Abstract
Background
Botulinum neurotoxin A (BoNTA), which alleviates overactive bladder symptoms, is thought to act predominantly via the inhibition of transmitter release from parasympathetic nerves. However, actions at other sites such as afferent nerve terminals are possible.
Objective
To evaluate the effects of BoNTA on bladder afferent neuropeptide release and firing.
Design, setting, and participants
One side of the bladder of control and chronic (1–2 wk) spinal cord transected (SCT; T8–T9) adult female mice was injected with BoNTA (0.5 U/5 µl saline). After 48 h, bladders with L6-S2 spinal nerves were prepared for in vitro recordings.
Outcome measurements and statistical analysis
In bladder preparations, tension and optical mapping of Ca2+ transients were used to measure intrinsic contractions, those evoked by capsaicin or the electrical stimulation of spinal nerves. Afferent firing was evoked by stretch or intrinsic bladder contractions. The numbers of responding units and firing rates were measured. Animal numbers were used to detect moderate to large between-group differences based on Cohen’s criteria. Two-way analysis of variance was used to test spatial/temporal differences in Ca2+ signals as mean plus or minus standard deviation. Differences between data sets were tested with the student t test and skewed data sets with a Mann-Whitney U test (significant when p < 0.05).
Results and limitations
In control and SCT bladders, BoNTA treatment decreased the contractions evoked by electrical stimulation of spinal nerves without altering intrinsic contractions. Afferent firing on untreated sides in response to stretch/intrinsic contractions was increased in SCTs versus controls. On BoNTA-treated sides, afferent firing rates were greatly attenuated in response to mechanical stimulation as were the capsaicin-evoked optical signals mediated by neuropeptide release.
Conclusions
SCT caused an increased sensitivity of afferent nerves to mechanical stimulation that was reduced by BoNTA treatment. Increased intrinsic activity after SCT was unaffected by the toxin. Thus BoNTA suppresses neurogenic detrusor overactivity by targeting afferent as well as efferent pathways in the bladder.
Keywords: Afferent nerve recording, Intrinsic activity, Neuropeptide release, Optical mapping
1. Introduction
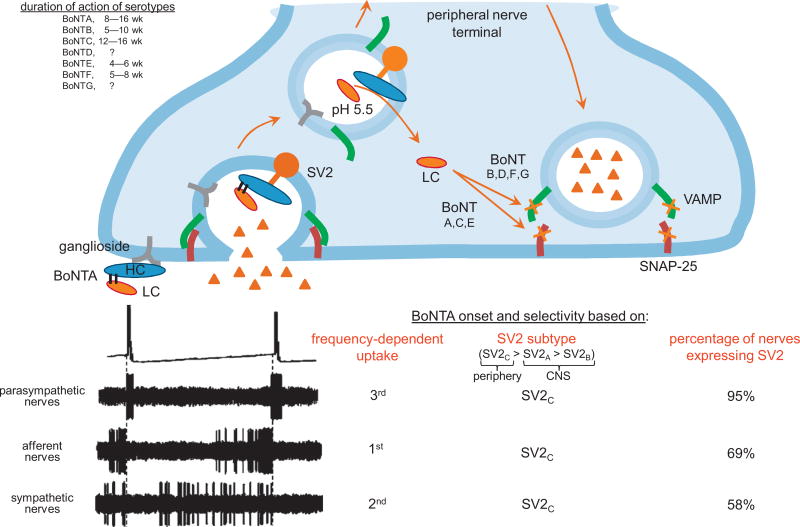
Botulinum neurotoxin is produced by Clostridium botulinum, and there are seven known serotypes [1]. These toxins target cholinergic nerve terminals by binding to synaptic vesicle protein type 2 (SV2) [2] and inhibiting acetylcholine release through the cleavage of synaptosome-associated protein of 25 kDa (SNAP-25) or vesical-associated membrane protein (Fig. 1) [3,4]. The longest acting serotype is botulinum toxin type A (BoNTA), which has been used clinically for various muscle spasticity disorders. BoNTA has also shown promise in treating neurogenic bladder overactivity [5] by reducing nonvoiding contractions, presumably inhibiting cholinergic efferent activity. Similar effects have been obtained in animal studies. Intravesical BoNTA reduced nonvoiding contractions and increased intercontractile intervals in spinal cord transected (SCT) rat bladders [6]. In addition, BoNTA decreased adenosine triphosphate (ATP) release from the bladder and spinal cord [7].
