Abstract
The cell membrane is a semi-fluid container that defines the boundary of cells, and provides an enclosed environment for vital biological processes. A sound excitable drug (SED) that is non-cytotoxic to cells is developed to disrupt the plasma membrane under gentle ultrasound insonation, 1 MHz, 1 W/cm2. The frequency and power density of insonation are within the physical therapy and medical imaging windows; thus the applied ultrasound is safe and not harmful to tissues. The insertion of SEDs into the plasma membrane is not toxic to cells; however, the intruding SEDs weaken the membrane’s integrity. Under insonation, the ultrasound energy destabilized the SED disrupted membranes, resulting in membrane rupture and eventual cell death. In a xenograft breast tumor model, the SED alone or the ultrasound alone caused little adverse effects to tumor tissue, while the combined treatment triggered necrosis with a brief local insonation of 3 minutes. The described sono-membrane rupture therapy could be a safe alternative to the currently used high-energy tissue ablation technology, which uses X-rays, gamma rays, electron beams, protons, or high- intensity focused ultrasound.
Keywords: Ultrasound, plasma membrane, rupture, sonodynamic therapy, tumor ablation
Graphical abstract
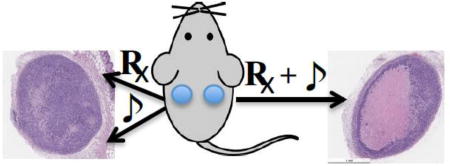
Introduction
Tumor ablation, which uses high-energy particles or waves, is an important and common means of cancer treatment. It is generally effective, but is often associated with severe side effects. The high-energy source causes undesired damage to the tissues along the radiation pathway. A low-energy technology, which could achieve the same ablation effect without damaging normal tissues, would be a preferred choice. In addition, thermal ablation that uses radiofrequency (RF) energy or high intensity focused ultrasound (HIFU) as the heat source are known to have limitations due to the convective cooling of blood flow, which can protect cancer cells near blood vessels from thermal damage. This sometimes results in recurring aggressive tumor growth. A technology based on similar modalities that does not mainly or entirely rely on thermal ablation is therefore highly desirable.
Ultrasound technology has been widely applied in diagnostic imaging, interventional guidance, and physical therapy. In addition to the routine imaging and medical applications, low-intensity ultrasound (<5.0 W/cm2) has been introduced in recent years to assist therapies.[1, 2] In contrast to the use of HIFU for direct thermal ablation, low-intensity ultrasound works together with chemical cytotoxic agents.[3, 4] A few recent studies have reported the use of the combination of chemotherapeutic agents with ultrasound enhances the drug’s anti-cancer effects, and sensitizes drug resistant cells. It was believed that ultrasound-induced cavitation weakens the cell membrane and facilitates the intracellular distribution of drugs.[5, 6] Direct tumor insonation could also loosen up tight tissue junctions in under-vascularized areas for better intratumoral drug dispersions.[7, 8] Most recently, an implantable ultrasound device was used to open up the blood-brain barrier in brain tumor patients to enhance drug delivery.[9] Drugs bound or loaded micrometer sized hollow microbubbles also have been used to deliver drugs.[1, 10] Alternatively, microbubbles in conjunction with other carriers, such as liposomes or micelles, could be burst to achieve a local drug release by a locally applied ultrasound energy.[11–13]
Sonodynamic therapy (SDT) is similar to the clinically used photodynamic therapy (PDT), but instead of light, ultrasound is used to activate therapeutic sensitizers.[14–16] PDT, which allows for the exclusive eradication of diseased tissue while sparing surrounding healthy cells from damage, suffers from poor tissue penetration and light diffusion. The substitution of ultrasound as the energy source in SDT allows the therapy to overcome these roadblocks. The physics of ultrasound propagation allows for a more favorable and direct deep tissue penetration compared to photons. Most of the reported sonosensitizers are also photosensitizers or derived from photosensitizers; therefore, the accepted mechanism of action of SDT is similar to that of PDT.[14, 17] Conversely, a few non-photosensitive sonosensitizers have also been identified.[18–20] These sonosensitizers cannot be excited by light; but under insonation, reactive oxygen species (ROS) were generated, triggering apoptosis. Intrigued by these prior arts, we sought to develop a novel non-toxic non-light-sensitive sound excitable drug (SED) to pair with a low-intensity ultrasound for tumor ablation.
Materials and Methods
Synthesis of RB4 (2,3,4,5-tetrachloro-6-(6-hydroxy-2,4,5,7-tetraiodo-3-oxo-3H-xanthen-9-yl)-N-(2-hydroxyethyl)-benzamide)
Rose Bengal (RB), O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU), and diisopropoyl ethyl amine (DIEPA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other solvents, including dimethyl formamide (DMF), dichloromethane (DCM), and methanol (MeOH), were purchased from Thermo Fisher (Waltham, MA).
