Abstract
Background
About 85.3% of hemolytic disease of the newborn (HDN) is caused by maternal-fetal ABO blood group incompatibility. However, there is currently no recommended “best” therapy for ABO incompatibility during pregnancy.
Objectives
To systematically assess the safety and effectiveness of oral Chinese herbal medicine (CHM) for preventing HDN due to ABO incompatibility.
Methods
The protocol of this review was registered on the PROSPERO website (No. CRD42016038637).Six databases were searched from inception to April 2016. Randomized controlled trials (RCTs) of CHM for maternal-fetal ABO incompatibility were included. The primary outcome was incidence of HDN. The Cochrane risk of bias tool was used to assess the methodological quality of included trials. Risk ratios (RR) and mean differences with 95% confidence interval were used as effect measures. Meta-analyses using Revman 5.3 software were conducted if there were sufficient trials without obvious clinical or statistical heterogeneity available.
Results
Totally 28 RCTs involving3413 women were included in the review. The majority of the trials had unclear or high risk of bias. Our study found that the rate of HDN and the incidence of neonatal jaundice might be 70% lower in the herbal medicine group compared with the usual care group (RR from 0.25 to 0.30).After treatment with herbal medicine, women were twice as likely to have antibody titers lower than 1:64 compared with women who received usual care(RR from 2.15 to 3.14) and the umbilical cord blood bilirubin level in the herbal medicine group was 4umol/L lower than in those receiving usual care. There was no difference in Apgar scores or birthweights between the two groups.
Conclusions
This review found very low-quality evidence that CHM prevented HDN caused by maternal-fetal ABO incompatibility. No firm conclusions can be drawn regarding the effectiveness or safety of CHM for this condition.
Introduction
Maternal-fetal incompatibility is one of the most common immunological causes for spontaneous abortions, with 15% being related to maternal-fetal ABO incompatibility [1]. About 85.3% of hemolytic disease of newborn (HDN) is also caused by maternal-fetal ABO incompatibility [2], which may result in neonatal jaundice. It is estimated that27% of newborns in China have ABO incompatibility compared with 15% worldwide [3]. During pregnancy, there are often no symptoms in the pregnant woman with ABO incompatibility, but 30% of babies born to these women have HDN, with 19% having moderately severe to severe hemolysis [4].
Intrauterine therapy such as maternal plasma exchange or intrauterine fetal blood transfusions are accepted therapies during pregnancy for maternal-fetal Rh incompatibility, as this condition has a higher risk of morbidity and mortality. Even though maternal plasma exchange is relatively safe, the procedure can be difficult and expensive [5]. On the other hand, intrauterine fetal blood transfusions have the potential risk for preterm pre-labor rupture of the membranes[6] (PPROM, 0.1%-2% risk), intra-amniotic infection (chorioamnionitis, 0.3%-1.2% risk), preterm labor, and other fetal complications (1.3%-2.5% risk).There is currently no recommended “best” therapy for preventing ABO incompatibility during pregnancy. In China intravenous injections of glucose and vitamin C in combination with oral Vitamin E are the commonly used for this condition.
ABO blood group incompatibility is recorded as Taihuang (fetal jaundice) or Tailou (threatened abortion) according to traditional Chinese medicine (TCM) diagnosis. In TCM theory, syndrome differentiation for this disease often referred to retained dampness-heat stagnation [7] and kidney-qi deficiency [8]. Consequently, clearing heat and draining dampness, warming the kidney and fortifying the spleen are the general treatment principles for this condition [9]. Previous studies published in China indicated that Herba Artemisiae Scopariae (Yinchen) and Rhizoma Rhei (Da Huang) have high contents of blood group A and B substances, which may resist antibodies from maternal-fetal incompatibility [10]. 6,7-dimethoxy coumarin, one of the ingredients of Herba Artemisiae Scopariae (Yinchen) is thought to protect the liver [11] and cause jaundice resolution [12]. A previous systematic review indicated a potential benefit for using combined therapy of Chinese and Western medicine to treat maternal-fetal ABO incompatibility [1]. However, due to the poor methodological quality of the included studies and obvious clinical heterogeneity between the trials, the study could not draw firm conclusions.
Objectives
To critically appraise the existing randomized controlled trials (RCTs) of Chinese herbal medicine (CHM) for treating ABO incompatibility during pregnancy to prevent hemolytic disease of the newborn, and provide evidence-based evaluation of the safety and effectiveness of the oral CHM.
Methods
The protocol of this review was registered on the PROSPERO website (No. CRD42016038637) and can be retrieved through https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016038637 (see S1 File).This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (see S2 File).
Criteria for considering studies for this review
Randomized controlled trials (RCTs) which compared oral administration of CHM (alone or in combination with other treatments) with other therapies (or no treatment)for treating maternal-fetal ABO incompatibility were included in our review. We defined ABO incompatibility as the situation when the maternal blood type is "O", and the paternal blood type is A, B or AB resulting in an antibody titer (IgG anti-A or anti-B) that is higher than 1:64. The primary outcome was the incidence of HDN; secondary outcomes of interest were: the level of antibody titer after treatment, the incidence of neonatal jaundice, neonatal bilirubin levels (including neonatal umbilical cord blood bilirubin and total serum bilirubin), other measurements of health status of the newborn (such as Apgar scores and birthweight) and adverse events. To be included in our evaluation, at least one of the above outcomes need to be reported in the trial.
Search methods for identification of studies
Two English databases and four Chinese databases were searched from inception to April 2016, including the Cochrane Central Register of Controlled Trials (CENTRAL), PubMED, China Network Knowledge Infrastructure(CNKI), Chinese Scientific Journals Database (VIP), WanFang Database (for unpublished graduate theses in China), and Chinese Biomedicine (CBM). Details of the search strategies are shown as below.
#1: "Medicine, Chinese Traditional" [Mesh]; #2: "Drugs, Chinese Herbal" [Mesh]; #3: "ABO incompatibility" [Mesh]; #4: “ABO hemolytic disease of newborn” [Mesh]; #5: (#1 OR #2) AND (#3 OR #4)
The above strategies were adapted for each specific database, with the use of Chinese characters for relevant key words when searching Chinese databases.
Study selection
Two review authors (Cao H and Wu R) independently assessed the titles, abstracts and keywords of every record retrieved to determine relevance and whether the study was an RCT according to the inclusion criteria. Full articles were retrieved for further assessment, with reasons for exclusion being recorded. Where differences in opinions existed, they were resolved by discussion with the other authors until a consensus was reached.
Data extraction and management
Data concerning details of the included studies were extracted independently by two review authors (Cao H and Wu R) using a piloted data extraction form. The data extraction form included general information, study design, participant information, interventions and outcomes. Authors of relevant studies identified were contacted if needed to obtain information regarding additional references, unpublished trials, or data missing from the original publication. Disagreements were resolved by consensus.
Assessment of risk of bias in included studies
Two authors (Cao H and Han M) assess the methodological quality of the included trials independently. Selection bias (random sequence generation and allocation concealment), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases (including sample size calculation, inclusion/exclusion criteria for patients' recruitment, comparability of baseline data, funding sources, and any other potential methodological flaws that may have influenced the overall results) were assessed according to the criteria from the Cochrane Handbook for Systematic Reviews of Intervention [13]. Since all of the outcomes of this review were measured objectively and were therefore unlikely to be influenced by the lack of blinding, and there was no ideal placebo control for Chinese herbal medicine (especially for the herbal decoction), we did not assess for performance bias (with blinding of participants and personnel). The level of bias was assessed for each trial (as low, high or unclear risk). A study was considered to have a low risk of bias if all seven risk of bias items met the criteria as “low risk”, a study was considered to have high risk of bias if at least one of the seven items was assessed as “high risk”.
Data analysis
Data were summarized using risk ratios (RR) with 95% confidence intervals (CI) for binary outcomes or mean difference (MD) with 95% CI for continuous outcomes. We used Revman 5.3 software from the Cochrane Collaboration for data analyses. Meta-analysis was used if there was acceptable homogeneity in the study design, participants, interventions, control, and outcome measures. Statistical heterogeneity was tested by examining I2 [14], with an I2greater than 50% indicating a possibility of statistical heterogeneity. Both fixed effects models and random effects models were used if there was a possibility of statistical heterogeneity between trials. Pooling analysis were not performed if there was large statistical heterogeneity (I2>75%). Publication bias was explored by funnel plot analysis. Subgroup analyses were conducted for different baseline characteristics or different treatment duration if data were sufficient. Sensitivity analyses were used to determine whether the conclusions differed if eligibility was restricted to studies without high risk of bias, or if a fixed effect/random effect model had been applied.
Results
Results of the search
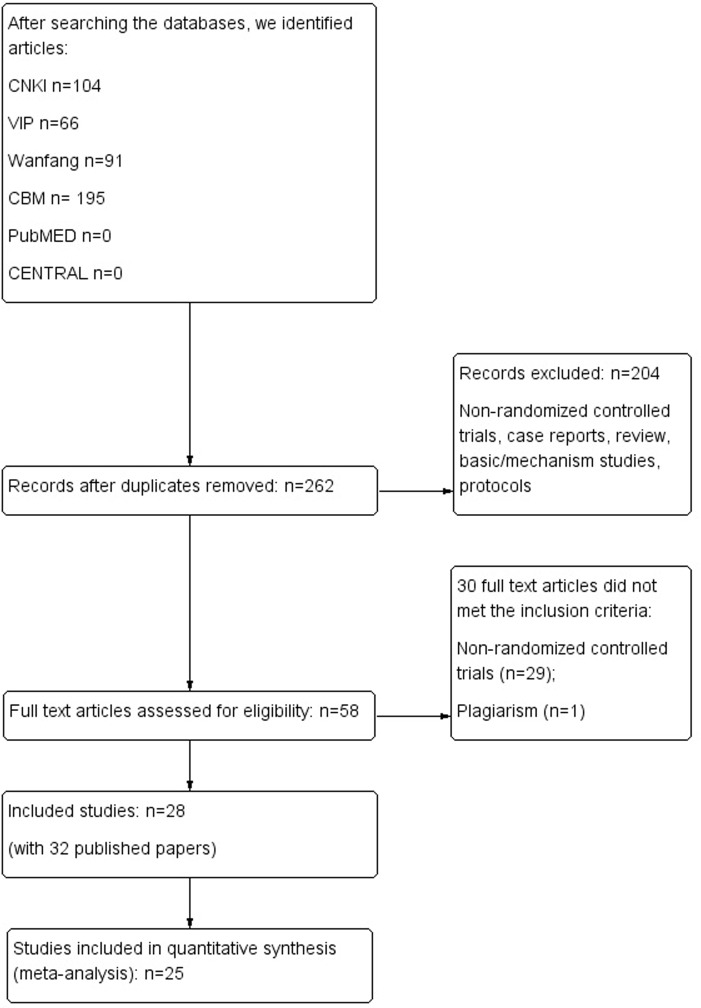
A totally of 262 papers were identified through literature searching, and 58 full text papers were retrieved for further assessment. Finally, 32 publications of 28trials [15–46] met our pre-defined criteria and were included in this review. There were four duplicate studies [16&17, 31&32, 33&34, 35&36]. One paper [47] was excluded due to plagiarism. All of the included trials were conducted and published in China. Details of the searching and selection of included studies are shown in Fig 1.