Fig. 1.
Mechanism of action of botulinum toxin type A (BoNTA) and its selectivity for different nerve terminals. Botulinum neurotoxins (BoNTs) initially bind to gangliosides expressed on the outer plasma membrane of nerve terminals [3]. During neurotransmitter release, the interior membrane of the vesicle is exposed, allowing BoNTs to attach to synaptic vesicle protein type 2 (SV2) and be transported into the nerve terminal via endocytosis. The low pH in the endosome causes dissociation of the di-chain polypeptide into its heavy chain (HC) and catalytic light chain (LC) components. The HC then inserts into the vesicle membrane allowing translocation of the LC into the cytosol where it is free to interact with and cleave SNARE proteins. The proteolytic activity and specificity of the LC differs among BoNT subtypes, targeting either synaptosomal-associated protein of 25 kDa (SNAP-25; serotypes A, C, and E) or vesical-associated membrane protein (VAMP; serotypes B, D, F, and G) of the synaptosomal complex.
The uptake of BoNTA depends on the frequency of nerve firing and the percentage of terminals expressing SV2. Although parasympathetic nerves are active only during bladder contraction, they have the highest percentage of nerves expressing SV2 [4]. Sympathetic neurons are active during most of the filling phase but have fewer nerves expressing SV2; this is preferable because their inhibition would decrease noradrenaline release and adversely affect bladder compliance. Afferent nerves progressively become more active as the bladder fills. They have the second least percentage of fibers expressing SV2, but their frequency of firing increases with sensitization, so they may have the highest likelihood of BoNTA uptake in pathology. CNS = central nervous system.
The ideal outcome for patients with overactive bladder treated with BoNTA injections is reduced urgency and involuntary bladder contractions. However, a potential side effect is urinary retention due to the excessive inhibition of transmission at the parasympathetic neuroeffector junction. Thus BoNTA was initially used in patients with neurogenic disorders such as spinal cord injury, where they overcome retention through catheterization [8]. BoNTA treatment has also been examined in patients with idiopathic bladder overactivity [9] and those with interstitial cystitis/painful bladder syndrome where there were mixed results [10].
Although BoNTA acts on efferent nerve terminals, there may be other sites of action including afferent nerves and the urothelium. A study by a coauthor, using reverse transcriptase polymerase chain reaction and Western blots, revealed SNAP-25 expression in human and mouse urothelium and BoNTA suppression of ATP release from urothelial cells. These data provide further support for the view that the urothelium has “neuronal-like” properties and may participate in bladder sensory mechanisms by releasing chemicals that modulate afferent nerve excitability [10]. However, another laboratory failed to detect SNAP-25 staining in human and rodent urothelium [11].
BoNTA may affect sensory nerves in the bladder through the inhibition of neuropeptide release [12,13] that can prevent autocrine stimulation of neurokinin 1 and 2 (NK1 and NK2) receptors on afferent terminals resulting in decreased afferent firing and sensitization [14]. Increased activity of afferents due to sensitization and bladder stretch may induce a greater uptake of BoNTA (Fig. 1) into afferent rather than efferent terminals that are only active during micturition. BoNTA may also elicit therapeutic effects by decreasing afferent sensitization and sprouting through alteration in afferent terminal receptor expression [15–17], inhibition of nerve growth factor [18], or inflammatory mediator [19] release.
Therefore, BoNTA may affect afferent and urothelial function directly and indirectly. In this study, we examined the effect of BoNTA injections on mechanosensitive afferent nerves in normal and SCT mouse bladders to determine if there is a direct effect that decreases antidromic neuropeptide release and/or firing rates.
2. Material and methods
2.1. Spinal cord transection
The T8–T9 SCT surgery was performed as previously described [20]. Animals were expressed by gentle abdominal compression for 1 wk following surgery and used for experiments 1–2 wk after surgery.