RB4 was synthesized following a published protocol.[21] To a solution of RB (509 mg, 0.5 mmol) in DMF (3 ml) and DIEPA (2 ml) was added HBTU (190 mg, 0.5 mmol) and stirred at room temperature (RT) for 4h, then 2-aminoethanol (91 µL, 1.5 mmol) was added and reacted overnight at RT. The solvent was removed under reduced pressure. The residue was extracted with DCM and washed with brine, dried over anhydrous sodium sulfate and concentrated, the residue was purified by silica gel column, eluted with DCM, DCM/MeOH=10/0.5 and 10/1(V/V) to give product as pale yellow solid (161mg, yield 31.7%). TCL: Rf=0.3, DCM/MeOH=10/1. 1H NMR (DMSO-6d, 300 MHz): δ 2.99 (s, 2H), 3.32 (s, 2H), 4.60 (br, 1H), 5.76 (s, 1H), 7.25 (s, 1H), 10.07 (s, 1H). ESI-MS: 1015.57 (M−H)−. The NMR and Mass spectra of RB4 are included in the Supplementary Materials and Methods.
Tumor cell culture and animals
MDA-MB-468 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and were maintained in Leibovitz’s L-15 medium (Corning, Manassas, VA, USA), and supplemented with 10% fetal bovine serum and antibiotics penicillin (100 µg/ml) and streptomycin (100 µg/ml) without CO2. HT1080-Luc2 cells obtained from Caliper (Hopkinton, MA, USA) were grown at 37 °C with 5% CO2 in Eagle's MEM from Corning (Manassas, VA, USA), and supplemented with 10% fetal bovine serum (Seradigm, Radnor, PA, USA) and antibiotics penicillin (100 µg/ml) and streptomycin (100 µg/ml) (Invitrogen, Carlsbad, CA, USA).
All animal studies were performed in compliance with the approved animal protocols and guidelines of the Institutional Animal Care and Use Committee of Weill Cornell Medicine. BALB/c Nu/Nu female nude mice (5–6 weeks) were purchased from Charles River (Wilmington, MA).
Ultrasound system
A portable bench top ultrasound system (Accusonic plus, Metron, Warrenville, IL) with 1–3 MHz, and 10, 20, 50 and 100% duty cycle capability was used to conduct all studies (Fig S1). Transducer one (5 cm2, Model# 901150, Metron) and transducer two (0.75 cm2, Model# 901175, Metron) were used in the cell culture and animal studies, respectively.
Sonotoxicity with RB4
MDA-MB-468 cells (5 × 104) in 24 well plates were incubated in complete media with 10 µM RB4 for 1 hour prior to the ultrasound treatment. The plates were then placed on a pre-cut gel pad (2cm × 3cm, Parker Laboratories, Fairfield, NJ, USA) with multipurpose ultrasound gel (Parker) over a mounted handheld ultrasound transducer (5 cm2) at 1MHz, 1 W/cm2 for 30s, 100% DC. Cells were checked under the EVOS® FL Auto Cell Imaging system microscope (Thermo Fisher). Cell viability at 24 h was quantitated using an MTS assay (Promega, Madison, WI, USA). The plate was incubated at 37 °C for 4 h. Absorbance was measured at 490 nm using a microplate reader (Infinite M1000 Pro, Tecan, Männedorf, Switzerland).
Mechanistic study of death process by flow cytometry
MDA-MB-468 cells (5 × 104) were incubated in complete media with or without 10 µM RB4 for 1 hour prior to ultrasound treatment. Then the plates were insonated at 1MHz, 1 W/cm2, 30sec, 100% duty cycle. Following treatment, cells were re-incubated for 1 day, 4 groups of cells (Control, RB4 alone, Ultrasound alone, and RB4 with Ultrasound) were collected and washed twice with pre-cooled PBS. Cells were stained with FITC-conjugated Annexin-V (Life Technologies) and propidium idodide (PI, Life Technologies) for 15min as per the manufacture’s instructions, and then analyzed by flow cytometry (Gallios, Beckman Coulter). The percentage of dead cells and those undergoing apoptosis were analyzed using Kaluza Software.
Chemical ROS assay
To study the insonation induced ROS generation in solution, RB4 was tested using a modified 2’, 7’–dichlorofluorescin diacetate (DCFH-DA) assay. DCFH-DA (1 ml, 1 mM in MeOH, Aldrich) was hydrolyzed in NaOH aqueous solution (0.01N, 4 mL) at RT for 30 min to yield a non-fluorescent DCFH intermediate. The solution was neutralized with 20 ml of NaH2PO4 (25 mM) and shielded with aluminum foil. The final solution of DCFH was around 40 µM. RB4 (1.0 mg) was dissolved in DMSO (1mL), and then diluted with water into a 20 µM solution. The RB4 solution (20 µM, 10mL) was mixed with a DCFH solution (40 µM, 10 ml) as the test solution. The test solution (0.5 mL) was placed into each well of a 24-well plate. The DCFH solution without RB4 was included as a background control. The plates were treated with ultrasound (0.4 −1.2 watt/cm2) one well by one well for 30 seconds; the insonated wells were then checked using a fluorescence plate reader, ex 485 nm/ em 520 nm.