Fig 1. Study flow chart.
Characteristics of the included studies
The 28 trials involved 3413 women, with an average of 57 women in each group. The average age of participants was 27.7 years, and the baseline gestational was around 25.0 weeks. Six of the trials [20, 27–29, 39, 41] enrolled pregnant women whose antibody titers were higher than 1:64 at baseline, and the remaining trials included women with antibody titers higher than 1:128.
Twenty-seven of the 28 included trials assessed the use of oral CHM for preventing hemolytic disease of the newborn due to ABO incompatibility, with 22 trials [15, 16, 18–20, 22–26, 29–33, 37, 39, 41, 43–46] using herbal decoctions, four [21, 27, 28, 40] using herbal liquid (patent) and one trial [36] using herbal granule. The remaining trial [42] assessed the use of oral administration and external washing of CHM. According to TCM theory, all of the included trials used clearing heat and draining dampness as their treatment principle. The top five most frequently used herbs were Herba Artemisiae Scopariae (Yin Chen, in 26 trials), Rhizoma Glycyrrhizae (Gan Cao, in 23 trials), Fructus Gardeniae (Zhi Zi, in 22 trials), Radix Scutellariae (Huang Qin, in 22 trials), and Radix et Rhizoma Rhei (Da Huang, in 22 trials). Except Rhizoma Glycyrrhizae, which was used for coordinating the drug actions of a prescription, the other four herbs were all used for clearing heat. Herba Artemisiae Scopariae and Fructus Gardeniae also works on draining dampness.
Twenty-three of the included trials compared herbal decoction with usual care, four trials [16, 21, 24, 28] assessed herbal decoction as an add-on treatment combined with usual care compared to usual care alone, and one trial [41] compared herbal decoction with no treatment. Usual care included vitamin supplements(in all trials: oral or intravenous vitamin C at 100-200mg three times daily and oral vitamin B6 at 20mg twice or three times daily), oxygen inhalation (in 18 trials, for 20-30minutes once daily) and oral phenobarbital (in 10 trials: at 20-30mg twice or three times daily for two to four weeks before delivery).
The treatment duration varied among the trials. Participants in five trials [19, 21, 22, 40, 44] received treatments for ten days during pregnancy. Treatment duration in 18 trials [18, 23, 24, 26, 27, 29–31, 33, 36–39, 41–43, 45, 46] was for at least two to six weeks depending on the level of the antibody titer. One trial [25] reported that the treatment was continued until labor. And four trials [15, 16, 20, 28] did not mention the length of treatment.
For the other outcomes, HDN was reported in 15 trials [16, 19, 20, 22, 23, 25, 26, 29–31, 33, 36, 41, 42, 46], the level of antibody titer after treatment was reported in 25 trials [18–31, 33, 36–40, 42–46], the incidence of neonatal jaundice was reported in 13 trials [15, 16, 18–20, 24–27, 29, 30, 36, 37], umbilical cord blood bilirubin was reported in seven trials [16, 18–20, 26, 36, 38], Apgar scores were reported in eight trials [18–21, 23, 26, 36, 40], birthweight was reported in six trials [18, 19, 21, 23, 36, 40], and adverse events were mentioned in eight trials [16, 21, 23, 30, 37, 38, 41, 42].
Details of the characteristics of the 28 included trials are shown in Table 1, and the components of the herbal prescriptions and adverse events are shown in Table 2.
Table 1. Characteristics of 28 included trials concerned Chinese herbal medicine for ABO incompatibility.
| Study ID | Number of the participants | Antibody titer at baseline | Age (yrs) | Gestational weeks | Intervention | Control | Outcome measurements |
|---|---|---|---|---|---|---|---|
| An 2014a [15] | 29:29 | >1:128 | T:30.8±11.3 C:31.4±10.7 | T:18.4±4.9 C:16.3±3.7 | Huangyin Antai Decoction, methods for administration were not reported | Vit C 100mg Tid, Vit E 100mg Tid, oxygen inhalation | Time for antibody titer less than 1:64, NJ |
| An 2014b [16] | 30:30 | >1:128 | T:23.5±6.1 C:22.1±5.3 | T:17.5±5.3 C:18.2±6.1 | Huangyin Antai Decoction plus control therapies, methods for administration were not reported | Vit C 100mg Tid, Vit E 100mg Tid, oxygen inhalation | NJ, HDN, UCBB, change of antibody titer, adverse events |
| Cai 2009 [18] | 30:30 | >1:128 | T:28.4±4.1 C:27.6±3.2 | T:27.1±6.3 C:25.4±5.5 | Lianhuang Decoction 250ml Bid for 2 weeks | Vit C 2g, Vit B6 0.2g intravenous injection, Vit E 100mg Bid, oxygen inhalation 30min once daily for 2 weeks. Phenobarbital30mg Bid for 10 days after 36 or 37 gestational weeks. | Apgar scores, NJ, Number with antibody titers lower than 1:64 post treatment, UCBB, birthweight |
| Chen 2011 [19] | 32:25:28 | >1:128 | 27.5±6.5 | >16 | T1: Gutai Yinchen Decoction 200ml Bid; T2: Yinchenhao Decoction 200ml Bid; Oral administration for 10 days at 26, 30, 34 gestational weeks | Vit C 100mg Tid, Vit E 50mg Tid, oral administration for 10 days at 26, 30, 34 gestational weeks. Pheonbarbital was given 30mg Bid for 10 days after 36 gestational weeks. | Apgar scores, NJ, HDN, number with antibody titers lower than 1:128 post treatment, UCBB, birthweight, adverse events |
| Cui 2014 [20] | 57:58 | >1:64 | T: 28.2±4.1 C:27.0±3.3 | T:26.9±5.8 C:25.3±4.8 | Baishao Gancao Decoction plus Yinchen Dazao Decoction, methods for administration were not reported | Vit C 0.2g Tid, Vit B6 20mg Tid, Vit E 50mg Bid, oxygen inhalation 30min daily for 10 days as a course of treatment. Phenobarbital30mg Bid for 10 days after 36 gestational weeks. | Apgar scores, NJ, HDN, number with antibody titers lower than 1:64 post treatment, UCBB |
| Ding 2002 [21] | 57:42 | >1:128 | Not reported | >16 | Kangtuihuang Oral Liquid 10ml Bid plus the control therapy. The treatment was given 10 days every 4 weeks. | Vit C 1g intravenous injection, Vit E 100mg Bid, oxygen inhalation 20min Bid. The treatment was given 10 days every 4 weeks. | Change in antibody titers, Apgar scores, birthweight, time and severity of the neonatal jaundice, 96 hour total serum bilirubin |
| Feng 2006 [22] | 148:140:123 | >1:128 | T1:27.5±3.8 T2:27.5±3.9 C:26.3±3.6 | T1:26±5.5 T2:27.5±5.2 C25.8±5.1 | T1: Decoction of Herba Arib (100g) 250ml daily; T2: Jigucao Decoction 250ml daily. The treatment was given 10 days every 4 weeks. | Vit C 0.2g Tid, Vit B6 20mg Tid, Vit E 50mg Bid, oxygen inhalation 30min daily. Oral administration for 10 days at 24, 30, 33 gestational weeks. Phenobarbital 30mg Bid for 10 days after 36 gestational weeks. | HDN, number with antibody titers lower than 1:64 post treatment |
| Hu 2014 [23] | 91:90:87 | >1:128 | 20–40 | 26–34 | T1: Quhuang Antai Decoction 200ml Bid; T2: Yinchenhao Decoction 200ml Bid. Treatment was given at least 28 days | Vit C 100mg Tid, Vit E 100mg Bid, oxygen inhalation 30min daily for 10 days every 4 weeks. Phenobarbital20-30mg Tid after 36 gestational weeks. Treatments were given at least 28 days | Apgar scores, time of the neonatal jaundice, HDN, number with antibody titers lower than 1:128 post treatment, birthweight, adverse events |
