome |
|---|---|---|---|---|---|
| Wang78 | 30 | Epilepsy (partial and generalized seizures) | Serum | MicroRNAs: let-7d-5p, miR-106b-5p, -130a-3p, 146a-5p | Up-regulated, miR-106b-5p 80.3% sensitivity and 81.2% specificity for epilepsy diagnosis |
| MicroRNAs: miR-15a-5p, -194-5p | Downregulated | ||||
| Chang76 | 34 | TLE | Serum | HSP70, S100β, NSE | Compared with the controls, the patients with TLE had poorer cognitive performances and higher HSP70 and S100βP levels |
| Stocklin77 | 161 | Children with febrile seizures | Serum | Copeptin and prolactin | Serum copeptin was significantly higher in children with febrile seizures compared to febrile controls |
| Diamond11 | 59 | Post-traumatic epilepsy | CSF/serum | IL1-β | Higher CSF/serum IL-1β ratios associated with increased risk of posttraumatic epilepsy over time (p = 0.08) |
| Mao10 | 70 | Symptomatic epilepsy | CSF/serum | IL-17A | Interictal serum IL-17A levels were significantly elevated in patients with epilepsy compared to controls. Levels correlated significantly with seizure frequency. |
| Lehtimaki9 | 74 | Developmental disorder with epilepsy | Serum | IL-6 | Patients showed significantly higher IL-6 levels than the controls (4.1 ± 4.5 pg/ml vs. 2.1 ± 1.0 pg/ml; p < 0.001). High seizure frequency and severe intellectual disability emerged as predictors for elevated serum levels of IL-6. |
| Riikonen74 | 30 | West syndrome | CSF | IGF-1, ACTH | Children with symptomatic infantile spasms had significantly low IGF-1, associated with poor cognitive outcome, poor response to therapy and high early insult/stress index. |
| Hulkkonen6 | 10 | Focal DRE | Serum | IL-1β, IL-6 and IL-1Ra | Highly pro-inflammatory cytokine profile (high IL-6, low IL-1RA, low IL-1RA/IL-1β ratio) in epilepsy group versus control |
| Vanhatalo67 | 31 | West syndrome | CSF | NO, nitrates and nitrites | Patients with a symptomatic etiology of WS had significantly higher nitrate levels than controls (p < 0.005) or than the patients with a cryptogenic etiology. |
N, number of patients in the study; DRE, drug-resistant epilepsy; TLE, temporal lobe epilepsy; HSP70, heat-shock protein 70; NSE, neuronal-specific enolase; CSF, cerebrospinal fluid; IL-1β, interleukin-1 β; IL-6, interleukin-6; IL1-Ra, interleukin-1 receptor antagonist; NO, nitric oxide; IGF-1, insulin-like growth factor-1; ACTH, adrenocorticotropic hormone; WS, West syndrome.
Inflammation
Recent experimental studies reveal that neurologic inflammation can both precipitate seizures and sustain seizure activity.3 Furthermore, peripheral inflammation can influence epileptic discharges through alterations in ion and glutamate homeostasis (reviewed in Ref 4). Consequently, biologic markers of inflammation represent a potential means to identify patients in whom aberrant inflammation plays a key role in epileptogenesis and/or maintenance of the epileptic state. Furthermore, immunomodulatory drugs, including steroids and intravenous immunoglobulins, have proven successful strategies in some children with epileptic encephalopathies that are otherwise intractable to conventional antiepileptic drugs (AEDs). This suggests that inflammation may be involved not only in the generation of seizures but also in the development of the drug-resistant phenotype. Surprisingly, even children with focal seizures not traditionally believed to be inflammatory in nature, responded to steroids. Experiments done in parallel with animal models suggested that the target of steroids appeared to be the blood–brain barrier.5 Targeting inflammation may represent a novel therapeutic strategy for the treatment of epilepsy, and circulating biomarkers able to demonstrate target engagement and treatment response are of high value in drug discovery. Individuals with focal drug-resistant epilepsy have been shown to exhibit a pro-inflammatory disequilibrium in the interleukin-1β/interleukin-1 receptor antagonist (IL-1β/IL-1Ra) ratio.6 IL-1β is a mediator of brain inflammation and is counteracted by its cognate anti-inflammatory receptor antagonist (IL-1Ra). In rodents, pharmacologic blockade of IL-1β biosynthesis significantly reduces seizures by targeting specifically the IL-1 converting enzyme responsible for production of the bioactive form.7 This “pro-inflammatory cytokine profile” in peripheral blood consisting of elevated IL-6 with low IL-1β)/IL-1Ra ratio, may indicate patients in whom persistent, unresolved inflammation leads to neuromodulation associated with alterations in neuronal excitability (reviewed in Ref 8). These findings are further supported by subsequent human epilepsy studies examining pro-inflammatory cytokines in peripheral blood.9,10 In addition, higher serum and CSF levels of IL-1β have been associated with an increased risk of developing epilepsy following moderate-to-severe brain injury.11 Furthermore, those with the C to T genotype rs1143634 displayed significantly lower serum IL-1β levels with higher IL-1β CSF/serum ratios, and this affected both seizure frequency and the probability of developing epilepsy (for commentary see Ref 12).