Cell based ROS scavenging assay
MDA-MB-468 cells (5 × 104) were treated with free radical scavengers, L-Histidine (10mM), D-Mannitol (100 mM), N-acetyl cysteine (NAC, 0.5mM), and superoxide dismutase (SOD, 100 µg/mL) for 30min. The treated cells were then incubated with a fresh media, containing RB4 (10 µM), for an hour. After incubation, the wells were insonated (1 MHz, 1 W/cm2, 30 sec, 100% DC) as described above. One day later, the cell’s viability was assessed using the MTS solution.
In vivo SMRT effect using preloaded tumor cells
MDA-MB-468 cells were suspended in PBS or RB4 (10 µM) in PBS for 1 day. The cells (5×106, 0.1 ml) were subcutaneously injected into both flanks. The left tumors, which were only treated with PBS, were the internal control; while the right tumors were treated with RB4 (10 µM) only, ultrasound only (1 MHz, 1 W/cm2, 100% DC, 3min), or an RB4/ultrasound combination (n=7). The transducer size is 0.75 cm2 (Metron). Tumor size was measured with slide calipers on days 7,10, 14,17,21,24, 28, and 36.
In vivo SMRT effect with intra-tumoral injected RB4
MDA-MB-468 cells (107, 0.1 ml PBS) were subcutaneously inoculated into both flanks of BALB/c Nu/Nu female nude mice (5–6 weeks). The RB4 injections and ultrasound therapies were performed when the tumors had grown to approximately 4–5 mm in diameter, 20–22 days after inoculation. The tumors were treated with RB4 (10 µM, 50 µl) only, ultrasound only (1 MHz, 1 W/cm2, 100% DC, 3 min), and a RB4/ultrasound combination (n=5). Under isoflurane anesthesia, RB4 was directly injected to the tumors of the mice. The ultrasound treatment was then performed immediately after the injection of RB4. The transducer size is 0.75 cm2 (Metron). The same RB4/ultrasound treatment was performed on the thigh muscles of the mice.
Histochemical analysis
Tumors and muscle tissue were harvested 2 days after therapy and the specimens were fixed in 10% formalin, cut in half, and embedded in paraffin. The paraffin sections (7 µm thick) were stained with hematoxylin-eosin (H&E). ImageJ 1.48 was used to measure the size of whole tumor and necrotic areas in the tumors,
Statistics
The measurement was performed three times for each group by an experienced pathologist. Statistical analysis was preformed with one and two-way ANOVA, followed by Bonferroni’s multiple comparison tests with the software Prism 7 (GraphPad Software, Inc). p values less than 0.05 were considered statistically significant.
Results and Discussion
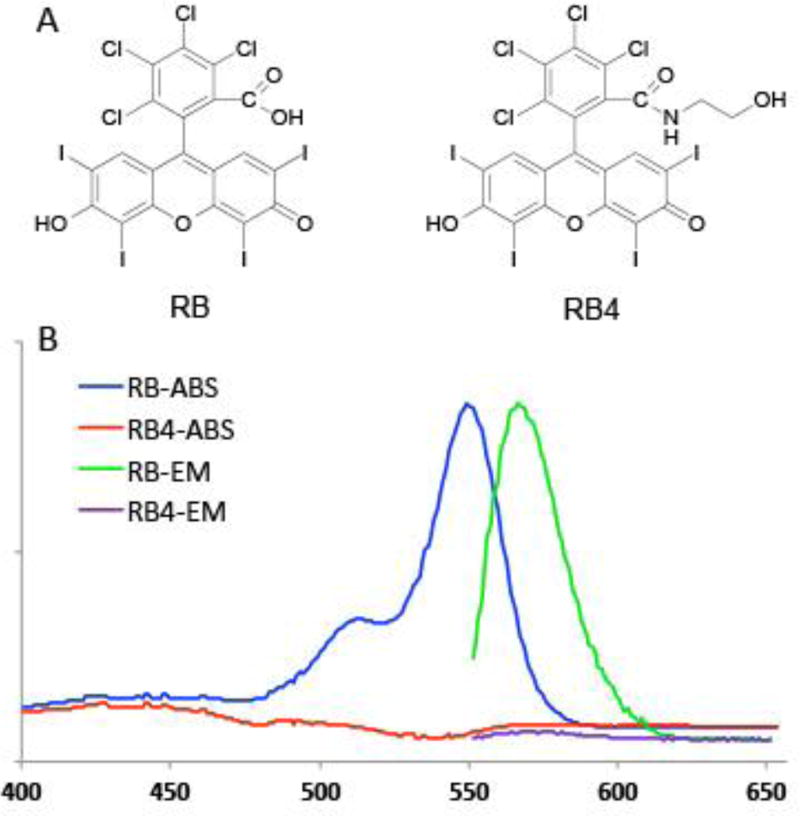