| Jin 2013 [24] | 30:30 | >1:128 | 22–40, average 27.6 | 13–36 | Self made prescription once daily for 4 weeks, plus the control therapy | Vit C100mg Tid, Vit E 100mg Tid. The treatment was given 10 days every 4 weeks. | NJ, number with antibody titers lower than 1:128 post treatment |
| Li 2013 [25] | 20:20 | >1:128 | 23–43 | Unclear | Modified Yinchenhao Decoction 200ml Bid. The treatment last until labor | Vit C 100mg Tid, Vit E 100mg Bid, oxygen inhalation 30min daily. Oral administration for 10 days at 28, 32, 36 gestational weeks. Phenobarbital 20mg Tid after 36 gestational weeks. | NJ, number with antibody titers lower than 1:128 post treatment, HDN |
| Li 2014 [26] | 28:28 | >1:128 | T:30.8±9 C:29.7±8.7 | T:17.9±4.5 C:16.9±3.7 | Modified Huangyin Antai Decoction Tid for 30 days | Vit E 100mg Tid, Vit C 100mg Tid, oxygen inhalation daily. | Apgar scores, NJ, HDN, number with antibody titers lower than 1:64 post treatment, UCBB, adverse events. |
| Li 2015 [27] | 45:45 | >1:64 | T:29.3±8.7 C:28.7±7.6 | T:21.6±7.3 C:22.4±6.3 | Yinzhihuang Oral Liquid 10ml Tid for at least 4 weeks | Vit C 2g intravenous injection, oxygen inhalation 30min daily for 4 weeks | NJ, number with antibody titers lower than 1:64 post treatment |
| Liu 2015 [28] | 42:42 | >1:64 | 23–39, average 25.6 | 14–36 | Yinzhihuang Oral Liquid 20ml Tid for 4 weeks, plus control therapy | Vit C 3g intravenous injection, Vit E 100mg Bid, for 10 days at 20, 34, 28 gestational weeks. | Time of neonatal jaundice, number with antibody titers lower than 1:64 post treatment, serum total bilirubin |
| Luo 2013 [29] | 50:50 | >1:64 | 26.8±3.1 | 12–33 | Herbal Decoction once daily for 14 days | Vit C 1g intravenous injection, Vit E 100mg Bid, oxygen inhalation 30min daily for 14 days | HDN, NJ, Number with antibody titers lower than 1:64 or 1:128 post treatments. |
| Lv 2012 [30] | 30:30 | >1:128 | T:28.17±5.15 C:28.03±5.25 | >12 | Yinchen Erdan Erhuang Decoction Tid for at least 4 weeks | Vit E 100mg Tid, Vit C 100mg Tid, oxygen inhalation 30min daily for at least 4 weeks | Number with antibody titers lower than 1:64/1:128 post treatments, HDN, NJ, time for taking medicine, adverse events |
| Mei 2006 [31] | 54:42 | >1:128 | 21–38 | Not reported | Yinqi Decoction once daily for 28 days per course, 1–2 courses | Vit E 100mg Tid, Vit C 100mg Tid, oxygen inhalation 30min daily. The treatment was given 10 days every 4 weeks. | HDN, Number with antibody titers lower than 1:64 post treatments. |
| Su 2005 [33] | 70:70:70 | >1:128 | T1:27.4±3.5 T2:27.6±3.8 C:27.3±3.7 | T1:26.1±5.6 T2:25.8±5.3 C:25.4±5.5 | T1: Jigucao Decoction for 20–30 days; T2: Fufang Yinchen Decoction for 20–30 days | Vit C 0.2g Tid, Vit B6 20mg Tid, Vit E 50mg Bid, for 20–30 days. Oxygen inhalation 30min daily for 10 days at 24, 30, 33 gestational weeks. Pheonbarbital30mg Bid for 10 days after 36 or 37 gestational weeks. | HDN, Number with antibody titers lower than 1:64 or 1:128 post treatments. |
| Sun 2008 [36] | 94:78 | >1:128 | 26.78±3.07 | 12–33 | Yinzhi Kangrong Granules Bid for 7–14 days | Vit C 1g intravenous injection, folic acid 5mg Tid, Vit B6 20mg Tid, Vit E 100mg Bid, oxygen inhalation 30min daily. Oral administration for 10 days at 24, 30, 33 gestational weeks. Pheonbarbital30mg Bid for 10 days after 39 gestational weeks. | Apgar scores, NJ, HDN, Number with antibody titers lower than 1:64 post treatment, UCBB, hemoglobin, birthweight, adverse events. |
| Xu B 2011 [37] | 83:83 | >1:128 | T:27.6±3.1 C:28.1±3.2 | T:25.2±2.4 C:24.8±2.4 | Modified Yinchenhao Decoction 200ml Bid. Pheonbarbital20-30mg Bid for 7 days after 36 gestational weeks. | Vit C 0.5g intravenous injection, Vit E 100mg Bid. Pheonbarbital 20-30mg Bid for 7 days after 36 gestational weeks. | NJ, Number with antibody titers lower than 1:64 post treatment, serum total bilirubin, adverse events. |
| Xu J 2009 [38] | 80:70 | >1:128 | T:28.8 C:26.5 | Not reported | Fangrong Antai Power 100ml Bid for at least 2 weeks | Vit C 1g intravenous injection, Vit E 100mg Bid for at least 2 weeks | Number with antibody titers lower than 1:128 post treatment, UCBB, adverse events |
| Xu L 2011 [39] | 30:30 | >1:64 | T:27.1 C:26.2 | T:22.6 C:23.1 | Taier Yixue Decoction 200ml Bid for at least 4 weeks | Vit C 1g intravenous injection, Vit E 100mg Bid, oxygen inhalation 20min Bid. Pheonbarbital20-30mg Tid after 38 gestational weeks. | Number with antibody titers lower than 1:64 post treatment |
| Yang 2015 [40] | 64:56:50 | >1:128 | T1: 27.5 T2:25.9 C:25.6 | T1:28.2 T2:28.8 C:29.8 | T1: Yinzhihuang Oral Liquid 20ml Tid; T2: Yinchenhao Decoction 50-200ml Bid. Treatment was given 10 days at 26, 30, 34 gestational weeks. | Vit C 2g intravenous injection, Vit E 100mg once daily, 10 days at 26, 30, 34 gestational weeks. | Number with antibody titers lower than 1:128 post treatment, Apgar scores, birthweight |
| Yu 2013 [41] | 92:90 | >1:64 | 28.3±6.7 | 16–36 | Self made prescription 3–4 times daily for 20–30 days, then once every two days | No treatment | Incidence, time and serious of the neonatal jaundice, HDN, adverse events |
| Zhang 2013 [42] | 60:60 | >1:128 | 20–39, average 27.6 | 16–36 | Modified Yinchenhao Decoction 200ml Bid for 2 weeks, plus herbal decoction external used once daily | Vit C 100mg Tid, Vit E 100mg Bid for 2 weeks | Number with antibody titers lower than 1:64 post treatment, Incidence of the neonatal jaundice, HDN, adverse events |
| Zhao 2012 [43] | 59:52:57 | >1:128 | T1:27.33±6.03 T2:27.14±6.12 C:27.52±6.43 | Not reported | T1: Xiaohuang Decoction 200ml Bid; T2: Yinchenhao Decoction 200ml Bid. At least 4 weeks treatment. | Vit C 1g intravenous injection, Vit E 100mg Bid, oxygen inhalation 30min once daily. At least 4 weeks treatment. | Number with antibody titers lower than 1:128 post treatment |
| Zhou 2007 [44] | 41:36 | >1:128 | T:26.3±2.8 C:27.4±3.2 | T:24.1±1.8 C:24.5±2.1 | Modified Yinchenhao Decoction Bid for 10 days, plus control therapy | Vit C 0.5g intravenous injection, Vit E 100mg Bid, oxygen inhalation 30min once daily for 10 days | Number with antibody titers lower than 1:64 post treatment |
| Zong 2012 [45] | 32:28 | >1:128 | 26–35 | 10–16 | Modified Erzhi Dihuang Yinchen Decoction 200ml Bid for 4 weeks | Vit C 100mg Tid, Vit E 100mg Bid for 4 weeks | Number with antibody titers lower than 1:64 post treatment |
| Zou 2001 [46] | 98:98 | >1:128 | Not reported | 16–34 | Yincan Erchen Decoction 200ml Bid for 2–6 weeks according to the baseline antibody titer | Vit C 0.2g Tid, Vit E 100mg Tid, oxygen inhalation 30min once daily for 2–6 weeks according to the baseline antibody titer | Number with antibody titers lower than 1:64 post treatment, HDN |
T: Treatment group; C: Control group; Vit: Vitamin; Tid: Three times daily; Bid: Twice daily; NJ: incidence of the neonatal jaundice; HDN: incidence of the newborn hemolytic disease; UCBB: umbilical cord blood bilirubin
Table 2. Components and adverse events of the herbal prescriptions in 28 included trials.