High-mobility group box-1 (HMGB1) is one of the earliest known mediators of neuroinflammation evoked by epileptogenic injuries and has been shown to be critically involved in the generation of seizures in preclinical epilepsy models.13 In its physiologic form, HMGB1 resides in the nucleus where it regulates transcription. Cytoplasmic translocation in response to immune activation is followed by acetylation of key lysine residues within the protein sequence. It is actively released from immune cells, either during infection or injury-induced sterile inflammation. Necrotic cell death leads to the passive release of nonacetylated HMGB1.14 The functional activity is then dictated by posttranslational redox modifications of the cysteine residues C23, C45, and C106. Disulfide HMGB1, containing a disulfide bond between C23 and C45,15 binds and signals via toll-like receptor-4 and induces pro-inflammatory and neuromodulatory effects via activation of nuclear factor kappa B (NF-κB).16,17 Fully reduced HMGB1 resides within the cell and upon release and acts as a chemoattractant via complex formation with CXCL12, binding exclusively via CXCR417. Experimental models of epilepsy suggest that the acetylated, disulfide form of HMGB1 is responsible for the detrimental inflammatory effects in epilepsy.18 Brain tissue taken at epilepsy surgery for drug resistance confirms the presence of inflammatory mediators,13,16 suggesting that persistent, unresolved inflammation may be important in the pathogenesis of symptomatic epilepsy occurring as a result of brain insult. A pilot study in patients with drug-resistant focal epilepsy suggests that HMGB1 isoforms may be candidate biomarkers for stratification in epilepsy (Walker et al., unpublished data). HMGB1 is, however, by no means specific for epilepsy, and has in fact shown promise as a sensitive and specific biomarker for stratification of subpopulations of patients in many conditions, including autoimmune and malignant diseases.19
Pharmacologic inhibition of HMGB1 has been successful in numerous experimental models of disease (strategies reviewed in Ref. 20). The interventions used have included direct inhibition using polyclonal and monoclonal antibodies, competitive inhibitors of the truncated HMGB1 A-Box, methods to sequester and degrade HMGB1 (recombinant soluble thrombomodulin), and inhibition of the NF-κB pathway to suppress downstream HMGB1 release (selective α7-nicotinic acetylcholine receptor agonists.) Because HMGB1 lacks brain specificity, it is unclear whether peripheral or CNS events are responsible for seizures or the therapeutic effects described earlier.
Blood–Brain Barrier
Blood–brain barrier dysfunction following prolonged seizures in animals has been recognized since the 1950s.21
Vasogenic edema due to disturbances in neurovascular units was first described by Klatzo and colleagues.22 In some cases, opening of the blood–brain barrier may acutely evoke seizures (for review see Ref. 23), whereas artificial opening by other means leads to delayed epileptogenesis.24 Blood–brain barrier opening due to a hypertensive crisis as in eclampsia and hypertensive encephalopathy may involve altered serum magnesium concentrations. Experimental opening of the blood–brain barrier25 caused a delayed appearance of seizures. Opening of the blood–brain barrier is common in different neurologic disorders such as encephalitis, meningitis, stroke, Alzheimer disease, and other diseases of the central nervous system.
There is little doubt that cerebrovascular dysfunction favors or sustains seizures.3,26–29 The role of cerebrovascular damage in central nervous system (CNS) disorders, including epilepsy, was in the past clinically accepted30 but only lately tested23,25,31,32 as a leading mechanism underlying epileptogenesis. Restoring cerebrovascular integrity has also been proposed as a complementary approach to traditional AEDs (for review see Ref. 3). In the case of human epilepsy, data are highly suggestive of loss of blood–brain barrier selective permeability in focal regions from which seizures originate.33
In addition, data from several groups support that the blood–brain barrier in patients with epilepsy presents a variety of molecular signatures that are in one way or another involved in the disease. These span from expression of multiple drug resistance–related transporters34 and enzymes,35 to abnormal levels of GLUT-1, a glucose transporter.36 Most of the human data derive from microscopic analysis of resected tissue, where expression of an array of drug extrusion molecules has been reported over a decade ago34 and leakage of capillaries or vessels reported by several groups after the original observation by Cornford.36 However, application of drug transport inhibitors does not help to control drug-resistant seizures in human specimens. Opening of the blood–brain barrier and extravasation of albumin may also lead to buffering of AEDs or extracellular γ-aminobutyric acid (GABA), thereby interfering with therapeutic effect or with some GABA-mimetic drug actions.