Rose Bengal (RB, Fig 1A), a known photosensitizer, has been used in photodynamic therapy.[22, 23] Interestingly, it was demonstrated that at a very high concentration (>100 µM) and under ultrasound insonation, RB could generate lethal reactive oxygen species (ROS) to kill cells in vitro,[24–26] and potentially in vivo.[27] It is believed that the inertial cavitation process, induced by the ultrasound, triggers sono-luminescence and pyrolysis.[14, 15] The associated light reacted with the photosensitive RB, resulting in ROS dependent cytotoxicity. Therefore, RB has been proposed as a sonoseneitizer. It has been modified[25] or conjugated to microbubbles[10, 28] for better sonotoxicity. We previously have learned that RB lost its photosensitivity after amidation; thus a search for photo-insensitive SED was extended to RB derivatives.[21] Among the tested molecules, an RB derivative, RB4 (2,3,4,5-tetrachloro-6-(6-hydroxy-2,4,5,7-tetraiodo-3-oxo-3H-xanthen-9-yl)-N-(2-hydroxyethyl)-benzamide), was identified to have the desired low-intensity ultrasound inducible cell killing capability, and it is not photosensitive (Fig 1A). The parent molecule, RB, has absorption and emission maximum of 549 nm and 565 nm, respectively, while RB4, the N-2-hydroxyethyl amidated derivative, has neither absorption nor emission above 400 nm (Fig 1B).
Figure 1.
The chemical structures (A) and absorption and emission spectra (B) of Rose Bengal (RB) and RB4.
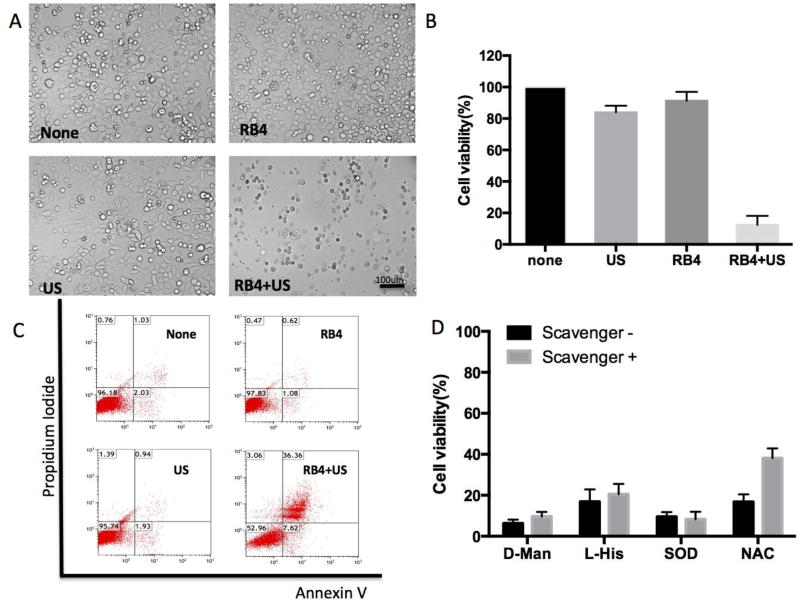
RB4’s sound sensitivity was validated with triple negative breast cancer cells, MDA-MB-468. The cells were incubated with/without RB4 (10 µM) for 60 min, and then insonated by a continuous ultrasound (1 MHz, 1 W/cm2) for 30 sec. Significant damage was observed in the combination wells after the treatment (Fig 2A). Round detached cells and high amounts of debris were observed floating in the media. Little difference could be seen amongst the untreated, RB4 treated, and ultrasound treated groups. The treated cells were cultured for an additional day and then checked for viability (Fig 2B). Ultrasound alone or RB4 alone had a mild effect on the cell’s viability (> 80% viable), while the RB4 combined with ultrasound treatment killed over 90% of cells. Based on these observations, the cell killing was rapid, and appeared to involve a complete loss of membrane integrity. A similar sono-sensitivity of RB4 was observed with a second cell line HT1080, fibrosarcoma, which resulted in 95% of the cells killed (Fig S2). RB4 is non-toxic to cells at the test concentration; its IC50 to MDA-MB-468 and HT-1080 was 71 and 157 µM, respectively (Fig S3).