| Study ID | Herbal Medicine | Component of Prescription | Adverse events |
|---|---|---|---|
| An 2014a [15] | Yinchen Antai Decoction | Herba Artemisiae Scopariae 10g, Radix Scutellariae 10g, Radix Rehmanniae 10g, Radix et Rhizoma Salviae Miltiorrhizae 10g, Cortex Phellodendri Chinensis 6g, Cortex Moutan 10g, Fructus Gardeniae 10g, Radix et Rhizoma Glycyrrhizae 3g, Cortex Lycii 10g, Radix et Rhizoma Rhei 5g | Not report |
| An 2014b [16] | Yinchen Antai Decoction | Radix et Rhizoma Rhei 5g, Radix et Rhizoma Salviae Miltiorrhizae 10g, Cortex Phellodendri Chinensis 6g, Radix et Rhizoma Glycyrrhizae 3g, Cortex Lycii 10g, Radix Rehmanniae 10g, Cortex Moutan 10g, Radix Scutellariae 10g, Herba Artemisiae Scopariae 10g, Fructus Gardeniae 10g; Generalized itching combine with Radix Angelicae Dahuricae; Yellow complexion combine with Rhizoma Polygoni Cuspidati; Lumbago combine with Herba Taxilli; Abdominal pain combine with Radix Paeoniae Alba | abortion or stillbirth(1/4) |
| Cai 2009 [18] | Lianhuang Decoction | Receptaculum Nelumbinis 10g, Radix Astragali 15g, Radix et Rhizoma Rhei 6g, Herba Artemisiae Scopariae 20g, Cortex Eucommiae 20g, Radix Aucklandiae 6g, Rhizoma Atractylodis Macrocephalae 10g, Herba Agrimoniae 20g | Not report |
| Chen 2011 [19] | GutaiYinchen Decoction | Semen Cuscutae 15g, Radix Dipsaci 15g, Rhizoma Atractylodis Macrocephalae15g, Herba Artemisiae Scopariae 12g, Radix Angelicae Sinensis 12g, Radix Paeoniae Alba12g, Radix et Rhizoma Rhei 6g, Radix et Rhizoma Glycyrrhizae 6g, Radix Scutellariae 9g, Fructus Gardeniae 10g | Not report |
| Cui L 2014 [20] | Baishaogancao Decoction combine with Yinchen Dazao Decoction | Radix Paeoniae Alba 30g, Herba Artemisiae Scopariae 30g, Radix et Rhizoma Glycyrrhizae Praeparata cum Melle 10g, 5 Fructus Jujubae | Not report |
| Ding 2002 [21] | Xiaokang Dihuang oral liquid | Herba Artemisiae Scopariae, Radix Gardeniae, Radix et Rhizoma Rhei, Fructus Jujubae, Rhizoma Atractylodis Macrocephalae, Radix Scutellariae, Radix Dipsaci, Herba Taxilli | Adverse event was not found in herbal medicine group |
| Feng 2006 [22] | T1:Jigucao;T2:Jigucao Decoction | T1 HerbaAbri;T2 Herba Abri 30g, Radix seu Caulis Berberidis Gagnepainii 15g, Poria 15g,3 Receptaculum Nelumbinis, Radix et Rhizoma Glycyrrhizae 8g | Not report |
| Hu 2014 [23] | Quhuang Antai mixture | Semen Cuscutae 30g, Cortex Eucommiae 10g, Rhizoma Atractylodis Macrocephalae 10g, Fructus Corni 10g, Fructus Lycii 10g, Radix Codonopsis 24g, Radix Polygoni Multiflori Praeparata cum Succo Glycines Sotae 24g, Fructus Alpiniae Oxyphyllae 10g, Fructus Citri Sarcodactylis 10g, Poria 10g, Herba Artemisiae Scopariae 10g, Fructus Gardeniae Praeparatus 10g, Radix Scutellariae 15g, Radix Angelicae Sinensis 15g, Pericarpium Citri Reticulatae 6g, Radix et Rhizoma Glycyrrhizae 5g | Adverse event was not found in herbal medicine group |
| Jin 2013 [24] | Self made prescription | Herba Artemisiae Scopariae, Fructus Gardeniae, Radix Scutellariae, Radix Codonopsis, Radix et Rhizoma Glycyrrhizae et al. kidney deficiency syndrome combine with Radix Dipsaci, Herba Taxilli, Cortex Eucommiae; Spleen deficiency syndrome combine with Radix Astragali, Rhizoma Atractylodis Macrocephalae, Rhizoma Dioscoreae, Radix Aucklandiae, Semen Coicis, Poria; Abdominal pain combine with Radix Angelicae Sinensis, Radix Paeoniae Alba; Vagina bleeding combine with Nodus Nelumbinis Rhizomatis, Herba Agrimoniae; Middle and late pregnancy combine with Radix et Rhizoma Salviae Miltiorrhizae, Radix Paeoniae Rubra, Herba Leonuri | Not report |
| Li 2013 [25] | Modified Yinchenhao decoction | Herba Artemisiae Scopariae 9g, Fructus Gardeniae Praeparatus 6g, Radix et Rhizoma Rhei 4g, Radix Scutellariae 9g, Rhizoma Atractylodis Macrocephalae 15g, Poria 10g, Radix et Rhizoma Glycyrrhizae 6g | Not report |
| Li 2014 [26] | HuangyinAntai decoction | Radix et Rhizoma Glycyrrhizae 3g, Radix et Rhizoma Salviae Miltiorrhizae 10g, Radix Scutellariae 10g, Fructus Gardeniae 10g, Cortex PhellodendriChinensis 6g, Radix Rehmanniae 10g, Radix et Rhizoma Rhei 5g, Cortex Moutan 10g, Cortex Lycii 10g, Herba Artemisiae Scopariae 10g; Yellow complexion combine with Rhizoma Polygoni Cuspidati; Abdominal pain combine with Radix Paeoniae Alba; Generalized itching combine with Radix Angelicae Dahuricae, white peony root, Herba Schizonepetae | Not report |
| Li 2015 [27] | T1:Yinzhihuang oral liquid; T2:Yinzhihuang oral liquid combine with Danshen | T1: Herba Artemisiae Scopariae, Fructus Gardeniae, baicalin, Flos Lonicerae Japonicae; T2:HerbaArtemisiaeScopariae, Fructus Gardeniae, baicalin, FlosLonicerae Japonicae, Radix et Rhizoma Salviae Miltiorrhizae | Not report |
| Liu 2015 [28] | Yinzhihuang Oral Liquid | Herba Artemisiae Scopariae, Fructus Gardeniae, baicalin, Flos Lonicerae Japonicae | Not report |
| Luo 2013 [29] | Self made prescription | Herba Artemisiae Scopariae, Radix et Rhizoma Salviae Miltiorrhizae, Eucommia ulmoides oliv, Semen Cuscutae, Radix Paeoniae Alba, Radix Dipsaci, Radix Astragali, Fructus Gardeniae, Radix Scutellariae, Radix et Rhizoma Rhei, Radix et Rhizoma Glycyrrhizae | Not report |
| Lv 2012 [30] | YinchenErdanErhuang Decoction | Herba Artemisiae Scopariae 10g, Fructus Gardeniae 10g, Radix et Rhizoma Rhei 3-6g, Cortex Moutan 10g, Radix et Rhizoma Salviae Miltiorrhizae 10g, Radix Scutellariae 10g, Cortex Phellodendri Chinensis 6g, Radix Rehmanniae 10, Cortex Lycii 10g; Generalized itching combine with Radix Angelicae Dahuricae, white peony root, Herba Schizonepetae; Lumbago combine with Radix Dipsaci, Herba Taxilli; Abdominal pain combine with Radix Paeoniae Alba, Radix et Rhizoma Glycyrrhizae Praeparata cum Melle; Yellow complexion combine with Rhizoma Polygoni Cuspidati | Adverse event was not found in herbal medicine group |
| Mei 2004 [31] | Yinqi mixture | Radix Astragali, Rhizoma Atractylodis Macrocephalae, Radix Dipsaci, Radix Scutellariae, Herba Artemisiae Scopariae, Fructus Gardeniae | Not report |
| Su 2005 [33] | T1:Jigucao Decoction; T2:Modified Yinchen Decoction | T1:HerbaAbri 30g, Radix seu Caulis Berberidis Gagnepainii 15g, Poria 15g, 3 Receptaculum Nelumbinis, Radix et Rhizoma Glycyrrhizae 8g; T2:Herba Artemisiae Scopariae 9g, Radix Scutellariae 9g, Radix et Rhizoma Rhei 4.5g, Radix et Rhizoma Glycyrrhizae 6g | Not report |
| Sun2008 [36] | Yinzhi Kangrong Decoction | Herba Artemisiae Scopariae 15g, Semen Cuscutae 10g, Fructus Gardeniae 10g, Radix Scutellariae 10g, Rhizoma Atractylodis Macrocephalae 10g, Radix et Rhizoma Salviae Miltiorrhizae 10g, Poria 10g, Polyporus 10g, Radix et Rhizoma Glycyrrhizae 3g | Not report |
| Xu J 2009 [37] | Fangrong Antai powder | Herba Artemisiae Scopariae 15g, Herba Taxilli 10g, Radix Paeoniae Alba 10g, Rhizoma Atractylodis Macrocephalae 10g, Radix et Rhizoma Rhei 5g, Radix Scutellariae 10g, Radix et Rhizoma Glycyrrhizae 5g; Threatened abortion combine with Radix Rehmanniae Praeparata 15g, Radix Astragali 12g, Semen Cuscutae 10g; Dampness-heat syndrome combine with Fructus Gardeniae10g, Herba Taraxaci 12g | abortion or stillbirth(0/3) |
| Xu B 2011 [38] | Modified Yinchenhao Decoction | Herba ArtemisiaeScopariae 15g, Fructus Gardeniae 12g, Radix et Rhizoma Rhei 3g, Radix Scutellariae 12g, FlosLonicerae Japonicae 12g, Herba Lysimachiae 12g, Radix Astragali 12g, Radix Codonopsis 12g, Radix Boehmeriae 10g, Radix et Rhizoma Glycyrrhizae 6g; Spleen deficiency syndrome combine with Rhizoma Dioscoreae 15g, Rhizoma Atractylodis Macrocephalae 12g; Kidney deficiency syndrome combine with Herba Taxilli 12g, Radix Dipsaci 15g, Cortex Eucommiae 15g; Vagina bleeding combine with Herba Agrimoniae 12g, Carbonizing Radix Rehmanniae 10g; Diarrhea reduce or stop using Radix et Rhizoma Rhei | Adverse event was not found in herbal medicine group |
| Xu L 2011 [39] | TaierYixue Decoction | Radix Rehmanniae 20g, Herba Artemisiae Scopariae 15g, Fructus Gardeniae 15g, Radix et Rhizoma Rhei 10g, Radix Scutellariae 10g, Rhizoma Coptidis 6g, Herba Leonuri 6g, Radix Angelicae Sinensis 15g, Rhizoma Chuanxiong 15g, Radix Paeoniae Alba 30g, Herba Agrimoniae 6g, Radix Aucklandiae 6g, Radix et Rhizoma Ginseng 6g, Radix et Rhizoma Glycyrrhizae 6g | Not report |
| Yang 2015 [40] | T1:Yinzhihuang Oral Liquid;T2:Yinchenhao Decoction | T1: Herba Artemisiae Scopariae, Fructus Gardeniae, baicalin, Flos Lonicerae Japonicae; T2: Herba Artemisiae Scopariae 9g, Fructus Gardeniae Praeparatus 10g, Radix Scutellariae 9g, Radix et Rhizoma Glycyrrhizae 6g, Radix et Rhizoma Rhei 4.5g | Not report |
| Yu 2013 [41] | Self made prescription | Herba Leonuri 30g, Radix Codonopsis 20g, Radix Angelicae Sinensis 15g, Herba Artemisiae Scopariae 15g, Semen Cuscutae 15g, Semen Persicae 12g, Rhizoma Cyperi 12g, Radix Curcumae 12g, Radix Scutellariae 12g, Rhizoma Atractylodis Macrocephalae 12g, Rhizoma Chuanxiong 9g, Radix et Rhizoma Rhei 9g, Radix et Rhizoma Glycyrrhizae Praeparata cum Melle 6g | abortion or stillbirth(2/10) |
| Zhan2013 [42] | Modified Yinchenhao Decoction | ORAL Herba Artemisiae Scopariae 15g, Fructus Gardeniae 9g, Radix et Rhizoma Rhei 3g, Herba Artemisiae Annuae 9g, Radix Scutellariae 9g, Cortex Eucommiae 15g, Semen Cuscutae 15g, Radix Dipsaci 15g, Endothelium Corneum Gigeriae Galli 9g, Radix et Rhizoma Glycyrrhizae 9g; Thirsty combine with Rhizoma Phragmitis 15g, Radix Glehniae 15g; Torpid intake combine with Fructus Amomi 6g, Pericarpium Citri Reticulatae; Sloppy stool stop using Radix et Rhizoma Rhei; Lumbago combine with Cortex Eucommiae Washing Herba Artemisiae Scopariae 30g, Fructus Gardeniae 15g, Radix et Rhizoma Rhei 10g, Herba Artemisiae Annuae 10g |
Adverse event was not found in herbal medicine group |
| Zhao 2012 [43] | T1: Xiaohuang Decoction T2:Yinchenhao Decoction | T1:HerbaArtemisiaeScopariae 10g, Fructus Gardeniae Praeparatus 10g, Radix et Rhizoma Rhei 5g, Radix Scutellariae 15g, Pericarpium Citri Reticulatae 10g, Herba Artemisiae Annuae 10g, Semen Cuscutae 30g, Cortex Eucommiae 20g, Rhizoma Atractylodis Macrocephalae 20g, Radix et Rhizoma Glycyrrhizae 12g; T2: Herba Artemisiae Scopariae 18g, Fructus Gardeniae Praeparatus 10g, Radix et Rhizoma Rhei 6g | Not report |
| Zhou 2007 [44] | Yinchen Decoction | Herba Artemisiae Scopariae 12g, Radix et Rhizoma Rhei 4g, Radix Scutellariae 9g, Radix et Rhizoma Glycyrrhizae 6g | Not report |
| Zong 2012 [45] | Modified Erzhi Dihuang Yinchen Decoction | Fructus Ligustri Lucidi 12g, Radix Scutellariae 12g, Rhizoma Alismatis 12g, Poria 12g, Herba Ecliptae 15g, Radix Rehmanniae Praeparata 15g, Semen Cuscutae 15g, Herba Artemisiae Scopariae 20g, Rhizoma Zingiberis Recens 6g, Radix et Rhizoma Glycyrrhizae 10g | Not report |
| Zou 2002 [46] | Yincan Erchen Decoction | Herba Artemisiae Scopariae, Faeces Bombycis, Sclerotium Poriae Pararadicis, Rhizoma Pinelliae Praeparatum, Pericarpium Citri Reticulatae | Not report |
Risk of bias of included studies
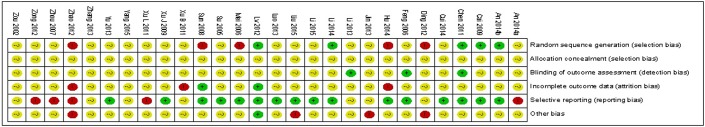
All of the included trials stated that the treatments were randomly assigned, however only five of them [16, 18, 19, 26, 30] were assessed as having low risk of selection bias because they reported that a random numbers table was used to generate random allocation. Five trials [21, 23, 31, 36, 43] had unequal numbers between the groups, with two of them [23, 43] reporting they excluded participants after randomization if the blood type of the newborn was ‘O’. These five trials were evaluated as having high risk of selection bias. The remaining seventeen trials did not provide sufficient information to judge their risk of selection bias. Furthermore, none of the included trials mentioned allocation concealment, thus all of the trials were evaluated to have unclear risk of bias regarding to this item.