2.2. Botulinum toxin type A injections
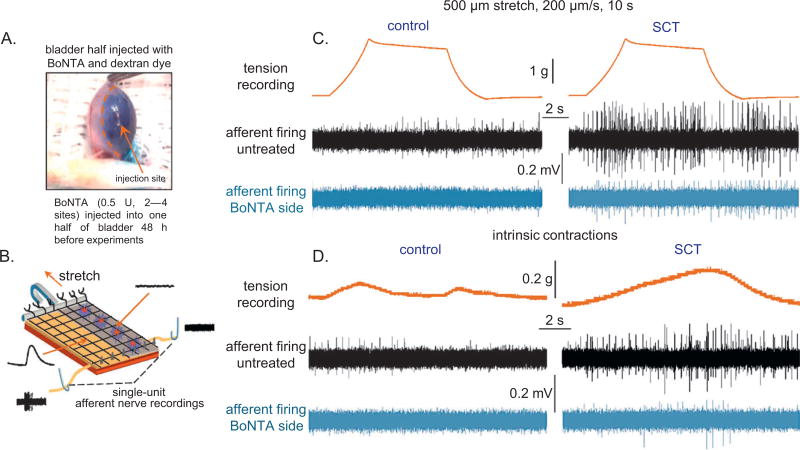
Mice were anesthetized with isoflurane, and a lower midline abdominal incision was made to expose the bladder. BoNTA (Botox, Allergan Inc; 100-U vials) was reconstituted in 1ml of sterile saline with a comparable weight (150 kDa) blue dextran dye (0.5 U of BoNTA in 5 µl saline/dextran; Fig. 2A) to track its distribution. Injections were made into the right half of the bladder wall at two to four serosal sites using a 32-gauge needle. The incision was sutured; animals were allowed to recover for 48 h. Based on 300 U/bladder for a 75-kg human, an equivalent dose for a 25-g mouse, which is 3000 times smaller, is 300 U/3000 = 0.1 U. Although 1 U is the median lethal dose for mice when injected intraperitoneally, our studies have demonstrated that it is safe to inject up to 2 U into the bladder wall.
Fig. 2.
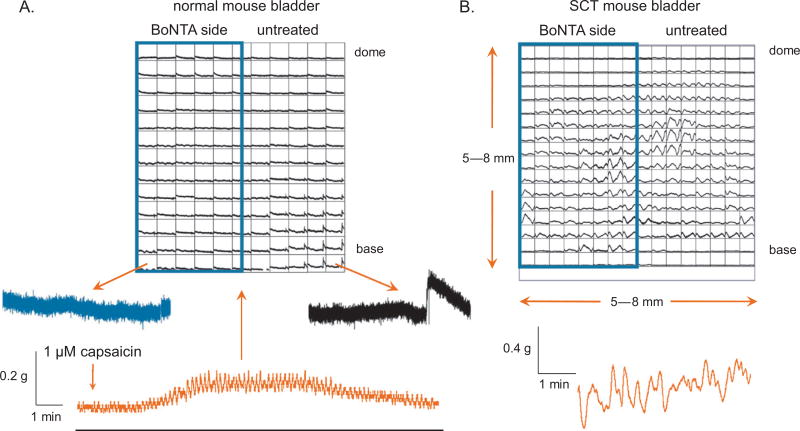
In vitro afferent nerve recordings from botulinum toxin type A (BoNTA)–treated mouse bladders. (A) Photo image of a mouse bladder injected across half its surface with 0.5 U of BoNTA in 5 ml of saline and blue dextran dye. (B) Schematic of the bladder-nerve preparation used for recording afferent nerve firing and optical mapping of intracellular Ca2+ transients. (C) Simultaneous afferent recordings from BoNTA-treated (blue traces) and nontreated (black traces) sides of the bladder in response to stepper motor-controlled stretches. In normal bladders, stretching resulted in moderate amounts of afferent firing from the control side but not the BoNTA-treated side. In spinal cord transected (SCT) mouse bladders, there was a significantly higher frequency of firing in response to the same stretch protocol; BoNTA inhibited stretch-evoked firing. (D) Intrinsic contractions evoked low-level afferent firing in control bladders. In SCT mouse bladders, the amplitude of intrinsic contractions was greater and resulted in increased frequency of firing compared with controls. BoNTA inhibited afferent firing due to intrinsic contractions in both control and SCT preparations.
2.3. In vitro afferent nerve recordings
Bladder L6-S2 spinal nerve preparations were isolated as previously described [20]. The preparation was mounted in a recording chamber (Fig. 2B and 3A), and nerves were passed through gates into oil-filled recording compartments. The bladder was superfused with oxygenated Krebs solution and maintained at 37 °C. For efferent nerve stimulation studies, the spinal nerves were positioned on bipolar platinum electrodes. Nerves were stimulated with a 3-s train at 20 Hz, 0.5-ms pulse width, and 20-V output. The same parameters were used for electric field stimulation studies with bladder cross sections. These parameters were determined to preferentially depolarize neurons and minimize the direct activation of the detrusor when tested in the absence and presence of 1 µM tetrodotoxin (not shown).