Figure 2.
In vitro SMRT effect. The MDA-MB468 cells were treated with RB4 (10 µM) and US (1 MHz, 1 W/cm2, 30 sec), RB4 alone (10 µM), US alone (1 MHz, 1 W/cm2, 30 sec), or none. (A) Microscopic images after treatment; (B) MTS viability assay at 24 hr; (C) FACS analysis after staining with a necrotic indicator, propidium iodide, and a apoptosis indicator, Annexin V, 24 hr after treatment. (D) ROS scavenger effect. Cells were pretreated with L-Histidine, D-Mannitol, superoxide dismutase, or N-acetyl cysteine (NAC) and then treated with RB4 (10 µM) for 1 hr and then subject to insonation (1 MHz, 1 W/cm2, 30 sec). Only NAC was able to rescue a fraction of cells.
The cell death process was studied by staining the treated cells with apoptosis and necrosis dyes, annexin V and propidium iodide (PI), respectively. As shown in Fig 2C, one day after treatment, RB4 treated or ultrasound treated cells were similar to the untreated group, mostly healthy. However, a high percentage of the cells treated with the RB4/ultrasound combination was annexin/PI double positive (36%), plus small fraction of annexin positive (8%) or PI positive (3%) cells. The actual dead cell population was larger than indicated in the FACS analysis plot (Fig 2C) because the fragments of the lysed cells could not be spun down during preparation.
The majority of the reported sonosensitizers used in sonodynamic therapy are also photosensitizers or are derived from photosensitizers.[14, 17] Yet, RB4 is not a photosensitive molecule. The photon theory of sonodynamic therapy might not be applicable in RB4. To study the mechanism of action, a quantitative fluorescence Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay was used to measure the ROS generation.[29] Freshly prepared DCFH and RB4 solutions were insonated with different ultrasound powers. However, the absorption measurement indicated that the ROS level of RB4 solution was not significantly different from the background level of the DCFH solution, indicating that insonated RB4 did not generate ROS in solution (Fig S4). The possible generation of ROS RB4 was then checked in MDA-MD-468 cells in the presence of various ROS scavengers, L-Histidine (L-His) for singlet oxygen plus hydroxyl radicals, D-Mannitol (D-Man) for hydroxyl radicals, superoxide dismutase (SOD) for superoxide radicals, and N-acetyl cysteine (NAC) for hydroxyl radicals plus hydrogen peroxide.[14] Among all the tested scavengers, none, with the exception of NAC, showed any protective effects (Fig 2D, and Fig S5). NAC was only able to rescue a small fraction (~20%) of cells from the RB4 and ultrasound combination treatments. These results suggest that hydrogen peroxide may only partially participate in cell toxicity, possibly as a side effect of cell lysis freeing it from intracellular compartments, rather than its direct generation by ultrasound. The majority of cells were killed through other mechanisms.
Based on these differences in mechanism studies, it is concluded that RB4 SED is not a typical sonosensitizer used in sonodynamic therapy.[14, 15] It produces little ROS, but kills cells almost instantly under insonation. The cell killing process is rapid, and appears to involve a complete loss of membrane integrity. We propose that RB4 acts as a membrane destabilizer, weakening the extracellular membrane by inserting itself into the membrane, and promoting the membrane lysis tendency. Assisted by an ultrasound pressure that oscillates between compression and expansion, the membrane bursts almost immediately. Similar ultrasound induced membrane damage effects have been suggested by other groups.[26, 30] This RB4 combination with low-intensity ultrasound could lead to a novel Sono-Membrane Rupture Therapy (SMRT) to ablate tumor tissues.