Given the importance of the blood–brain barrier for seizure disorders and epilepsy, it is not surprising that biomarkers for this aspect of epileptic pathophysiology have been pursued and developed.29 In general, there are three approaches to measure blood–brain barrier function in epilepsy; these are not different from what has been used clinically to measure cerebrovascular integrity in other neurologic diseases. Historically, the ratio of serum albumin-to-CSF albumin has been the first approach used. The rationale for this approach is essentially analogous to all methods of clinical detection of the blood–brain barrier. This vascular barrier protects the brain from harmful substances of the bloodstream, while supplying the brain with the nutrients required for proper function and strictly regulating the trafficking of cells and molecules from the blood into the brain. When doing so it also segregates impermeant macromolecules (>~500 Da) in the brain and blood compartments. Thus, albumin, which is 10 times more concentrated systemically, will give a fairly constant blood-to-CSF ratio when the blood–brain barrier is intact. A similar principle, yet applied to an entirely different mode of detection, is contrast-enhanced magnetic resonance imaging (MRI). Here the “ratio” between brain and blood is measured topographically, and the fate and distribution of a marker injected in blood is visualized in the brain. Absence of extravasation indicates intact blood–brain barrier, while the opposite can be quantified and compared across hemispheres, and so on.
The last approach is based on serum markers of the blood–brain barrier first described >10 years ago37 and reviewed in Ref.38 The brain produces specific proteins that are “segregated” in the CNS in conditions of intact blood–brain barrier. During blood–brain barrier opening, proteins normally present in high concentrations in the CNS are free to diffuse into the blood following their concentration gradients. An ideal peripheral marker of clinical significance should be the following: (1) a protein (or molecule) present at low or undetectable levels in serum of normal subjects; (2) present in brain and CSF and have a higher concentration in the brain parenchyma than in plasma; (3) available for extravasation in case of blood–brain barrier opening; and (4) further released by brain cells in response to brain damage (e.g., during reactive gliosis). Several proteins, including S100B, neuron-specific enolase (NSE), and glial fibrillary acidic protein (GFAP) have been evaluated for this purpose, and S100B suits all the above-mentioned characteristics. Available imaging techniques for human research or clinical care lack the high resolution necessary to “visualize” this structure, albeit contrast agents have been used to measure blood–barrier integrity. Functional assessment of blood–brain barrier status by calculation of the CSF-serum albumin quotient (QA) and contrast-enhanced MRI are widely accepted as the gold standards for blood–brain barrier permeability.39 A recent paper40 has shown that serum S100B correlates with QA, thus allowing measurement of CSF protein indirectly and without a spinal tap. We and others have shown equivalence between negative predictive value of S100B and contrast MRI.
Relevant to the issue of delayed epileptogenesis after traumatic brain injury (TBI) is the fact that serum S100B is the best studied marker of concussion.41 Concussions, or TBI in general, are associated with a rapid loss of BBB integrity followed (or not) by the development of brain damage.42 S100B has emerged as a candidate peripheral biomarker of blood–brain barrier permeability. Elevation of S100B serum levels reflect the presence of a damaged blood–brain injury and may predict or rule out brain injury.40 Most importantly, S100B increases also after TBI characterized by computed tomography (CT) changes consistent with intracranial events. In studies where S100B serum levels were compared to CT-based diagnosis of mTBI, a negative predictive value of >95% was reported.38,43 Because serious intracranial events are associated with an increased risk for seizures, it is possible that S100B will also prove utility in detecting individuals at low risk versus those who will likely develop posttraumatic sequelae. An important feature of S100B is its excellent negative predictive value for sequelae of blood–brain barrier disruption or TBI.41 In contrast, other markers are more geared toward a good positive predictive value. For example, the meta-analysis for the marker ubiquitin carboxy-terminal hydrolase L1 (UCHL-1) recently published, stated that in studies with a total of 1,138 TBI cases and 1,373 controls there was a significant increase in serum UCH-L1 levels in patients with TBI compared to controls (weighted mean difference 0.96, 95% confidence interval [CI] 0.31–1.61; p = 0.004).44 Two independent meta-analyses for S100B in TBI concluded that “Low serum S100B levels accurately predict normal CT findings after mTBI and that S100B sampling within 3 h of injury should be considered when no focal neurological deficit, or significant extracerebral injury is present.” These studies recommend a cut-off for omitting CT set at <0.10 ng/ml.45 There is therefore an opportunity to produce a test with high negative predictive value as a point-of-care device so that many unnecessary scans can be avoided, and separately, a laboratory-based positive predictive value test to diagnose complications after TBI. A recent paper describes the validation of S100B negative predictive value in TBI in some detail.41
Redox and Metabolic Factors