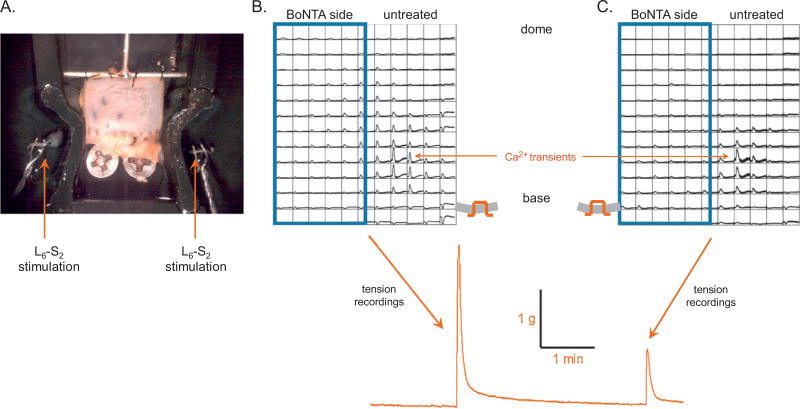
Fig. 3.
Effect of botulinum toxin type A (BoNTA) on nerve-mediated bladder contractions. (A) Photo image of a bladder-nerve preparation with associated L6-S2 spinal roots draped over stimulation electrodes. (B) Ca2+ optical map from the mucosal surface of a control mouse bladder treated with BoNTA (blue boxed area). Stimulation of nerve roots on the untreated side resulted in a large contraction with the spread of Ca2+ activity across the bladder. (C) Nerve stimulation on the BoNTA-reated side resulted in a much smaller contraction and propagation only on the untreated side due to activation of crossed efferent pathways.
For afferent recordings, the spinal nerves were split into smaller bundles and the receptive fields of mechanosensitive fibers located by passing a soft brush along the length of the bladder and then by probing using a von Frey hair. Electrical signals were sampled at 20 kHz, amplified and filtered using a WPI DAM 80 AC differential amplifier interfaced with an AD Instruments PowerLab 8/30. Data were acquired and stored on a computer for offline analysis using LabChart v.7. Electrical signals were fed to an audio monitor to facilitate real-time detection and discrimination of active units.
2.4. Optical imaging of bladder preparations
Bladder sheets were incubated with the Ca2+-sensitive fluorescent dye rhod-2-AM (1 µM) at physiologic temperature for 5 min and imaged using an optical mapping system described previously [21].
2.5. Statistical analysis
Quantitative data are shown as mean plus or minus standard deviation. Differences between normal data sets were tested with the student t test; the null hypothesis was rejected when p was < 0.05.
3. Results
3.1. Effect of botulinum toxin type A on mechanically stimulated afferent firing in control versus spinal cord transected mouse bladders
Afferent firing was simultaneously recorded from BoNTA-treated and -untreated sides of the bladders from 10 control and 9 SCT mice in response to controlled stretches (Fig. 2C) and intrinsic bladder contractions (Fig. 2D). Bladders were stretched 500 µm at 10 µm/s and held for 30 s before being relaxed to starting length. On the untreated sides of control bladders, in response to stretch, the firing of a total of 59 units was recorded from six responsive nerves at an average firing rate of 5 ± 0.4 Hz. The intrinsic contraction induced the firing of 48 units at the same rate. BoNTA-treated sides of control bladders were unresponsive to mechanical stimulation. On untreated sides of SCT mouse bladders, both stretch and contraction induced considerably higher activity (stretch: 132 units firing at an average rate of 16 ± 1.8 Hz; contraction: 109 units firing at an average rate of 22 ±s 2.3 Hz from six responsive nerves). However, afferent activity on the BoNTA-treated sides of the SCT bladders was significantly decreased (p < 0.01): Stretch induced the firing of 42 units at an average of 5 ± 1 Hz, and contraction induced firing at an average of 2 ± 1 Hz in 35 units from four responsive nerves. Control and SCT mouse bladders injected only with dye showed no significant difference in afferent firing compared with nontreated halves (not shown).