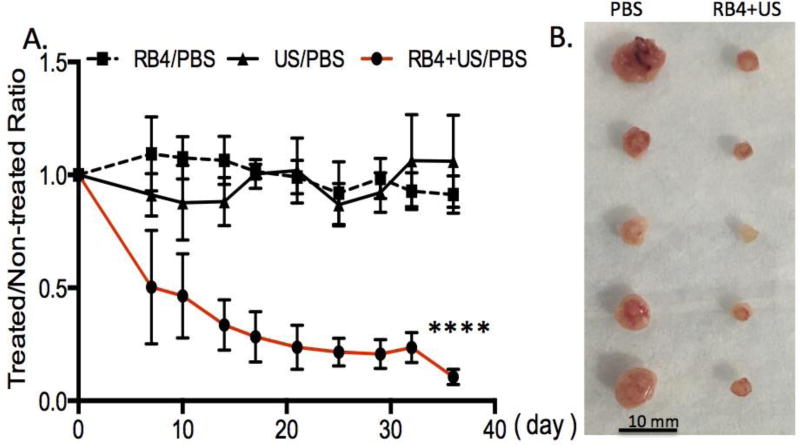
The potential for tumor ablation using the RB4/ultrasound combination was evaluated in a triple negative MDA-MD-468 breast cancer xenograft model. To minimize the variation between animals, each animal was inoculated with two tumors. The treatment was then only applied to one of the tumors. The other tumor that had not received treatment served as an internal reference. The tumors were either treated with ultrasound alone (1 MHz, 1 W/cm2, 3 min), RB4 alone (10 µM), or an RB4/ultrasound combination. For the experiments that required RB4, the cells (5 × 106) were pre-incubated with RB4 (10 µM) for 1 day, and then subcutaneously inoculated into the flank. A handheld ultrasound transducer was placed on top of the injection site for 3 min with a continuous ultrasound (1 MHz, 1 W/cm2). This gentle ultrasound insonation did not cause visible negative effects to the contacted skin. The SMRT treatment effect was investigated non-invasively by measuring the tumor sizes twice a week up to 36 days (Fig 3A). As expected, there were no significant differences in tumor size among the untreated, RB4 treated, and ultrasound treated groups, which have similar growth rate ratios (≈ 1). In contrast, the RB4 assisted sonotherapeutic effect was significant throughout the tested period. The average size of the RB4/ultrasound treated tumors was only about 20 % of the control untreated tumor (Fig 3A and 3B). This data clearly showed that growth inhibition happened only with the combination of RB4 and ultrasound. The drug alone or ultrasound alone offered no appreciable inhibition effect. These long-term inhibition effects further support that the RB4/ultrasound combination could be a new way to ablate tumors.
Figure 3.
In vivo tumor inhibition effect of SMRT and controls. (A) Significant grow arrest was seen in the SMRT combination group which was treated with RB4 (10 µM) and US (1 MHz, 1 W/cm2, 3 min). While the size of tumors of the control RB4 alone (10 µM) or US alone (1 MHz, 1 W/cm2, 3 min) groups were about the same with the non-treated group. N = 7, p < 0.0001. (B). A representative image of the excised tumors.
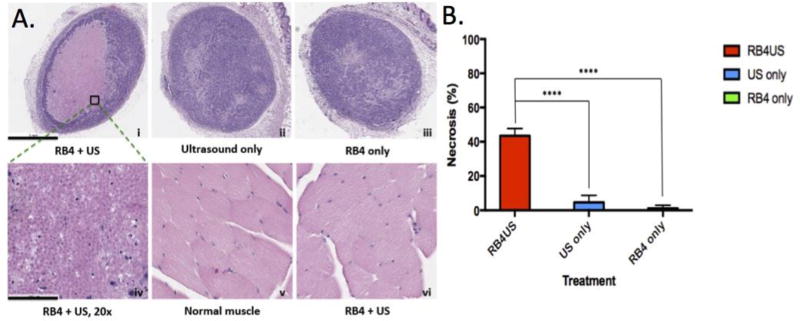
With this encouraging RB4 pretreatment result, the RB4/ultrasound effect was further tested by direct intratumoral injection when tumors were about 4–5 mm in size. RB4 (50 µl, 10 µM) was injected directly into the tumor and followed by a local insonation (1 MHz, 1 W/cm2, 3 min). Two days after treatment, the tumors were excised, sectioned, and checked for cell death (Fig 4). Pathological analysis showed a large necrotic area (~40%) caused by the RB4 and ultrasound combination treatment (Fig 4A–i and 4A–iv). Importantly, when the same treatment was applied to normal muscle tissue (Fig 4A–v and 4A–vi), no damaged cells were found. Similar to the PBS treated group (Fig S6), little tissue damage was observed with either ultrasound alone or RB4 alone in vivo (Fig 4A–ii and 4A–iii). Both pre-treatment and direct intratumoral injection experiments support the clinical potential of low energy ultrasound induced tissue ablation.
Figure 4.
SMRT effect in the MDA-MB468 xenograft tumor model. RB4 (50 µl, 10 µM) was injected directly into tumor and followed by a local insonation (1 MHz, 1 W/cm2, 3 min). Treated and control tumors were excised two days later for pathological analysis. The same RB4/US treatment was applied to the thigh muscle as another control. (A) HE staining of the tissues. A large necrotic area was found only in the tumor with RB4 and ultrasound combination therapy (i & iv). Little effect was found with only ultrasound or RB4 treatment (ii – iii). The myocytes maintained their normal structures without damage after RB4 and ultrasound combination therapy (v–vi). Original magnification: i–iii 4X, iv–vi: 20X. Scale bar: i–iii 1 mm, iv–vi 100 µm. (B) Statistic analysis of the necrotic area. The difference between the combination therapy and US alone or RB4 alone is significant (n=5, p < 0.0001).
