Abstract
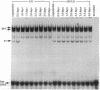
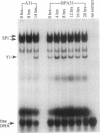
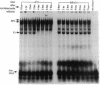
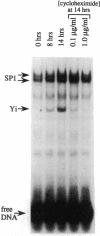
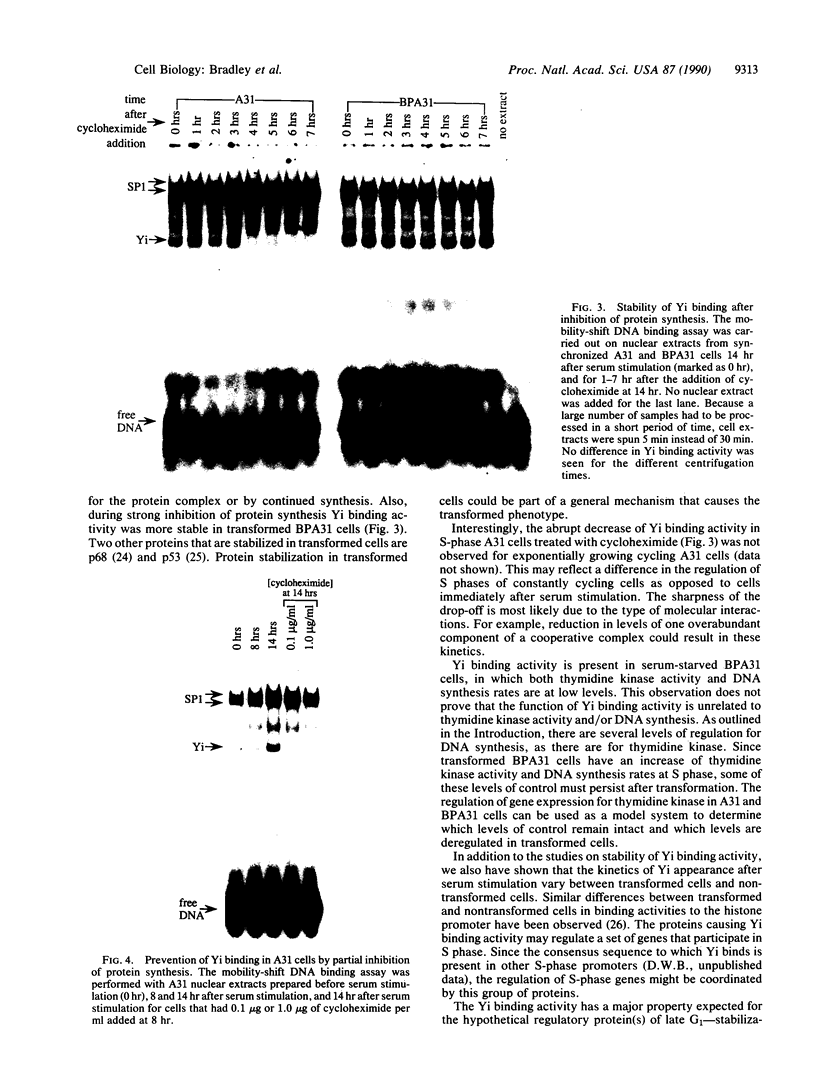
A DNA binding activity to an upstream region of the murine thymidine kinase gene is regulated differently in a transformed and nontransformed cell line pair. Differences in regulation were observed (i) after serum levels were reduced, (ii) when serum levels were returned to initial high levels, and (iii) while protein synthesis was inhibited. After reduction of serum levels, the binding activity was unstable in nontransformed BALB/c 3T3 clone A31 cells but was significantly more stable in benzo[a]pyrene-transformed BALB/c 3T3 cells. After serum concentration was returned to high levels, the kinetic pattern of the binding activity differed between nontransformed and transformed cells. While protein synthesis was inhibited, the binding activity was unstable in nontransformed cells and stable in transformed cells. Partial inhibition of protein synthesis--a more stringent condition to test instability--prevented the induction of the binding activity in nontransformed cells. Previously, the labile protein hypothesis set forth the criterion that a protein regulating the onset of DNA synthesis should be unstable in nontransformed cells and stable in transformed cells. The DNA binding activity described here satisfies this criterion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E., Morreo G., Pardee A. B. Restriction point control of cell growth by a labile protein: evidence for increased stability in transformed cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):436–440. doi: 10.1073/pnas.79.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Morreo G., Pardee A. B. Kinetics of G1 transit following brief starvation for serum factors. Exp Cell Res. 1984 Jun;152(2):459–466. doi: 10.1016/0014-4827(84)90647-5. [DOI] [PubMed] [Google Scholar]
- Cherington P. V., Smith B. L., Pardee A. B. Loss of epidermal growth factor requirement and malignant transformation. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3937–3941. doi: 10.1073/pnas.76.8.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Regulation of thymidine kinase activity in the cell cycle by a labile protein. J Cell Physiol. 1985 Aug;124(2):269–274. doi: 10.1002/jcp.1041240215. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Pardee A. B. Enhanced synthesis and stabilization of Mr 68,000 protein in transformed BALB/c-3T3 cells: candidate for restriction point control of cell growth. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4699–4703. doi: 10.1073/pnas.80.15.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Hall A., Oren M. Stabilization of the p53 transformation-related protein in mouse fibrosarcoma cell lines: effects of protein sequence and intracellular environment. Mol Cell Biol. 1989 Aug;9(8):3385–3392. doi: 10.1128/mcb.9.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J., Owen T. A., van Wijnen A. J., Wright K. L., Ramsey-Ewing A., Kennedy M. B., Carter R., Cosenza S. C., Soprano K. J., Lian J. B. Tumor cells exhibit deregulation of the cell cycle histone gene promoter factor HiNF-D. Science. 1990 Mar 23;247(4949 Pt 1):1454–1457. doi: 10.1126/science.247.4949.1454. [DOI] [PubMed] [Google Scholar]
- Knight G. B., Gudas J. M., Pardee A. B. Coordinate control of S phase onset and thymidine kinase expression. Jpn J Cancer Res. 1989 Jun;80(6):493–498. doi: 10.1111/j.1349-7006.1989.tb01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Fairman M. P., Blow J. J. S phase of the cell cycle. Science. 1989 Nov 3;246(4930):609–614. doi: 10.1126/science.2683076. [DOI] [PubMed] [Google Scholar]
- Lieberman H. B., Lin P. F., Yeh D. B., Ruddle F. H. Transcriptional and posttranscriptional mechanisms regulate murine thymidine kinase gene expression in serum-stimulated cells. Mol Cell Biol. 1988 Dec;8(12):5280–5291. doi: 10.1128/mcb.8.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano E. E., Pardee A. B. Prevalent deficiency in tumor cells of cycloheximide-induced cycle arrest. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4123–4126. doi: 10.1073/pnas.77.7.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu H., Berezney R. Mapping replicational sites in the eucaryotic cell nucleus. J Cell Biol. 1989 Jan;108(1):1–11. doi: 10.1083/jcb.108.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Coppock D. L., Yang H. C. Regulation of cell proliferation at the onset of DNA synthesis. J Cell Sci Suppl. 1986;4:171–180. doi: 10.1242/jcs.1986.supplement_4.11. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Riddle V. G., Lehtomaki D. M. Growth arrest states of RNA virus- and chemically transformed mouse cells. Cancer Res. 1981 May;41(5):1778–1783. [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]