3.2. Effects of botulinum toxin type A treatment on efferent pathways in spinal cord transected and normal mouse bladders
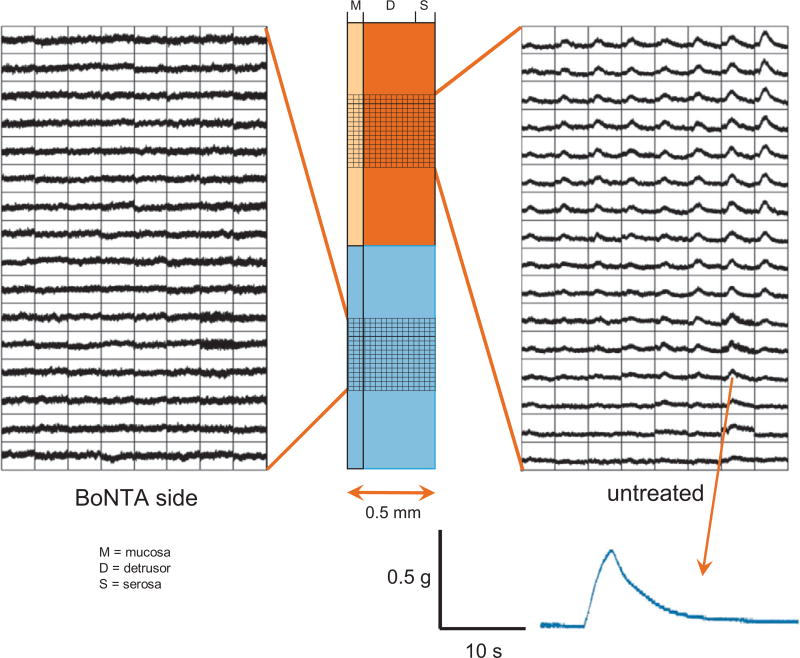
Efferent activity was evaluated during electrical stimulation of the left or right spinal nerves, and muscle activity was monitored using Ca2+ optical mapping from the mucosal surface along with tension measurements from the sheet. In normal mouse bladders (n = 11), nerve stimulation on the untreated side elicited Ca2+ transients and tension in the range of 3.03 ± 0.07 g. In contrast, on the BoNTA-treated side, Ca2+ transients were essentially abolished, and tension decreased to 1.51 ± 0.56 g (p < 0.05) compared with the untreated side (Fig. 3B and 3C). Tension generated when stimulating the spinal nerve on the BoNTA-treated side of the bladder was due to the activation of crossed efferent pathways that project to the untreated side. Similar results were obtained from SCT mouse bladders (n = 7). When a bladder cross section was imaged during electrical stimulation, spread of Ca2+ activity was not observed in the BoNTA-treated half of the bladder (Fig. 4; n = 4 control and 3 SCT bladders). Ca2+ activity was suppressed through all the layers of the BoNTA-treated tissue.
Fig. 4.
Ca2+ optical maps from wall cross-sections of normal mouse bladders. The bladder was imaged across the wall, from mucosa to serosa, and stimulated using electric field stimulation. The Ca2+ transients elicited spread through the untreated half of the bladder but not through the botulinum toxin type A (BoNTA)–treated side. Thus although BoNTA can be localized to one half of the bladder wall circumferentially by injections at two to four serosal sites (see Methods), it readily crosses from serosa to mucosa, at least in a 0.5-mm-thick mouse bladder.
M = mucosa, D = detrusor and S = serosa.
3.3. Effect of botulinum toxin type A treatment on antidromic neuropeptide release and intrinsic bladder contractions
Capsaicin (1 µM) was applied to evoke neuropeptide release from transient receptor potential vanilloid (TRPV) 1–containing afferent nerves while mapping Ca2+ transients in the muscle that functioned as a sensor (Fig. 5A). Bladders from control mice (n = 9) were used to avoid the complication of neuropeptide release due to large amplitude intrinsic contractions. On the untreated side, Ca2+ transients were detected mostly near the base/trigone region where most afferent nerves enter the bladder wall. On the BoNTA-treated side, this activity was absent. A second application of capsaicin 15 min later did not elicit a response (not shown), presumably due to the depletion of transmitter and/or the desensitization of TRPV1 receptors, demonstrating that the muscle signals were due to neuropeptides released from afferent terminals.