Our result indicated that the RB4/ultrasound combination therapy was selective between tumor and muscle. Several cell elastic property studies reported that cancer cells are at least 70% softer than healthy cells.[31–36] Although the exact composition difference and role of the softness in cancer cells is still obscure, it has been suggested to be a factor in driving tumor metastasis.[37] The intrusion of the bulky RB4 might weaken the cancer cell’s plasma membrane, and then promote the ultrasound induced membrane lysis tendency. The healthy cells, which are much stiffer, may resist the insertion of RB4 and are less prone to ultrasound oscillation. This cell membrane selectivity could be a critical contributor making SMRT a unique, safe and effective ablation method.
In this study, a new low-intensity ultrasound assisted SMRT is demonstrated with a Rose Bengal derivative, RB4. RB4 alone does not lyse the plasma membrane. However, it has been shown to cause membrane rupture when used together with an ultrasound wave.
The cells with broken plasma membrane die near instantly. The frequency and intensity of the ultrasound are all within the medical imaging window; thus, the applied ultrasound is safe and causes no damage to tissues. As the wavelength of ultrasound is too long to be absorbed by chemical bonds, it would not interact directly with SED to induce any chemical reactions. The cell death is thus likely a result of the physical oscillation. Instead of targeting the traditional therapeutic targets such as pathways, receptors, enzymes, or genes, SMRT acts on the container, the plasma membrane. The treatment is expected to be safe, because it requires no toxic ingredients, nor high-energy exposure, as well as specific because it requires the coexistence of SED and ultrasound. Since the proposed SMRT does not act on the typical therapeutic targets, it could also overcome many existing drug resistance issues. Membranes, comparing to other biomolecules, are less prone to mutation; thus, SMRT is expected to be a new therapeutic combination for a local low-energy tumor ablation.
Supplementary Material
Acknowledgments
This research was supported in part by NIH GM094880.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
The authors declare no competing financial interests.
References
- 1.Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–928. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trendowski M. Using the Promise of Sonodynamic Therapy in the Clinical Setting against Disseminated Cancers. Chemother Res Pract. 2015;2015:316015. doi: 10.1155/2015/316015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feril LB, Jr, Kondo T. Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. Journal of radiation research. 2004;45:479–489. doi: 10.1269/jrr.45.479. [DOI] [PubMed] [Google Scholar]
- 4.Emoto M. Development of a Cancer Treatment with the Concomitant Use of Low-Intensity Ultrasound: Entering the Age of Simultaneous Diagnosis and Treatment. Diagnostics. 2014;4:47–56. doi: 10.3390/diagnostics4020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Fan H, Wang Z, Zheng J, Cao W. Potentiation of scutellarin on human tongue carcinoma xenograft by low-intensity ultrasound. PLoS One. 2013;8:e59473. doi: 10.1371/journal.pone.0059473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nomikou N, McHale AP. Exploiting ultrasound-mediated effects in delivering targeted, site-specific cancer therapy. Cancer Lett. 2010;296:133–143. doi: 10.1016/j.canlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Nomikou N, Li YS, McHale AP. Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett. 2010;288:94–98. doi: 10.1016/j.canlet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Watson KD, Lai CY, Qin S, Kruse DE, Lin YC, Seo JW, Cardiff RD, Mahakian LM, Beegle J, Ingham ES, Curry FR, Reed RK, Ferrara KW. Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res. 2012;72:1485–1493. doi: 10.1158/0008-5472.CAN-11-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre JY, Idbaih A. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Science translational medicine. 2016;8:343re342. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 10.McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, Coussios CC, Borden M, Nomikou N, McHale AP, Callan JF. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Control Release. 2015;203:51–56. doi: 10.1016/j.jconrel.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Escoffre JM, Mannaris C, Geers B, Novell A, Lentacker I, Averkiou M, Bouakaz A. Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:78–87. doi: 10.1109/TUFFC.2013.2539. [DOI] [PubMed] [Google Scholar]
- 12.Lin CY, Li JR, Tseng HC, Wu MF, Lin WL. Enhancement of focused ultrasound with microbubbles on the treatments of anticancer nanodrug in mouse tumors. Nanomedicine. 2012;8:900–907. doi: 10.1016/j.nano.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Yan F, Li L, Deng Z, Jin Q, Chen J, Yang W, Yeh CK, Wu J, Shandas R, Liu X, Zheng H. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J Control Release. 2013;166:246–255. doi: 10.1016/j.jconrel.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Costley D, Mc Ewan C, Fowley C, McHale AP, Atchison J, Nomikou N, Callan JF. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31:107–117. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- 15.Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014;33:143–160. doi: 10.1007/s10555-013-9461-5. [DOI] [PubMed] [Google Scholar]