In focal epilepsies, it is long known that glucose hypometabolism occurs in areas indicative of seizure foci as determined by positron emission tomography (PET) studies.46,47 The reason could lie in altered neurovascular coupling, reduced glucose uptake into the brain, or alteration in mitochondrial function as indicated by reduced nicotinamide adenine dinucleotide (NAD(H)) and the semiquinone form of flavin adenine dinucleotide (FAD(H)) autofluorescence signals. Initially during seizures there is an NAD(P)H dip followed by an overshoot, which is lacking in human temporal lobe epilepsy tissue,48 potentially caused by alterations in lactate delivery by astrocytes, lactate dehydrogenase, glucose uptake into neurons, or in mitochondrial energetics. The acceleration in tricarboxylic acid (TCA) cycle throughput is likely not only determined by retrograde signals such as ATP/ADP ratio but also by calcium, since certain mitochondrial enzymes such as pyruvate dehydrogenase are calcium dependent.49 Calcium accumulation in the cytosol can in turn cause mitochondrial depolarization with less-effective respiration and partial reduction of oxygen, resulting in increased steady-state levels of superoxide (O2−). Increased steady-state levels of O2−, hydrogen peroxide (H2O2), and ultimately iron-catalyzed hydroxyl radical (.OH) formation result in mitochondrial oxidative stress, which can damage proximal vulnerable targets such as mitochondrial proteins, lipids, and DNA. Some evidence suggests that this causes mitochondrial gene alterations.50 Subunits of the complex 1, for example, are differentially distributed between cells and thus might contribute to differential vulnerability between cell types. In addition to mitochondria, seizure activity can result in reactive oxygen species (ROS) production via the pentose pathway,51 xanthine oxidase, and NADPH oxidase family of enzymes.52,53 Furthermore, microglia are associated with seizure-induced ROS production due to acidosis or harboring phagocytic nitric oxide (Nox) enzymes.52
Regardless of the sources and sites of ROS production in the epileptic brain, it is now recognized that oxidative stress and metabolic dysfunction are activated by epileptogenic injuries and can contribute to seizures and comorbidities. A number of useful biomarkers indicative of oxidative stress have been developed in the area of redox biology (see review by Ref. 54). Tissue, plasma, and urine 8-hydroxy-2-deoxyguanosine (8OHdG) and F2-isoprostanes (F2-IsoP) are two important validated biomarkers of oxidative damage to a DNA base and lipids, respectively, in humans and experimental models. Both 8OHdG and F2-IsoPs have been shown to increase in vulnerable brain regions of animals after status epilepticus55,56 and human epilepsy subjects.57,58 A more common method of assessing redox imbalance is measurement of interconvertible redox couples such as NAD(P)H/NAD(P)+, cysteine/cystine, and glutathione/glutathione disulfide (GSH/GSSG). Imaging of brain GSH found depletion in epilepsy patients59 and in hippocampus of animals following status epilepticus.60 Collectively, redox alterations in peripheral tissue or those amenable to imaging may be useful as biomarkers for epilepsy development, progression, and/or comorbidities. Plasma biomarkers of metabolic perturbations such as vitamin D metabolites based on mass spectrometry and other analytical methods have been identified in animal models of acquired epilepsy.61 These biomarkers can provide important information regarding the epileptogenic potential of an insult, disease progression, and/or drug resistance.
Hormones and Growth Factors
Recent works have shown that biomarkers may be used to assess vulnerability to a given phenotype. For example, after an intense stress (social defeat), all animals display a depression-like phenotype, oxidative stress, and low serum brain-derived neurotrophic factor (BDNF) levels. After a couple of weeks, none of the animals display a depression-like profile, but half of them maintain low serum BDNF levels and oxidative stress.62 When challenged with a minor stress, animals (called vulnerable) with sustained low serum BDNF levels show a depression-like profile, whereas those with normal BDNF levels (called nonvulnerable) do not display any phenotype.62 Hence, the original stressful event sensitized a proportion of the animals, making them vulnerable if they happen to encounter a second hit. Serum BDNF levels can be used as a predictive biomarker of vulnerability.62 Finally, injection of a BDNF mimetic normalizes the vulnerable population, making it nonvulnerable.62 Replacing the second hit (minor stress) by an epileptogenic insult (kainic acid–induced status epilepticus) showed that vulnerable animals have a lower threshold for status epilepticus, develop epilepsy much faster, and display severe cognitive deficits as well as a depression-like profile.63 The nonvulnerable population develops epilepsy on a slower time scale, and does not display strong comorbidities.63 Hence, serum BDNF level is predictive for the vulnerability to develop epilepsy after an insult, and for the development of comorbidities (depression and cognitive deficits) during the chronic phase with spontaneous seizures. Note that these results do not contradict the large body of literature reporting increased tissue levels of BDNF once epilepsy has developed.64 In the former case, BDNF serum levels are assessed before the epileptogenic insult. This work fully justifies clinical studies, since not all individuals develop epilepsy and/or comorbidity after an insult. In addition to its possible translation to the clinic, this work also shows that experimental animals are not biologically equivalent, as they react differently to the same insult. This is an important factor to consider, as the expression of other classes of biomarkers may depend on the specific biologic group of a given animal.