Fig. 5.
Optical mapping of capsaicin-evoked Ca2+ transients from the mucosal surface of control and spinal cord transected (SCT) mouse bladders treated with botulinum toxin type A (BoNTA). (A) Capsaicin (1 µM) caused an increase in baseline and contractile amplitudes in control mouse bladders. Optical mapping demonstrated that capsaicin-evoked Ca2+ transients only occurred in the untreated half of the bladder smooth muscle. A second dose of capsaicin did not evoke transients (not shown), presumably due to the depletion of neuropeptides and/or desensitization of transient receptor potential vanilloid-1 channels, demonstrating that the muscle signals are due to neuropeptides released from afferent terminals. (B) Intrinsic contractile activity was not inhibited by BoNTA treatment in SCT or control (not shown) mouse bladders.
Following SCT, there was a significant increase in the amplitude of intrinsic bladder contractions compared with normal bladders (peak amplitude from baseline tension: 0.10 ± 0.04 g control vs 0.55 ± 0.21 g SCT; n ≥6; p = 0.05). BoNTA treatment did not reduce the amplitude or frequency of these contractions compared with untreated SCT bladders (not shown). The mapping of intracellular Ca2+ transients responsible for intrinsic contractions also demonstrated that these are not affected by BoNTA (Fig. 5B). This is supported by previous findings where we showed that intrinsic activity is not blocked by tetrodotoxin and therefore not mediated by action potentials in nerves [21].
4. Discussion
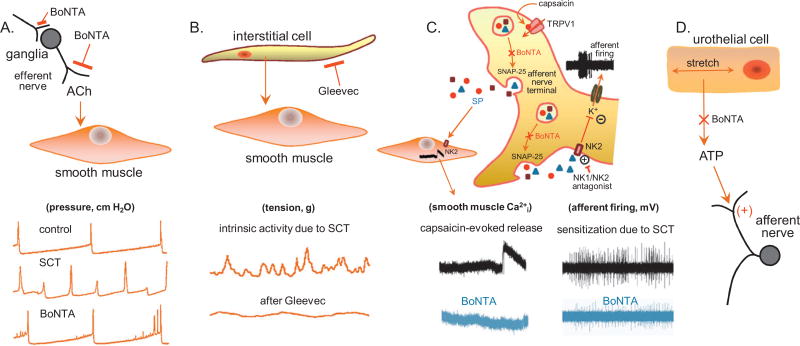
This study demonstrates that 48 h after the injection of BoNTA into the walls of control or SCT mouse bladders, afferent activity evoked by mechanical or chemical stimulation, or efferent activity evoked by electrical stimulation of the spinal nerves, was suppressed. However, intrinsic contractile activity was unaffected, suggesting this activity is nonneuronal and therefore not a target for BoNTA; this activity was inhibited by Gleevec (Fig. 6B). In addition, the effect of BoNTA did not appear to be limited to the sites of injection because tissue responses were markedly reduced throughout the layers of the bladder wall (Fig. 4).
Fig. 6.
Schematic of different cell targets and mechanisms of action of botulinum toxin type A (BoNTA) in the urinary bladder. (A) Efferent nerve terminals: BoNTA can affect transmitter release from pre- and postganglionic parasympathetic nerve terminals. In the bladder, inhibition of acetylcholine (Ach) release from efferent nerves reduces detrusor contractility during micturition. This can be reflected in cystometric measurements as a decrease in reflex bladder contractions and intradetrusor pressures, and an increase in intercontractile intervals. (B) Interstitial cells: These cells are believed to promote intrinsic contractile activity of the detrusor, especially under pathologic conditions such as spinal cord injury or bladder outlet obstruction. (C) Afferent nerve terminals: BoNTA can reduce afferent sensitization by inhibiting neuropeptide release and decreasing firing frequency. (D) Urothelium: Urothelial cells release various factors including adenosine triphosphate (ATP) in response to stretch. Increased urothelial ATP release in pathology may contribute to afferent sensitization by acting on P2X2/P2X2/3 receptors. Because ATP release occurs through a vesicular mechanism, BoNTA may inhibit its release. NK = neurokinin.