Except for three trials [19, 22, 25] which employed a third party to measure treatment outcomes, the other twenty-five trials were assessed as having unclear risk of detection bias.
Two trials [30, 36] were evaluated as having low risk of attrition bias because they clearly explained the reasons for drop outs, with equal numbers of drop outs (1 or 2 cases) in each group which is unlikely to impact on the final results. Three trials were evaluated as having high risk of attrition bias: one trial [37] reported a few cases of drop outs due to high and unchanged antibody titers during treatment and two other trials [23, 43] excluded participants after randomization if the blood type of the new born were ‘O’.
Since the protocols of the trials were unavailable, we determined the risk of reporting bias according to whether the trial reported all the key outcomes (HDN and antibody titer after treatment). Therefore, sixteen trials [16, 18–20, 22, 23, 26–31, 33, 36, 38, 41] had low risk of reporting bias and five trials [15, 39, 43–45] had high risk of reporting bias.
Only one trial [30] was assessed as having low risk of other bias, as this trial was well designed with an appropriate sample size calculation. Two trials [21, 24] had only one author and another two trials [28, 43] had poor methodology which may influence the results. These four trials were defined as having high risk of other bias.
In summary, twelve of the included trials [15, 21, 23, 31, 36, 37, 38, 43–45] had poor methodological quality due to the potential high risk of selection, attrition, reporting or other bias. Only five trials [16, 18, 19, 26, 30] were assessed as having low risk of bias because of adequate randomization and completeness of the report. The remaining trials all had unclear risk of bias. The risk of bias summary for each trial is showed in Fig 2.
Fig 2. Risk of bias summary for each trial.
Effects of interventions
Primary outcomes
Hemolytic Disease of the Newborn
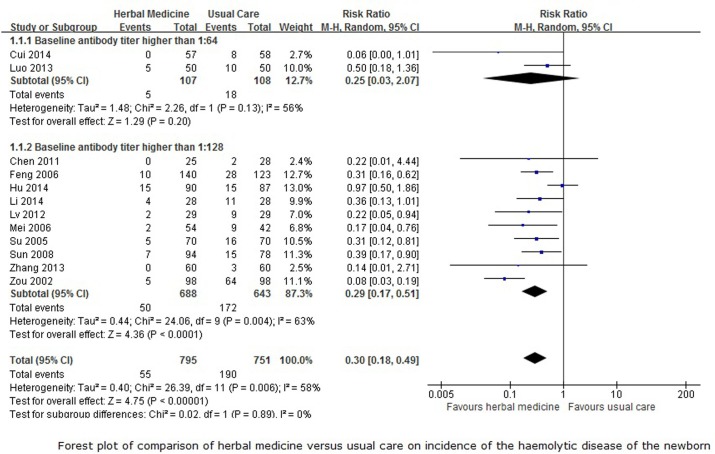
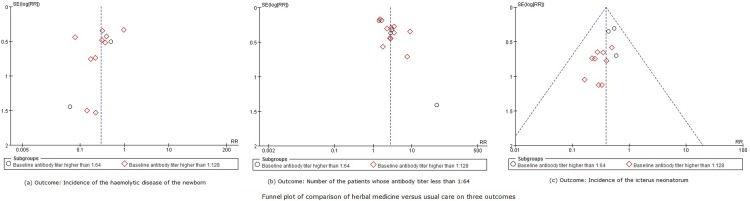
Data from twelve trials that reported the rate of HDN and compared CHM with usual care were pooled in a meta-analysis. The baseline antibody titer in two of the trials[20, 29] were higher than 1:64, and in the other ten trials [19, 22, 23, 26, 30, 31, 33, 36, 42, 46] were higher than 1:128, thus we performed subgroup meta-analyses on these 12 trials. With potential statistical heterogeneity between trials (50%< I2<75%), the subgroup analysis of participants with a baseline antibody titer that was higher than 1:64showed no difference between herbal medicine and usual care in decreasing the incidence of the HDN (RR 0.25, 95%CI 0.03 to 2.07, 215 participants, 2 trials, I2 = 56%, random-effect model, p = 0.20), however, herbal medicine may superior to the usual care when the baseline antibody titer of the participants was higher than 1:128 (RR 0.29, 95%CI 0.17 to 0.51, 1331 participants, 10 trials, I2 = 62%, random-effect model, p<0.0001). The overall meta-analysis (Fig 3) showed that CHM was better at reducing the incidence of HDN compared to usual care (RR 0.30, 95%CI 0.18 to 0.49, 1546 participants, 13 trials, I2 = 58%, random-effect model, p<0.00001). The funnel plot for this comparison showed considerable asymmetry (Fig 4A), which indicates that publication bias cannot be ruled out for this outcome.
Fig 3. Forest plot for incidence rate of HDN.
Fig 4. Funnel plot for three outcomes.
One trial [25] also compared CHM with usual care and reported that no case of HDN occurred. One other trial [41] found CHM was superior to no treatment in reducing the number of HDN (RR 0.36, 95%CI 0.14 to 0.87, 170 participants). Another trial [16] reported no difference between herbal medicine plus usual care and usual care alone in reducing the incidence of the HDN when the baseline antibody titer of participants was higher than 1:128 (RR 0.42, 95%CI 0.17 to 1.04, 60 participants).
Secondary outcomes
Antibody titer after treatment
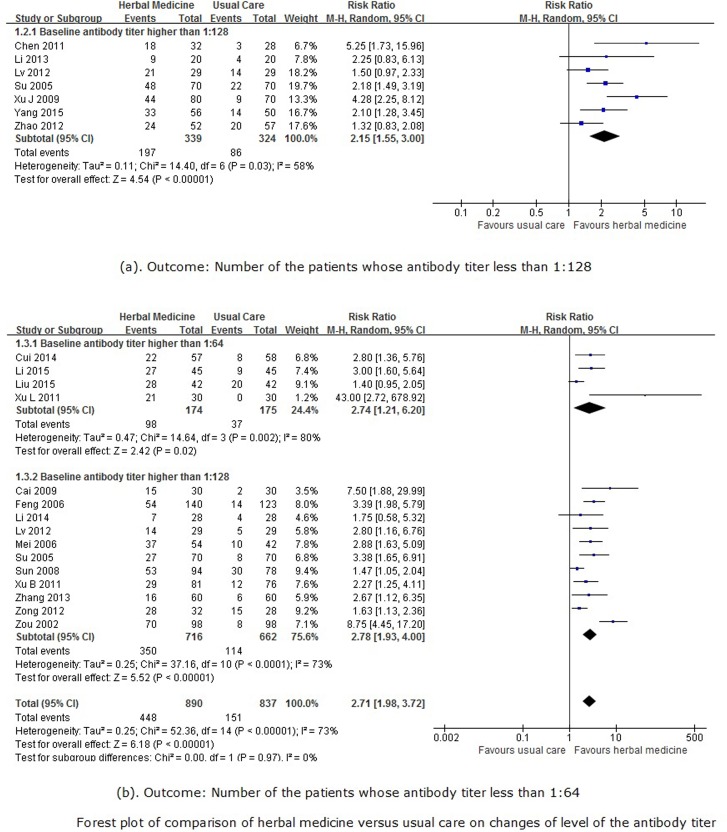
Seven trials [19, 25, 30, 33, 38, 40, 43] reported the number of patients with post-treatment antibody titers of less than 1:128. The meta-analysis of these studies (Fig 5A) showed that herbal medicine was superior to usual care in reducing the antibody titer to below 1:128(RR 2.15, 95%CI 1.55 to 3.00, 663 participants, 7 trials, I2 = 58%, random-effect model, p<0.00001). Another trial [24] found herbal medicine combined with usual care was superior to usual care alone for reducing antibody titers to below 1:128 (RR 3.14, 95%CI 1.59 to 6.23, 60 participants).