West syndrome is a rare epileptic disorder with onset usually prior to the age of 2, which is characterized by clusters of infantile spasms (IS) with hypsarrhythmia or modified hypsarrhythmia on interictal electroencephalography (EEG).65 The syndrome is classified into symptomatic, genetic, and cryptogenic groups, depending on the known etiology. A newer classification66 divides IS into structural-metabolic, cryptogenic, and genetic groups.
Nitric oxide metabolites, nitrates, and nitrites in the CSF of children with IS could differentiate symptomatic from cryptogenic etiologies, although they could not estimate the duration of symptoms or predict the prognosis of mental development.67
The mechanism of the disorder is currently unclear; however, early life stress has been proposed as the trigger.68 Corticotropin-releasing factor (CRF) is a strong convulsive neuropeptide during early brain development. Although CRF acutely stimulates hypopituitary adrenocorticotropic hormone (ACTH) secretion, chronically elevated brain CRF desensitizes CRF receptor and eventually decrease ACTH release.69 When stress is repetitive, it affects synthesis of insulin-like growth factors (IGF-1), because IGFs need a continuous influx of steroids. IGFs are important trophic factors during early brain development. Lack of IGF-1 leads to synaptic impairment, the effect of which ranges from reduction of certain cognitive functions to epileptic encephalopathy. An early initial brain-damaging insult may trigger a cascade of molecular and cellular changes. Epileptic process is considered to consist of three phases: initial insult, latency period (epileptogenesis), and recurrent seizures.70 An initial brain damaging insult is often seen pre-peri-, or postnatally followed some months later by infantile spasms. This latent process could be a target for therapeutic intervention.
First-line treatment of infantile spasms includes the immunosuppressive agent synthetic ACTH. The therapeutic action of ACTH in this disorder is unknown, but it might downregulate the secretion of CRF and other stress hormones.
IGFs influence the entire process of neurogenesis. Brain growth is extremely sensitive to levels of IGF-1. IGF-1 also reduces neuroinflammation.71 ACTH, glucocorticoids, and the ketogenic diet all affect IGF levels72,73 and have all been used for the treatment of IS. In children with symptomatic IS it has been demonstrated that they display markedly low CSF IGF-1 concentrations, in combination with significantly low ACTH concentrations when compared with those with an idiopathic form of the disease.74 Symptomatic IS are characterized by a history of pre, peri-, or postnatal damage. Prenatal stress has been shown in animals to decrease IGF-1.75 In patients with IS, low CSF IGF-1 correlated with a poor response to therapy and poor cognition. The brain cannot produce steroids, which stimulate secretion of IGF-1, an essential growth factor for survival of synapses, high-lighting its potential use as a biomarker for disease severity. Patients with low CSF-1 do not respond to therapy and there is an association between IGF-1 levels and later worsening of mental retardation.74 In IS, CSF IGF-1 at the time of presentation seems to be a biomarker of treatment response and progression of epilepsy, and of later cognitive outcome. It should be noted that while we emphasize hormones and neurotrophic factors associated with disease pathogenesis here, a number of additional proteins have been identified in the serum of epilepsy patients such as HSP7076 and copeptin77 (Table 1), the etiologic roles of which, if any, remain to be determined.
Conclusion
Epilepsy represents a therapeutic area for which there is a large unmet clinical need in terms of drug resistance, where personalized therapy needs to be developed. Identification of biomarkers predicting both the development of disease following first seizure and the likelihood of drug resistance could have a significant impact on the clinical course. It should be recognized that many of the biomarkers discussed in this review have been implicated in other diseases including non-neurologic diseases. Future research will be needed to identify individual or panels of biomarkers that discriminate epilepsies from other diseases. New therapeutic strategies need to involve integrating clinical information, including electroencephalography and neuroimaging, with novel molecular and cellular biomarkers and genomic information.
Identification of biomarkers of aberrant inflammation could potentially stratify patients, early in the course of disease, in whom inflammation contributes to maintenance of the epileptic state. Deconstructing the role that inflammation plays in epilepsy may stimulate novel therapeutic strategies to arrest progression to the drug-resistant phenotype. This is in its infancy, but one can imagine the potential for novel drug-diagnostic combination products in this area.
Markers of blood–brain barrier integrity are useful tools for the determination of sequelae in a variety of neurologic diseases or acute events (stroke, TBI). How these markers may aid in the prognosis and diagnosis of seizure disorders is currently being investigated, but from an experimental point of view, these markers have already demonstrated that the blood–brain barrier is breached at the time of seizures and that blood–brain barrier disruption is epileptogenic. Furthermore, when the barrier is damaged to such an extent that albumin enters the interstitial space, this may impact the effectiveness of some drugs, and, thereby, markers of blood–brain barrier integrity may be helpful for therapeutic decision-making.
Better tools to predict the onset of epilepsy, following brain insult, for example, could lead to the development of new therapeutic strategies for epilepsy prevention, potentially in the form of immune-modulatory intervention. Furthermore, early prediction of drug resistance would mean that patients could be assessed for neurosurgery at an earlier stage, thereby avoiding multiple trials of AEDs, with associated side effects, that are inevitably doomed to failure.