The effect of BoNTA in decreasing afferent nerve firing induced by mechanical stimuli (stretch or intrinsic contractions) may involve multiple mechanisms. It has been demonstrated that NK receptor agonists inhibit K+ currents in capsaicin-sensitive dorsal root ganglion neurons [22]. Assuming that these receptors and currents are also present in the nerve terminals, auto feedback stimulation would result in depolarization and enhanced afferent firing. BoNTA could decrease this firing by inhibiting the autoactivation of afferent NK2 receptors in rodents and NK1 receptors in humans (Fig. 6C). Alternatively, one could argue that BoNTA limits trafficking of voltage-gated Ca2+ channels to the membrane in afferent terminals [23], thereby limiting depolarization and afferent firing.
BoNTA attenuated contractile and Ca2+ activity in response to electric stimulation (field and direct nerve; Figs. 3 and 4). It is unlikely that there could have been a direct inhibition of the detrusor smooth muscle because BoNTA requires the SV2 protein to be transported inside a cell. In addition, we used stimulation parameters that we determined to preferentially depolarize nerves rather than smooth muscle cells (see Methods). Therefore, the inhibitory effects of BoNTA observed are neutrally mediated. The dosage used in this study is approximately five times that given clinically [8], so it is expected there would be a greater degree of parasympathetic inhibition. To determine if BoNTA had a direct effect on afferent-mediated neuropeptide release, we initially performed electrical stimulation in the presence of atropine and pyridoxal-phosphate-6-azophenyl-2′-4′-disulfonate (PPADS) to block muscarinic and purinergic receptors (not shown). As further confirmation that these electrically evoked Ca2+ transients resulted from neuropeptides released from afferents, we stimulated bladder tissues with capsaicin.
The suppression of capsaicin-induced Ca2+ signals in bladder smooth muscle provides further evidence that BoNTA blocks the release of neuropeptides from TRPV1-expressing afferent nerves. Capsaicin acts intracellularly on these channels to increase cytosolic Ca2+ and release neuropeptides (eg, substance P) that can activate neurokinin receptors in smooth muscle to elicit contractile activity. The effect of BoNTA to block the capsaicin responses is attributable to a direct action on afferent nerves because bladder smooth muscle does not contain SV2 receptors and is therefore not a site of action for BoNTs. Under pathologic conditions, bladder sensory nerves fire more frequently, thus increasing the likelihood of BoNTA binding to afferent SV2 proteins. A number of studies have examined the potential of targeting BoNTA to afferent nerves through modification of its protein structure [24,25], which would offer increased efficacy and a reduction in adverse side effects (eg, urinary retention).
Finally, the urothelium is a source of stretch-induced ATP release [26,27] that may act on P2X2/P2X2/3 receptors [28,29] on afferent nerves to promote their sensitization and increase bladder hyperreflexia [30]. Because urothelial ATP release is vesicular and SNAP-25 (pers. comm., L. Birder, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA) and SV2 [31] have been identified in human urothelial cells, BoNTA may also act therapeutically on the urothelium (Fig. 6D).
5. Conclusions
BoNTA appears to elicit its suppressant effects on neurogenic detrusor overactivity in mice via the inhibition of neurotransmitter release from afferent as well as efferent nerve terminals without altering the intrinsic contractile activity of the detrusor. This could partly explain the effects of BoNTA treatment in reducing sensory symptoms in patients with bladder dysfunction.
Acknowledgments
Funding/Support and role of the sponsor: This research was funded by a grant to Anthony J. Kanai from Allergan, Inc., which approved the manuscript. The sponsor also kindly provided BoNTA (Onabotulinumtoxin A, or Botox).
Footnotes
Author contributions: Anthony J. Kanai had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kanai.
Acquisition of data: Ikeda, Zabbarova, McCarthy, Kanai.
Analysis and interpretation of data: Birder, de Groat, Kanai.
Drafting of the manuscript: Ikeda, Zabbarova, Kanai.
Critical revision of the manuscript for important intellectual content: Birder, de Groat, Hanna-Mitchell.
Statistical analysis: Ikeda, Zabbarova, McCarthy, Kanai.
Obtaining funding: Kanai.
Administrative, technical, or material support: None.
Supervision: Kanai.
Other (specify): None.
Financial disclosures: Anthony J. Kanai certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Anthony J. Kanai is funded by Allergan and Astellas. Irina V. Zabbarova is funded by Pfizer. Lori A. Birder is a consultant for Astellas and funded by Pfizer. William C. de Groat is a consultant for Allergan, Eli Lilly, Endo, Ethicon, and Medtronic and is funded by Eli Lilly, Helsinn, and Takeda. The other authors have nothing to disclose.