- 16.Sadanala KC, Chaturvedi PK, Seo YM, Kim JM, Jo YS, Lee YK, Ahn WS. Sono-photodynamic combination therapy: a review on sensitizers. Anticancer research. 2014;34:4657–4664. [PubMed] [Google Scholar]
- 17.Xiong W, Wang P, Hu J, Jia Y, Wu L, Chen X, Liu Q, Wang X. A new sensitizer DVDMS combined with multiple focused ultrasound treatments: an effective antitumor strategy. Sci Rep. 2015;5:17485. doi: 10.1038/srep17485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hachimine K, Shibaguchi H, Kuroki M, Yamada H, Kinugasa T, Nakae Y, Asano R, Sakata I, Yamashita Y, Shirakusa T. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na(I), which is devoid of photosensitivity. Cancer Sci. 2007;98:916–920. doi: 10.1111/j.1349-7006.2007.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuru H, Shibaguchi H, Kuroki M, Yamashita Y, Kuroki M. Tumor growth inhibition by sonodynamic therapy using a novel sonosensitizer. Free Radic Biol Med. 2012;53:464–472. doi: 10.1016/j.freeradbiomed.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Ninomiya K, Noda K, Ogino C, Kuroda S, Shimizu N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: its application to targeted sonodynamic therapy. Ultrason Sonochem. 2014;21:289–294. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Rubio V, Qi J, Xia R, Shi ZZ, Peterson L, Tung CH, O'Neill BE. Cancer treatment using an optically inert Rose Bengal derivative combined with pulsed focused ultrasound. J Control Release. 2011;156:315–322. doi: 10.1016/j.jconrel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachter E, Dees C, Harkins J, Scott T, Petersen M, Rush RE, Cada A. Topical rose bengal: pre-clinical evaluation of pharmacokinetics and safety. Lasers in surgery and medicine. 2003;32:101–110. doi: 10.1002/lsm.10138. [DOI] [PubMed] [Google Scholar]
- 23.McCaughan B, Rouanet C, Fowley C, Nomikou N, McHale AP, McCarron PA, Callan JF. Enhanced ROS production and cell death through combined photo- and sono-activation of conventional photosensitising drugs. Bioorg Med Chem Lett. 2011;21:5750–5752. doi: 10.1016/j.bmcl.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Umemura S, Yumita N, Umemura K, Nishigaki R. Sonodynamically induced effect of rose bengal on isolated sarcoma 180 cells. Cancer Chemother Pharmacol. 1999;43:389, 393. doi: 10.1007/s002800050912. [DOI] [PubMed] [Google Scholar]
- 25.Sugita N, Kawabata K, Sasaki K, Sakata I, Umemura S. Synthesis of amphiphilic derivatives of rose bengal and their tumor accumulation. Bioconjugate chemistry. 2007;18:866–873. doi: 10.1021/bc060189p. [DOI] [PubMed] [Google Scholar]
- 26.Hiraoka W, Honda H, Feril LB, Jr, Kudo N, Kondo T. Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason Sonochem. 2006;13:535–542. doi: 10.1016/j.ultsonch.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K, Fukushima T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer research. 2009;29:943–950. [PubMed] [Google Scholar]
- 28.Nomikou N, Fowley C, Byrne NM, McCaughan B, McHale AP, Callan JF. Microbubble-sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chemical communications. 2012;48:8332–8334. doi: 10.1039/c2cc33913g. [DOI] [PubMed] [Google Scholar]
- 29.Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, Gombau L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro. 2013;27:954–963. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Krasovitski B, Frenkel V, Shoham S, Kimmel E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci U S A. 2011;108:3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 32.Fraldi M, Cugno A, Deseri L, Dayal K, Pugno NM. A frequency-based hypothesis for mechanically targeting and selectively attacking cancer cells. J R Soc Interface. 2015;12:20150656. doi: 10.1098/rsif.2015.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ketene AN, Schmelz EM, Roberts PC, Agah M. The effects of cancer progression on the viscoelasticity of ovarian cell cytoskeleton structures. Nanomedicine. 2012;8:93–102. doi: 10.1016/j.nano.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Lekka M, Pogoda K, Gostek J, Klymenko O, Prauzner-Bechcicki S, Wiltowska-Zuber J, Jaczewska J, Lekki J, Stachura Z. Cancer cell recognition--mechanical phenotype. Micron. 2012;43:1259–1266. doi: 10.1016/j.micron.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Rebelo LM, de Sousa JS, Mendes Filho J, Radmacher M. Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology. 2013;24:055102. doi: 10.1088/0957-4484/24/5/055102. [DOI] [PubMed] [Google Scholar]
- 36.Prabhune M, Belge G, Dotzauer A, Bullerdiek J, Radmacher M. Comparison of mechanical properties of normal and malignant thyroid cells. Micron. 2012;43:1267–1272. doi: 10.1016/j.micron.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Swaminathan V, Mythreye K, O'Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075–5080. doi: 10.1158/0008-5472.CAN-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.