Key Points.
The field of epilepsy suffers from a lack of reliable peripheral biomarkers for predication, prognostication, and pharmacovigilance.
Several candidate molecular and cellular biomarkers of inflammation, blood–brain barrier dysfunction, redox, metabolic, hormone, and growth factors are in development
Acknowledgments
The review is devised as an extended account of the discussion that took place during the XIII WONOEP (Heybeliada Island, Istanbul, Turkey, August 31st–September 4th, 2015) organized and supported by the Neurobiology Commission of the International League Against Epilepsy and generously sponsored by Harinarayan Family Foundation (US) Cyberonics Inc. (US), Insys Therapeutics Inc. (US), Astellas Pharma (Japan), MSD (Japan), and Meiji Seika Pharma (Japan). LW was supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which was funded by the Medical Research Council (grant number G1000417), ICON Developmental Solutions, GlaxoSmithKline, AstraZeneca, and the Medical Evaluation Unit.
Biography
Dr Lauren Walker is a National Institute for Health Research (NIHR) Academic Clinical Lecturer in Clinical Pharmacology & Therapeutics.
Footnotes
Disclosure of Conflicts of Interest
DJ holds a patent for the use of S100B in traumatic brain injury and stroke. MP is a consultant for Aeolus Pharma, which is developing catalytic antioxidants for human diseases. The remaining authors have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Hulka B. Overview of biological markers. In: Hulka BS, Ja G, Hulka Barbara S, Wilcosky Timothy C, Griffith Jack D, editors. Biological markers in epidemiology. New York: Oxford University Press; 1990. pp. 3–15. [Google Scholar]
- 2.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 3.Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janigro D. Are you in or out? Leukocyte, ion, and neurotransmitter permeability across the epileptic blood-brain barrier. Epilepsia. 2012;53(Suppl 1):26–34. doi: 10.1111/j.1528-1167.2012.03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchi N, Granata T, Freri E, et al. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS ONE. 2011;6:e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulkkonen J, Koskikallio E, Rainesalo S, et al. The balance of inhibitory and excitatory cytokines is differently regulated in vivo and in vitro among therapy resistant epilepsy patients. Epilepsy Res. 2004;59:199–205. doi: 10.1016/j.eplepsyres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Maroso M, Balosso S, Ravizza T, et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzani A, French J, Bartfai T, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtimaki KA, Liimatainen S, Peltola J, et al. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res. 2011;95:184–187. doi: 10.1016/j.eplepsyres.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Mao LY, Ding J, Peng WF, et al. Interictal interleukin-17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia. 2013;54:e142–e145. doi: 10.1111/epi.12337. [DOI] [PubMed] [Google Scholar]
- 11.Diamond ML, Ritter AC, Failla MD, et al. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 2014;55:1109–1119. doi: 10.1111/epi.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wathen C, Janigro D. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 2014;55:1313. doi: 10.1111/epi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 14.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Lundback P, Ottosson L, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–259. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zurolo E, Iyer A, Maroso M, et al. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134:1015–1032. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]
- 17.Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balosso S, Liu J, Bianchi ME, et al. Disulfide-containing high mobility group box-1 promotes N-Methyl-d-aspartate receptor function and excitotoxicity by activating toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal. 2014;21(12):1726–40. doi: 10.1089/ars.2013.5349. [DOI] [PubMed] [Google Scholar]
- 19.Pilzweger C, Holdenrieder S. Circulating HMGB1 and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics (Basel) 2015;5:219–253. doi: 10.3390/diagnostics5020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther. 2014;141:347–357. doi: 10.1016/j.pharmthera.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Fregni R, De Poli A. Convulsive state produced by various types of shock; conduct of three barriers (blood-aqueous, blood-labyrinthine fluids, and blood-liquor [spinal fluid]) with reference to some convulsive states. AMA Arch Otolaryngol. 1954;60:149–153. doi: 10.1001/archotol.1954.00720010155004. [DOI] [PubMed] [Google Scholar]