Fig 5. Forest plot for antibody titer value.
Another fifteen trials [18, 20–23, 26, 27, 29, 31, 36, 37, 39, 42, 45, 46] reported the number of patients with post-treatment antibody titers of less than 1:64.In the subgroup meta-analysis, herbal medicine was superior to usual care in reducing antibody titers to below 1:64 (RR 2.71, 95%CI 1.98 to 3.72, 1727 participants, 15 trials, I2 = 73%, random-effect model, p<0.00001), regardless of whether the baseline antibody titer was higher than 1:64 (RR 2.74, 95%CI 1.21 to 6.20, 349 participants, 4 trials, I2 = 80%, random-effect model, p = 0.02) or 1:128 (RR 2.78, 95%CI 1.93 to 4.00, 1378 participants, 11 trials, I2 = 73%, random-effect model, p<0.00001) (Fig 5B). There was also evidence of considerable asymmetry in the funnel plot (Fig 4B) for this comparison. The other two trials [28, 44] found there were no difference between herbal medicine plus usual care and usual care alone (RR 1.62, 95%CI 1.09 to 2.39, 161 participants, 2 trials, I2 = 27%, random-effect model, p = 0.02).
Incidence of the neonatal jaundice
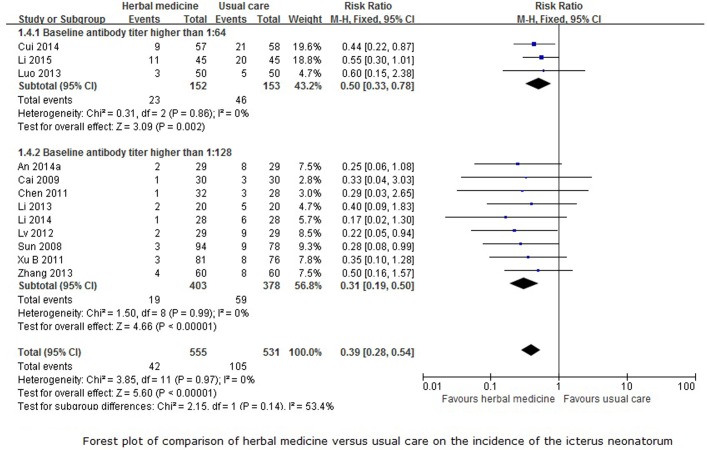
Twelve trials [15, 18–20, 25–27, 29, 30, 36, 37, 42] reported the incidence of the neonatal jaundice of the newborn with comparison between herbal medicine and usual care. The subgroup analysis showed that herbal medicine was more effective at reducing the incidence of neonatal jaundice compared to usual care whether the baseline antibody titer was higher than 1:64 (RR 0.50, 95%CI 0.33 to 0.78, 305 participants, 3 trials, I2 = 0%, fixed-effect model, p = 0.002) or 1:128 (RR 0.31, 95%CI 0.19 to 0.50, 781 participants, 9 trials, I2 = 0%, fixed-effect model, p<0.0001). The overall meta-analysis (Fig 6) also showed the same results (RR 0.39, 95%CI 0.28 to 0.54, 1086 participants, 12 trials, I2 = 0%, fixed-effect model, p<0.00001) for this outcome. The funnel plot for this comparison also showed considerable asymmetry (Fig 4C).
Fig 6. Forest plot for incidence of neonatal jaundice.
Another trial [16] reported no difference between herbal medicine plus usual care and usual care alone on reducing the incidence of neonatal jaundice when the baseline antibody titer of participants was higher than 1:128 (RR 0.25, 95%CI 0.06 to 1.08, 60 participants).
Umbilical cord blood bilirubin
One trial [20] where participants’ antibody titers were higher than 1:64 before treatment reported that herbal medicine may be superior to usual care in reducing umbilical cord blood bilirubin (MD -5.40 umol/L, 95%CI -7.76 umol/L to -3.04 umol/L, 115 participants). Similar results were reported in another five trials [18, 19, 26, 36, 38] where the baseline antibody titer of participants was higher than 1:128 (MD -4.05 umol/L, 95%CI -5.81 umol/L to -2.29 umol/L, 493 participants, 5 trials, I2 = 33%, random-effect model, p<0.00001). The overall meta-analysis showed that herbal medicine was superior to usual care in reducing unbilical cord blood bilirubin (MD -4.33 umol/L, 95%CI -5.84 umol/L to -2.82 umol/L, 608 participants, 6 trials, I2 = 35%, random-effect model, p<0.00001).
Another trial [16] reported an even larger effect when comparing herbal medicine as add-on compared with usual care alone (MD -10.10 umol/L, 95%CI -10.53 umol/L to -8.67 umol/L, 60 participants).
Apgar scores
There was no difference in the newborn’s Apgar scores between the herbal medicine and usual care groups in six trials [18–21, 23, 26, 36, 40] (MD 0.10, 95%CI -0.06 to 0.26, 572 participants, 6 trials, I2 = 0%, fixed-effect model, p = 0.24), irrespective of whether the baseline antibody titer was higher than 1:64 (MD 0.10, 95%CI -0.08 to 0.28, 457 participants, 5 trials, I2 = 0%, fixed-effect model, p = 0.29) or 1:128 (MD 0.10, 95%CI -0.27 to 0.47, 115 participants).
Another two trials [21, 23] only reported the numbers of the newborn with Apgar scores of less than 8, with no difference between the CHM and usual care groups (RR 0.97, 95%CI 0.29 to 3.22, 177 participants) and between CHM plus usual care and usual care alone (RR 0.67, 95%CI 0.12 to 3.82, 98 participants).
Birthweight
Five trials [18, 19, 23, 36, 40] reported the birthweight of the newborn, with no difference between the herbal medicine and usual care groups demonstrated in the meta-analysis (MD 0.06kg, 95%CI -0.04kg to 0.15kg, 578 participants, 5 trials, I2 = 41%, random-effect model, p = 0.27). Another trial [21] also found no difference in birthweight between herbal medicine plus usual care and usual care alone groups (MD 0.05kg, 95%CI -0.42kg to 0.52kg, 98 participants).
Adverse events
Twenty of the 28 included trials did not report whether there were any adverse events during the treatment period. Six [21, 23, 30, 38, 41, 42] of the remaining eight trials found no adverse events in herbal medicine group. Only two trials [16, 37] mentioned the adverse events which were present in both herbal medicine and control groups. One reported a case (3.33%) of abortion/stillbirth in herbal group and four cases (13.33%) of abortion/stillbirth in control group, and the other trial reported two cases (2.17%) of abortion/stillbirth in herbal group and ten cases (11.11%) of abortion/stillbirth in control group.
For the meta-analyses in which the fix-effects model and random effects model were both used as sensitive analysis, the results were consistent irrespective of potential heterogeneity between trials. Only the results of the meta-analysis using the random-effects model were reported in this review.
Discussion
Summary of main results
This review included 28 randomized controlled trials which assessed the effects of herbal medicine for preventing hemolytic disease of the newborn in pregnant women with ABO incompatibility and antibody titers that were higher than 1:64. The majority of the trials had unclear or high risk of bias according to the mentioned assessment criteria. The main results found that the rate of HDN and the incidence of neonatal jaundice in herbal medicine group might be 70% lower compared than in the usual care group (25.3% in usual care group, RR from 0.25 to 0.30); twice as many women taking herbal medicine (RR from 2.15 to 3.14) had post-treatment antibody titers lower than 1:64 compared with those receiving usual care, and umbilical cord blood bilirubin in herbal medicine group was 4 umol/L less than in the usual care group. There was no difference in Apgar scores and birthweights between groups. However, considering the poor methodological quality of the included trials, there remains some uncertainty about whether Chinese herbal medicine prevents HDN due to the ABO incompatibility.
Only a few included trials mentioned adverse events, with six trials reporting no adverse event in the herbal medicine group, and another two trials reported lower rates of fetal loss in the herbal medicine group (3%) than in the control group (13%). Due to insufficient information regarding the safety of herbal medicine for pregnant women, more trials will be needed to confirm its safety.
Overall completeness and applicability of evidence
We searched two English databases and four Chinese databases without language restrictions. However, all of the 28 included trials from this review were conducted and published in China. In countries outside of China, there is currently no standard therapy for treating maternal-fetal ABO incompatibility during pregnancy, with therapeutic procedures only being provided if the newborn was diagnosed with HDN after birth. Thus, the results of this review may have significant implications for providing a preventative therapy for HDN caused by ABO incompatibility.
Quality of the evidence
As previously mentioned, only five trials were evaluated as having low risk of bias. The remaining 23 trials all have poor methodology and have high or unclear risk of selection, attrition, reporting and other bias.
We employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria ratings to assess the quality of the evidence; factors that downgraded the quality include imprecision, inconsistency, indirectness, limitations and bias of the evidence. For the meta-analyses in this review, we downgraded the level of evidence mainly because of the potential for performance, attrition, and reporting bias and the inconsistency of the results between the included studies. Subsequently, we could find only very low-quality evidence that CHM may reduce the incidence of HDN and reduce antibody titers, and low-quality evidence that CHM may reduce the incidence of neonatal jaundice and umbilical cord blood bilirubin levels (Table 3 Summary of finding table).