References
- 1.Foran PG, Mohammed N, Lisk GO, et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–71. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- 2.Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–6. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 3.Rummel A, Hafner K, Mahrhold S, et al. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem. 2009;110:1942–54. doi: 10.1111/j.1471-4159.2009.06298.x. [DOI] [PubMed] [Google Scholar]
- 4.Coelho A, Dinis P, Pinto R, et al. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol. 2010;57:884–90. doi: 10.1016/j.eururo.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Smith CP. Botulinum toxin in the treatment of OAB, BPH, and IC. Toxicon. 2009;54:639–46. doi: 10.1016/j.toxicon.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology. 2005;66:208–12. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 7.Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–93. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55:100–20. doi: 10.1016/j.eururo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Dmochowski R, Chapple C, Nitti VW, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184:2416–22. doi: 10.1016/j.juro.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charrua A, Avelino A, Cruz F. Modulation of urinary bladder innervation: TRPV1 and botulinum toxin A. Handb Exp Pharmacol. 2011:345–74. doi: 10.1007/978-3-642-16499-6_17. [DOI] [PubMed] [Google Scholar]
- 12.Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644–50. doi: 10.1016/j.eururo.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Lucioni A, Bales GT, Lotan TL, McGehee DS, Cook SP, Rapp DE. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–70. doi: 10.1111/j.1464-410X.2007.07312.x. [DOI] [PubMed] [Google Scholar]
- 14.Morrison J. The activation of bladder wall afferent nerves. Exp Physiol. 1999;84:131–6. doi: 10.1111/j.1469-445x.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 15.Apostolidis A, Popat R, Yiangou Y, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–82. doi: 10.1097/01.ju.0000169481.42259.54. discussion 982–3. [DOI] [PubMed] [Google Scholar]
- 16.Roosen A, Datta SN, Chowdhury RA, et al. Suburothelial myofibroblasts in the human overactive bladder and the effect of botulinum neurotoxin type A treatment. Eur Urol. 2009;55:1440–9. doi: 10.1016/j.eururo.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Datta SN, Roosen A, Pullen A, et al. Immunohistochemical expression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive human bladders, and changes with botulinum neurotoxin administration. J Urol. 2010;184:2578–85. doi: 10.1016/j.juro.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Ochodnicky P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30:1227–41. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 19.Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB. Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56:159–67. doi: 10.1016/j.eururo.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy CJ, Zabbarova IV, Brumovsky PR, Roppolo JR, Gebhart GF, Kanai AJ. Spontaneous contractions evoke afferent nerve firing in mouse bladders with detrusor overactivity. J Urol. 2009;181:1459–66. doi: 10.1016/j.juro.2008.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai A, Roppolo J, Ikeda Y, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065–72. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sculptoreanu A, Artim DE, de Groat WC. Neurokinins inhibit low threshold inactivating K+ currents in capsaicin responsive DRG neurons. Exp Neurol. 2009;219:562–73. doi: 10.1016/j.expneurol.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson MT, Todorovic SM. Is there a role for T-type calcium channels in peripheral and central pain sensitization? Mol Neurobiol. 2006;34:243–8. doi: 10.1385/MN:34:3:243. [DOI] [PubMed] [Google Scholar]
- 24.Duggan MJ, Quinn CP, Chaddock JA, et al. Inhibition of release of neurotransmitters from rat dorsal root ganglia by a novel conjugate of a Clostridium botulinum toxin A endopeptidase fragment and Erythrina cristagalli lectin. J Biol Chem. 2002;277:34846–52. doi: 10.1074/jbc.M202902200. [DOI] [PubMed] [Google Scholar]
- 25.Meng J, Ovsepian SV, Wang J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981–92. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–9. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 27.Wang EC, Lee JM, Ruiz WG, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–22. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlaskovska M, Kasakov L, Rong W, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–7. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cockayne DA, Dunn PM, Zhong Y, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–39. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 31.Giannantoni A, Proietti S, Vianello A, et al. Assessment of botulinum A toxin high affinity SV2 receptors on normal human urothelial cells [abstract 793]. Poster presented at: American Urological Association annual meeting; May 14–19, 2011; Washington, DC, USA. [Google Scholar]