- 22.Klatzo I, Piraux A, Laskowski EJ. The relationship between edema, blood-brain-barrier and tissue elements in a local brain injury. J Neuropathol Exp Neurol. 1958;17:548–564. doi: 10.1097/00005072-195810000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 24.de Vries HE, Kooij G, Frenkel D, et al. Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53(Suppl 6):45–52. doi: 10.1111/j.1528-1167.2012.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorter JA, van Vliet EA, Aronica E. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 2015;49:13–16. doi: 10.1016/j.yebeh.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Tomkins O, Feintuch A, Benifla M, et al. Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy. Cardiovasc Psychiatry Neurol. 2011;2011:765923. doi: 10.1155/2011/765923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raabe A, Schmitz AK, Pernhorst K, et al. Cliniconeuropathologic correlations show astroglial albumin storage as a common factor in epileptogenic vascular lesions. Epilepsia. 2012;53:539–548. doi: 10.1111/j.1528-1167.2012.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchi N, Granata T, Ghosh C, et al. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–1886. doi: 10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penfield W. The Wesley M. Carpenter lecture: the influence of the diencephalon and hypophysis upon general autonomic function. Bull N Y Acad Med. 1933;9:613–637. [PMC free article] [PubMed] [Google Scholar]
- 31.Janigro D, Leaman SM, Stanness KA. Dynamic modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm Sci Technolo Today. 1999;2:7–12. doi: 10.1016/s1461-5347(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 32.Marchi N, Angelov L, Masaryk T, et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigau V, Morin M, Rousset MC, et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 34.Dombrowski SM, Desai SY, Marroni M, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–1506. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh C, Puvenna V, Gonzalez-Martinez J, et al. Blood-brain barrier P450 enzymes and multidrug transporters in drug resistance: a synergistic role in neurological diseases. Curr Drug Metab. 2011;12:742–749. doi: 10.2174/138920011798357051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornford EM, Hyman S, Cornford ME, et al. Interictal seizure resections show two configurations of endothelial Glut1 glucose transporter in the human blood-brain barrier. J Cereb Blood Flow Metab. 1998;18:26–42. doi: 10.1097/00004647-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Kapural M, Krizanac-Bengez L, Barnett G, et al. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- 38.Marchi N, Cavaglia M, Fazio V, et al. Peripheral markers of blood-brain barrier damage. Clin Chim Acta. 2004;342:1–12. doi: 10.1016/j.cccn.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann A, Bredno J, Wendland MF, et al. Validation of in vivo magnetic resonance imaging blood-brain barrier permeability measurements by comparison with gold standard histology. Stroke. 2011;42:2054–2060. doi: 10.1161/STROKEAHA.110.597997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blyth BJ, Farhavar A, Gee C, et al. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. 2009;26:1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unden L, Calcagnile O, Unden J, et al. Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med. 2015;13:292. doi: 10.1186/s12916-015-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27:1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 43.Biberthaler P, Mussack T, Kanz KG, et al. Identification of high-risk patients after minor craniocerebral trauma. Measurement of nerve tissue protein S 100. Unfallchirurg. 2004;107:197–202. doi: 10.1007/s00113-004-0730-1. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Yu C, Sun Y, et al. Serum ubiquitin C-terminal hydrolase L1 as a biomarker for traumatic brain injury: a systematic review and meta-analysis. Am J Emerg Med. 2015;33:1191–1196. doi: 10.1016/j.ajem.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Heidari K, Vafaee A, Rastekenari AM, et al. S100B protein as a screening tool for computed tomography findings after mild traumatic brain injury Systematic review and meta-analysis. Brain Inj. 2015;11:1–12. doi: 10.3109/02699052.2015.1037349. [DOI] [PubMed] [Google Scholar]
- 46.Bernardi S, Trimble MR, Frackowiak RS, et al. An interictal study of partial epilepsy using positron emission tomography and the oxygen –15 inhalation technique. J Neurol Neurosurg Psychiatry. 1983;46:473–477. doi: 10.1136/jnnp.46.6.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henry TR, Engel J, Jr, Mazziotta JC. Clinical evaluation of interictal fluorine-18-fluorodeoxyglucose PET in partial epilepsy. J Nucl Med. 1993;34:1892–1898. [PubMed] [Google Scholar]
- 48.Schuchmann S, Kovacs R, Kann O, et al. Monitoring NAD(P)H autofluorescence to assess mitochondrial metabolic functions in rat hippocampal-entorhinal cortex slices. Brain Res Brain Res Protoc. 2001;7:267–276. doi: 10.1016/s1385-299x(01)00080-0. [DOI] [PubMed] [Google Scholar]
- 49.Denton RM, McCormack JG. The calcium sensitive dehydrogenases of vertebrate mitochondria. Cell Calcium. 1986;7:377–386. doi: 10.1016/0143-4160(86)90040-0. [DOI] [PubMed] [Google Scholar]
- 50.Flynn JM, Czerwieniec GA, Choi SW, et al. Proteogenomics of synaptosomal mitochondrial oxidative stress. Free Radic Biol Med. 2012;53:1048–1060. doi: 10.1016/j.freeradbiomed.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malkov A, Ivanov AI, Popova I, et al. Reactive oxygen species initiate a metabolic collapse in hippocampal slices: potential trigger of cortical spreading depression. J Cereb Blood Flow Metab. 2014;34:1540–1549. doi: 10.1038/jcbfm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel M, Li QY, Chang LY, et al. Activation of NADPH oxidase and extracellular superoxide production in seizure-induced hippocampal damage. J Neurochem. 2005;92:123–131. doi: 10.1111/j.1471-4159.2004.02838.x. [DOI] [PubMed] [Google Scholar]