Table 3. Summary finding table with herbal medicine vs. usual care for maternal and infant blood type incompatibility.
| Patient or population: Pregnant women with maternal and infant blood type incompatibility | |||||
| Settings: The outpatient department of the traditional Chinese medicine hospital | |||||
| Intervention: Herbal medicine versus usual care | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| (95% CI) | (GRADE) | ||||
| Usual care | Chinese herbal medicine | ||||
| Incidence of the hemolytic disease of the newborn | 253 per 1000 | 76 per 1000 | RR 0.3 | 1546 | ⊕⊝⊝⊝ |
| (46 to 124) | (0.18 to 0.49) | (12 studies) | very low1,2,3 | ||
| Number of the patients whose antibody titer less than 1:128 | 265 per 1000 | 571 per 1000 | RR 2.15 | 663 | ⊕⊝⊝⊝ |
| (411 to 796) | (1.55 to 3) | (7 studies) | very low1,2,3 | ||
| Number of the patients whose antibody titer less than 1:64 | 180 per 1000 | 489 per 1000 | RR 2.71 | 1727s) | ⊕⊝⊝⊝ |
| (357 to 671) | (1.98 to 3.72) | (15 studies | very low1,3,4 | ||
| Incidence of the neonatal jaundice | 198 per 1000 | 77 per 1000 | RR 0.39 | 1086 | ⊕⊕⊝⊝ |
| (55 to 107) | (0.28 to 0.54) | (12 studies) | low1,3 | ||
| Umbilical cord blood bilirubin (umol/L) | The mean umbilical cord blood bilirubin (umol/l) in the control groups was32.35 umol/L | The mean umbilical cord blood bilirubin (umol/l) in the intervention groups was4.33 lower(5.84 to 2.82 lower) | 608 | ⊕⊕⊝⊝ | |
| (6 studies) | low1,3 | ||||
| Apgar scores(Scale from0 to 10) | The mean Apgar scores in the control groups was9.23 | The mean Apgar scores in the intervention groups was0.1 higher(0.06 lower to 0.26 higher) | 572 | ⊕⊕⊝⊝ | |
| (6 studies) | low1,3 | ||||
| Weigh of newborn (kg) | The mean Birthweight in the control groups was3.10 | The mean Birthweight in the intervention groups was0.06 higher(0.04 lower to 0.15 higher) | 578 | ⊕⊝⊝⊝ | |
| (5 studies) | very low1,2,3 | ||||
*The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
1 Majority trials had unclear risk of selection, detection, attrition and reporting bias
2 The I square test showed a significant statistical heterogeneity among trials (I-square larger than 50%)
3 The asymmetric funnel plot indicated the possibility of publication bias.
4 The I square test showed a significant large statistical heterogeneity among trials (I square larger than 70%)
Agreements and disagreements with other studies or reviews
Two other reviews [1, 48] were found on this topic, which were all dissertations for masters’ degrees and written in Chinese, with one of them being published in 2015 [1]. One review included eleven trials and the other included 20 trials. Both of them used a total effective rate as the primary outcome measure, which is a composite outcome index combining the reduction of antibody titers, rate of HDN and adverse birth outcomes post treatment. Although this outcome index is commonly used in China, it is difficult to determine its clinical relevance since the definition of “effectiveness” is inconsistency between studies. These two reviews also conducted meta-analyses to investigate the effects of CHM in reducing the rate of HDN, with results consistent with our studies, showing that CHM was more likely to reduce the incidence of HDN compared to usual care (RR = 0.19 or 0.25, p<0.05). One of the reviews also reported there was no difference between CHM and usual care in Apgar scores and birthweight.
Our review included 28 trials and assessed the effects of CHM for reducing the incidence of HDN, the antibody-titer, the incidence of neonatal jaundice and umbilical cord blood bilirubin. Subgroup-meta analyses were conducted for the participants with different baseline antibody titer values. We found similar results to the previous reviews, showing that CHM is effective in reducing the incidence of HDN (RR = 0.30, p<0.05), with the overall quality of the evidence appraised according to the GRADE criteria.
Implications for practice
Maternal-fetal ABO incompatibility may be asymptomatic during pregnancy, and patients may not be aware they have this condition. As there are currently no guidelines or standard therapies to prevent HDN caused by ABO incompatibility during pregnancy, this study may have identified a possible therapy for preventing this condition.
According to the review, five herbs are frequently used to prevent HDN caused by maternal-fetal ABO incompatibility, including Herba Artemisiae Scopariae (Yin Chen), Rhizoma Glycyrrhizae (Gan Cao), Fructus Gardeniae (Zhi Zi), Radix Scutellariae (Huang Qin), and Radix et Rhizoma Rhei (Da Huang). Among these five herbs, Yin Chen has been commonly used for treating jaundice and HDN.A laboratory study [49] found that liver fibrosis induced by dimethylnitrosamine in rats was improved by the administration of Yinchenhao Decoction (in which Yin Chen was the primary active ingredient), with a reduction in hydroxyproline and a significant improvement in liver function and hepatic histology after 2 weeks of treatment. Da Huang was found to promote the secretion of bile, causing gallbladder and biliary sphincter relaxation which may relieve jaundice. The potential mechanism may be through increased expression of transport proteins associated with the metabolism of bile acids to reduce the accumulation of bile acids and other toxic compounds in the liver [50, 51], which could improve the ultrastructure of liver cells, thus affecting the intracellular enzyme activity and the concentration of calcium ions in the cells [52]. Huang Qin is thought to be a heat-clearing, phlegm-removing herb, traditionally used to cool heat, clear damp-heat, and calm the fetus [53]. It is also found to contain compounds with anti-inflammatory and antimicrobial effects [54].Zhi Zi has effects on the contraction of gallbladder, containing iridoid and Saffron glucoside which may increase the secretion of bile [55].Even though there are some studies (cited as above) tried to investigate the potential mechanism of the specific herbs in treating HDN, we need to aware that the mechanisms of how the Chinese herbal medicine could eliminate maternal IgG antibodies were not evidenced.
Regarding safety considerations, medications used during pregnancy require extra caution. According to Chinese herbal medicine traditions, herbs that work on clearing heat are usually forbidden during pregnancy, as it is believed that oral intakes of these herbs may cause abortions and other serious adverse birth outcomes. However, four of the top five most frequently used herbs in the included trials from this review are herbs that have the function of clearing heat [53]. The majority of trial participants took the herbal decoction for at least half of their pregnancy until labor, with no or less adverse events (such as fetal loss) reported in the treatment group compared with the usual care group. This finding suggests that it may be safe for pregnant women with ABO incompatibility to use CHM with herbs that have the function of clearing heat and draining dampness, under the guidance of herbal medicine practitioners. According to our study, the incidence of HDN from ABO incompatibility may decrease from 25.3% to 7.6% when CHM is used during pregnancy, compared to usual care. However, we have low confidence in certainty of the safety issue using Chinese herbal medicine to prevent or treat ABO HDN based on the current evidence.
Conclusions
This review of28 included trials found very low-quality evidence that CHM using herbs with the function of clearing heat and draining dampness may prevent hemolytic disease of the newborn when used in pregnant women with ABO incompatibility. Due to insufficient information, no firm conclusions can be draw based on this review’s results regarding the effectiveness of CHM for this condition, as well as the safety of the relevant herbal medicine for pregnant women.
Supporting information
(PDF)
(DOCX)
Acknowledgments
Huijuan Cao is supported by the Beijing Municipal Organization Department talents project (Grant No. 2015000020124G083) and the Research Capacity Establishment Grant (No. 2015-JYB-JSMS037) from the Beijing University of Chinese Medicine. Jian-Ping Liu is supported by the Research Capacity Establishment Grant (No. 201207012) from the State Administration of Traditional Chinese Medicine.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work. Hui-Juan Cao is supported by the Beijing Municipal Organization Department talents project (Grant No. 2015000020124G083) and the Research Capacity Establishment Grant (No. 2015-JYB-JSMS037) from the Beijing University of Chinese Medicine. Jian-Ping Liu is supported by the Research Capacity Establishment Grant (No. 201207012) from the State Administration of Traditional Chinese Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huang J, Sun HQ. Systematic review on Chinese herbal medicine for maternal-fetal ABO blood group incompatibility. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2015; 21(2): 55–59; [Google Scholar]
- 2.Song XC, Li YJ, Xu J, Hu AH. Correlation analysis of antenatal IgG antibody titer determination of pregnant woman of blood type O and Hemolytic disease of newborn. J Clin Hemotal (Chinese). 2015; 28(4): 333–335; [Google Scholar]
- 3.Cao ZY. Obstetrics and Gynecology (2nd Edition). Beijing: People’s Medical Publishing House; 2005: 680–689; [Google Scholar]
- 4.Akanmu AS, Oyedeji OA, Adeyemo TA, Ogbenna AA. Estimating the risk of ABO hemolytic disease of the newborn in Lagos.Journal of Blood Transfusion. 2015; 2015: 1–5; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng YJ, Shen K. Gynecotokology. Beijing: People’s Medical Publishing House; 2010: 88–90; [Google Scholar]
- 6.Dodd JM, Windrim RC, Kamp IL. Techniques of intrauterine fetal transfusion for women with red-cell isoimmunisation for improving health outcomes.Cochrane Databases of Systematic Reviews. 2012; (9): doi: 10.1002/14651858.CD007096.pub3 [DOI] [PubMed] [Google Scholar]
- 7.Bian J. Clinical experience of Zhao Yongmei in treating maternal-fetal blood group incompatibility.Chinese Journal of Information on TCM (Chinese).1998; (5): 42; [Google Scholar]
- 8.Yang ZM. Kidney Nourishing therapy for 200 cases of functional sterility. Acta Academiae Medicinae Jiangxi.1991; (2):151; [Google Scholar]
- 9.Li Q, Li GX, Ding HY. Prenatal treatment with Chinese herbal medicine for 65 cases of maternal-fetal ABO blood group incompatibility. Liaoning Journal of Traditional Chinese Medicine (Chinese).2006; 33(11):1442; [Google Scholar]
- 10.Huh Keun, Park Jong-Min, Shin Uk-Seob, et al. Effect of scoparone on the hepatic microsomal UDP glucuronyl transferase activity in Mice. Arch Pharm Res. 1990; 13 (1): 51–54; [Google Scholar]
- 11.Wang XJ, Sun WJ, Liu L, et al. Influence on constituents in blood with different compatibility of Yinchenhao Decoction. Chinese Journal of Natural Medicines (Chinese). 2008; 6(1): 43–47; [Google Scholar]
- 12.Han LS, Hu H, Wang F, Ma YT, Protective effects of zang ying chen on liver and multiple organs injury by CCL4. Journal of Medicine & Pharmacy of Chinese Minorities (Chinese). 2005; (03): 20–21; [Google Scholar]
- 13.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2.The Cochrane Collaboration. 2009;
- 14.Higgins JPT, Thompson SG, Quantifying heterogeneity in a meta-analysis. Statist in Med 2002; 21(11): 1539–1558; [DOI] [PubMed] [Google Scholar]
- 15.An WQ, Li F, Li J, Chen XJ, Zhang JL. Impact of ABO blood type incompatibility on the newborn with the comparative of treatment of Huangyin Antai Decoction and western medicine based on the antibody titer level. Modern Journal of Integrated Traditional Chinese and Western Medicine (Chinese). 2014; 23(26): 2929–2931; [Google Scholar]
- 16.An WQ, Li F, Li J, Chen XJ, Zhang JL. Combination of Huangyin Antai Decoction and western medicine for changes of antibody titer value of the pregnant women with ABO incompatibility and the blood bilirubin of their newborns. Modern Journal of Integrated Traditional Chinese and Western Medicine (Chinese). 2014; 23(23): 2572–2573; [Google Scholar]
- 17.An WQ, Li F, Li J, Chen XJ, Zhang JL. Investigation of the clinical implication of combination of Huagyin Antai Decoction and western medicine on preventing the hemolytic disease of the newborn due to ABO incompatibility. Yi Xue Mei Xue Mei Rong (Chinese). 2014; (9): 11; [Google Scholar]
- 18.Cai ZH, Chen YY, Zeng Y, Qian DL, Xie LH, Zhou SC. Effects of Lianhuang Decoction in treating fetomaternal ABO blood group incompatibility. Zhong Guo Zhong Xi Yi Jie He Za Zhi (Chinese). 2009; 29(2): 156–158; [PubMed] [Google Scholar]
- 19.Chen XY, Gong Y, Zhou QX. Observation of the effect of Gutai Yinchen Decoction on preventing 32 cases of maternal-fetal ABO blood type incompatibility. Journal of New Chinese Medicine (Chinese). 2011; 43(8): 79–80; [Google Scholar]
- 20.Cui L, Tao C. Clinical observation of Chinese herbal medicine for ABO incompatibility. Journal of Community Medicine (Chinese). 2014; 12(23): 35–36, 44; [Google Scholar]
- 21.Ding SJ. Integrative medicine for 57 cases of ABO incompatibility. Journal of Sichuan of Traditional Chinese Medicine. 2002; 20(11): 61–62; [Google Scholar]
- 22.Feng HJ, Zhou XR, Su XJ. Observation of therapeutic effect of herba abriin treatment of 148 cases with ABO blood group incompatibility in pregnant women and infant. Maternal and Child Health Care of China (Chinese). 2006; (21): 1712–1714; [Google Scholar]
- 23.Hu FY, Lv L. Clinical observation of Quhuang Antai Decoction on hemolytic disease caused by maternal-fetal ABO incompatibility. Chinese Journal of Traditional Medical Science and Technology (Chinese). 2014; 21(6): 660–662; [Google Scholar]
- 24.Jin HY. Observation on effect of combination of traditional Chinese and western medicine treatment of 30 cases of blood group incompatibility. Journal of Liaoning University of TCM (Chinese). 2013; 15(5): 197–198; [Google Scholar]
- 25.Li XH. Effect of modified Yinchenhao Decoction for 40 cases of maternal-fetal blood group incompatibility. Guangming Journal of Chinese Medicine (Chinese). 2013; 28(7): 1351–1352; [Google Scholar]
- 26.Li F, An WQ. Comparative between Huangyin Antai Decoction and western medicine for hemolytic disease of the newborn caused by ABO incompatibility. Modern Journal of Integrated Traditional Chinese and Western Medicine (Chinese). 2014; 23(32): 3573–3574; [Google Scholar]
- 27.Li F, An WQ, Li J, Chen XJ, Zhang JL. Yinzhihuang oral solution combined with Salviain treating maternal-fetal ABO blood group incompatibility (revised). World Journal of Integrated Traditional and Western Medicine (Chinese). 2015; 10(11): 1583–1586; [Google Scholar]
- 28.Liu XQ, Zhe KE, Li QM. Clinical observation of the effect of Yinzhihuang oral liquid for maternal-fetal ABO blood group incompatibility. Chinese Journal for Clinicians (Chinese). 2015; 43(2): 83–85; [Google Scholar]
- 29.Luo M. Clinical observation of the combination of Chinese herbal medicine and Danshen injection for maternal-fetal blood group incompatibility. Hebei Medicine. 2013; 19(6): 908–910; [Google Scholar]
- 30.Lv YD. Randomized, parallel, controlled clinical observational study o Yin Chen Er Dan Er Huang Decoction treat in maternal-fetal ABO blood group incompatibility of the syndrome of damp-heat complex. Dissertation for Master Degree of Chengdu University of Chinese Medicine. 2012;
- 31.Mei RS. Effect of integrative medicine for 50 cases of maternal-fetal blood group incompatibility. New Journal of Traditional Chinese Medicine (Chinese). 2004; 36(12): 42–43; [Google Scholar]
- 32.Mei RS, Wu EP, Zhou MH, Hu JS, Wang JX. Clinical study of Yinchen Heji for decreasing incidence rate of hemolytic disease of the newborn. Chinese Archives of Traditional Chinese Medicine (Chinese). 2006; 24(8): 1486–1487; [Google Scholar]
- 33.Su XJ, Feng HJ. Jigucao Decoction for 70 cases of maternal-fetal ABO blood group incompatibility. New Journal of Traditional Chinese Medicine (Chinese). 2005; 37(7): 47–48; [Google Scholar]
- 34.Zhou XR, Su XJ, Feng HJ, Feng XP. Clinical observation on treatment of ABO blood group incompatibility in pregnant women and infant by Jigucao Tang. Hebei Medicine (Chinese). 2004; 10(12):1089–1090; [Google Scholar]
- 35.Ouyang X. Study on Yinzhikangrongtang prevent and treat ABO materal-fetal blood group incompatibility hemolytic disease. Dissertation for Master Degree of Guangxi Traditional Chinese Medical University. 2005;
- 36.Sun QH, Ouyang X. Yinzhi Kangrong Decoction for 94 cases of maternal-fetal blood group incompatibility. Shanxi Journal of Traditional Chinese Medicine (Chinese). 2008; 29(8): 1030–1032; [Google Scholar]
- 37.Xu J, Li M, Wang Q. Summary of the effect of Fangrong Antai Powder for 80 cases of maternal-fetal ABO blood group incompatibility. Hunan Journal of Traditional Chinese Medicine (Chinese). 2009; 25(5): 28–29; [Google Scholar]
- 38.Xu BH, Li MQ. Modified Yichehao Decoction for 81 cases of maternal-fetal ABO incompatibility. J Tradit Chin Med (Chinese). 2011; 52(16): 1418–1419; [Google Scholar]
- 39.Xu LR, Liu SY, Qiao ZH, Zhao XY. Clinical observation of Chinese herbal medicine on preventing hemolytic disease caused by maternal-fetal ABO incompatibility. Hebei J TCM (Chinese). 2011; 33(3): 368–369; [Google Scholar]
- 40.Yang SZ, Wei JH. Effect of Yinzhihuang oral liquid for maternal-fetal blood group incompatibility. Health Vocational Education (Chinese). 2015; 33(5): 146–147; [Google Scholar]
- 41.Yu LP. Clinical observation of Chinese herbal medicine for hemolytic disease caused by maternal-fetal ABO incompatibility. Journal of New Chinese Medicine (Chinese). 2013; 45(8): 115–116; [Google Scholar]
- 42.Zhang YH, Liu WJ. Curative effect observation of flavored Artemisiacapillaries soup with Chinese medicine external treatment of mother washing son ABO blood group. China Modern Doctor (Chinese). 2013; 51(18): 97–99; [Google Scholar]
- 43.Zhao L, Yin H, Lv L, Hu FY, Yu JW. Research the clinical prevention and treatment of Xiaohuangyin for the ABO maternal-fetal blood group incompatibility. Journal of Zhejiang University of Chinese Medicine (Chinese). 2012; 36(6): 630–632; [Google Scholar]
- 44.Zhou J, Huang YJ, Liu XL, Huang YP. Clinical observation of Yinchen Decoction for 41 cases maternal-fetal ABO blood group incompatibility. Zhejiang Journal of Chinese Medicine (Chinese). 2007; 42(12): 712; [Google Scholar]
- 45.Zong Y. Effect of modified Erzhi Dihuang Yinchen Decoction for pregnant women with maternal-fetal ABO incompatibility. Journal of New Chinese Medicine (Chinese). 2012; 44(11): 76–77; [Google Scholar]
- 46.Zou XY, Wang JX. Observation of Yincan Erchen Decoction on decreasing hemolytic disease of the newborn due to ABO incompatibility. Beijing Journal of Chinese Medicine (Chinese). 2002; 21(2): 69–70; [Google Scholar]
- 47.Liu FJ, Luo AN. Observation of the effect of Gutai Yinchen Decoction on preventing 32 cases of maternal-fetal ABO blood type incompatibility. Yunnan Journal of Traditional Chinese Medicine (Chinese). 2011; 32(9): 37–38; [Google Scholar]
- 48.Du X. Meta-analysis of Chinese herbal medicine for maternal-fetal ABO incompatibility. Dissertation for Master Degree of Liaoning University of Chinese Medicine. 2014;
- 49.Bian YQ, Ning BB, Cao HY, Lu Y, Liu C, Chen GF, Liu J, Liu P, Sun MY. Formula-syndrome correlation study of three classical anti-jaundice formulas in inhibition of liver fibrosis induced by dimethylnitrosamine in rats. J Chin Integr Med (Chinese). 2012; 10(12): 1405–1412; [DOI] [PubMed] [Google Scholar]
- 50.Zhou F, Xu HM. Effect of comdin on P-gp expression in intrahepatic cholestatic rats. Zhongguo Zhong Yao Za Zhi (Chinese). 2010; 35(7): 908–911; [DOI] [PubMed] [Google Scholar]
- 51.Luo YL, Zeng JW, Yu M, Wei YL, Qu SY, Li W, Zheng TZ. Effect of rhubarb on contractile response of gallbladder smooth muscle strips isolated from guinea pigs. World J Gastroenterol (Chinese). 2005; 11(6): 863–866; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu X, Li BP, Zhang HF. Advancement of the study on rhubarb. Shanghai Zhong Yi Yao Za Zhi (Chinese). 2003; 37(4): 56–59; [Google Scholar]
- 53.Pharmacopoeia of the People's Republic of China (Chinese). Beijing: People's Medical Publishing House; 2005;
- 54.Muluye RA, Bian Y, Alemu PN. Anti-inflammatory and Antimicrobial Effects of Heat-Clearing Chinese Herbs: A Current Review. J Tradit Complement Med. 2014; 4(2): 93–98; doi: 10.4103/2225-4110.126635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L. The modern research of Yinchenhaotang’s treating jaundice. Dissertation for Master Degree of Shandong University of Traditional Chinese Medicine (Chinese). 2000.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.