- 53.Kovac S, Domijan AM, Walker MC, et al. Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 2014;5:e1442. doi: 10.1038/cddis.2014.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalle-Donne I, Rossi R, Colombo R, et al. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 55.Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 56.Patel M, Liang LP, Roberts LJ. Enhanced hippocampal F2-isoprostane formation following kainate-induced seizures. J Neurochem. 2001;79:1065–1070. doi: 10.1046/j.1471-4159.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- 57.Grosso S, Longini M, Rodriguez A, et al. Oxidative stress in children affected by epileptic encephalopathies. J Neurol Sci. 2011;300:103–106. doi: 10.1016/j.jns.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Tanuma N, Miyata R, Nakajima K, et al. Changes in cerebrospinal fluid biomarkers in human herpesvirus-6-associated acute encephalopathy/febrile seizures. Mediators Inflamm. 2014;2014:564091. doi: 10.1155/2014/564091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mueller SG, Trabesinger AH, Boesiger P, et al. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- 60.Liang LP, Patel M. Seizure-induced changes in mitochondrial redox status. Free Radic Biol Med. 2006;40:316–322. doi: 10.1016/j.freeradbiomed.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Heischmann SQK, Cruickshank-Quinn C, Liang L-P, et al. Metabolic changes in rat plasma and hippocampus in the kainic acid model of acquired epilepsy determined by LC-MS metabolomics analysis. American Epilepsy Society Abstracts. 2014 [Google Scholar]
- 62.Blugeot A, Rivat C, Bouvier E, et al. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Becker C, Bouvier E, Ghestem A, et al. Predicting and treating stress-induced vulnerability to epilepsy and depression. Ann Neurol. 2015;78:128–136. doi: 10.1002/ana.24414. [DOI] [PubMed] [Google Scholar]
- 64.Liu G, Gu B, He XP, et al. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79:31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel J, Jr International League Against E. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 66.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 67.Vanhatalo S, Riikonen R. Nitric oxide metabolites, nitrates and nitrites in the cerebrospinal fluid in children with west syndrome. Epilepsy Res. 2001;46:3–13. doi: 10.1016/s0920-1211(00)00154-6. [DOI] [PubMed] [Google Scholar]
- 68.Baram TZ, Mitchell WG, Snead OC, 3rd, et al. Brain-adrenal axis hormones are altered in the CSF of infants with massive infantile spasms. Neurology. 1992;42:1171–1175. doi: 10.1212/wnl.42.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunson KL, Khan N, Eghbal-Ahmadi M, et al. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol. 2001;49:304–312. [PMC free article] [PubMed] [Google Scholar]
- 70.Pitkanen A. Drug-mediated neuroprotection and antiepileptogenesis: animal data. Neurology. 2002;59:S27–S33. doi: 10.1212/wnl.59.9_suppl_5.s27. [DOI] [PubMed] [Google Scholar]
- 71.Corvin AP, Molinos I, Little G, et al. Insulin-like growth factor 1 (IGF1) and its active peptide (1–3)IGF1 enhance the expression of synaptic markers in neuronal circuits through different cellular mechanisms. Neurosci Lett. 2012;520:51–56. doi: 10.1016/j.neulet.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 72.Cheng CM, Kelley B, Wang J, et al. A ketogenic diet increases brain insulin-like growth factor receptor and glucose transporter gene expression. Endocrinology. 2003;144:2676–2682. doi: 10.1210/en.2002-0057. [DOI] [PubMed] [Google Scholar]
- 73.Agha A, Monson JP. Modulation of glucocorticoid metabolism by the growth hormone - IGF-1 axis. Clin Endocrinol (Oxf) 2007;66:459–465. doi: 10.1111/j.1365-2265.2007.02763.x. [DOI] [PubMed] [Google Scholar]
- 74.Riikonen RS, Jaaskelainen J, Turpeinen U. Insulin-like growth factor-1 is associated with cognitive outcome in infantile spasms. Epilepsia. 2010;51:1283–1289. doi: 10.1111/j.1528-1167.2009.02499.x. [DOI] [PubMed] [Google Scholar]
- 75.Szczesny E, Basta-Kaim A, Slusarczyk J, et al. The impact of prenatal stress on insulin-like growth factor-1 and pro-inflammatory cytokine expression in the brains of adult male rats: the possible role of suppressors of cytokine signaling proteins. J Neuroimmunol. 2014;276:37–46. doi: 10.1016/j.jneuroim.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Chang CC, Lui CC, Lee CC, et al. Clinical significance of serological biomarkers and neuropsychological performances in patients with temporal lobe epilepsy. BMC Neurol. 2012;12:15. doi: 10.1186/1471-2377-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stocklin B, Fouzas S, Schillinger P, et al. Copeptin as a serum biomarker of febrile seizures. PLoS ONE. 2015;10:e0124663. doi: 10.1371/journal.pone.0124663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Yu JT, Tan L, et al. Genome-wide circulating microRNA expression profiling indicates biomarkers for epilepsy. Sci Rep. 2015;5:9522. doi: 10.1038/srep09522. [DOI] [PMC free article] [PubMed] [Google Scholar]