Abstract
Synaptic loss is a symptom of Alzheimer’s disease (AD) that is associated with the onset of cognitive decline and the loss of executive function. The strongest genetic risk factor for AD is the APOE4 allele, which results in both a greater risk of developing AD as well as an earlier age of onset of AD. Dendritic spines, the anatomical substrate of the excitatory synapse, are reduced in the cortex of humanized APOE4 mice but the reason for this synaptic decline is unknown.
Calcineurin, a calcium/calmodulin dependent phosphatase, is a mediator of dendritic spine retraction. We used humanized APOE mice to examine how APOE genotype altered calcineurin activity and found that APOE4 mice have 35% higher cortical calcineurin activity compared to APOE3 mice. This occurred in the absence of any increase in calcineurin protein levels or mRNA expression. The elevation in calcineurin was associated with 10% fewer dendritic spine number in Layer II/III of the cortex. Treatment with the calcineurin inhibitor FK506 reduced calcineurin activity by 64% and resulted in normalization of dendritic spine numbers in APOE4 mice.
In conclusion we find that the APOE4 gene in mice is associated with elevated calcineurin activity and fewer dendritic spine numbers compared to APOE3 mice. Importantly, calcineurin in APOE4 remains sensitive to pharmacological inhibition and spine density can be rescued by treatment with FK506.
Keywords: APOE genotype, PP2B, dendritic spine, FK506, Alzheimer’s disease
INTRODUCTION
Dendritic spines are the physical structure that makes up the post-synaptic synapse. They contain glutamate receptors, and therefore are recognized as excitatory synapses. Dendritic spines can be visualized in the central nervous system (CNS) by examining the small membranous protrusions arising from the dendritic arbors of neurons that provide the anatomical architecture for synaptic transmission. Dendritic spines also serve to increase the surface area of the postsynaptic density and the number of possible contacts between neurons.1 Dendritic spines are plastic during neurodevelopment, arising and retracting frequently, but in adult brains, spines remain very stable, especially in the cortex.2 However, in vivo live imaging studies have revealed that spine turnover continues into adult life.3 While one-month old mice display a net loss of cortical spine density over a 2-week period, adult mice display a consistent 3–5% turnover of cortical dendritic spines.3 Over the course of 18 months 26% of spines in adult mice that were present at the start of the experiment were eliminated, and 19% new spines had formed, indicating that while the majority of cortical synapses are stable there remains a significant proportion that show continuous plasticity throughout life.3 Synaptic plasticity can also occur through changes in spine morphology, and there is a strong correlation between the size of the spine and the strength of the synapse. Dendritic spines that are short and stubby are considered to be more mature than those that are long and thin.4
Changes in spine morphology are associated with developmental disorders such as autism spectrum disorders and fragile X syndrome. In these cases, dendritic spines display long, thin, filapodia-type structure associated with immature spines.5 Changes in dendritic spine density are associated with neurodegenerative diseases including Alzheimer’s disease.6 Interestingly the apolipoprotein E (APOE) epsilon 4 (APOE4) gene has been associated with AD for over 20 years7 with the APOE4 allele conveying higher risk of developing AD, as well as earlier age of onset.7 The APOE4 allele impacts multiple pathways relevant to Alzheimer’s disease pathogenesis including impaired amyloid clearance from the brain,8 neuroinflammation,9 reduced synaptic activity,10 and reduced dendritic spine density in Layer II/III of the cortex.11 Dendritic spine loss is an early event in APOE4 mice and is apparent by 3 months of age,11 although the mechanisms remain unknown. While the apoE protein is produced in astrocytes in the brain,12 many apoE receptors are located on the post-synaptic synapse.13,14 These apoE receptors can modify synaptic plasticity by mediating NMDA receptor activation and activation of subcellular signaling cascades.15
One of the major regulators of dendritic spine stability is the calcium/calmodulin dependent phosphatase, calcineurin (PP2B). Activation of calcineurin results in dephosphorylating of the GluR1 subunit of the AMPA receptor, and consequential collapse of the post-synaptic density can occur.16 In this study we aimed to explore the mechanisms underlying the reduction in dendritic spine density in APOE4 mice. We examined dendritic spine number and morphology in C57Bl/6, APOE3, and APOE4 mice with a focus on calcineurin activity. We used the calcineurin inhibitor FK506 to reduce the activity of this phosphatase and quantify the downstream effects on dendritic spines. We found that APOE4 mice have elevated calcineurin activity that correlates with reduced spine numbers compared to APOE3 mice. We further found that APOE4 mice are responsive to calcineurin inhibition with spine number normalizing after 4 days of treatment with FK506.
EXPERIMENTAL PROCEDURES
Ethical Statement
Procedures were carried out in accordance with protocols approved by Georgetown University Animal Care and Use Committee, and adhered to all federal regulations.
Study Design
Study 1
Four-month old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were randomly treated with vehicle or FK506 for 4 days (n = 4). Bisected PBS perfused brains were used for calcineurin assay or Golgi staining. For Golgi counts an average of 18 neurons were counted for each animal.
Study 2
Four-month old APOE3 and APOE4 targeted replacement mouse brains were probed for calcineurin activity or fixed for Golgi staining and dendritic spine counts as described below (n = 5 for each genotype). For Golgi Counts an average of 11 neurons were counted for each animal. A separate cohort of mice (n = 5 for each genotype) were used for mRNA and protein studies.
Study 3
Littermates from humanized APOE3 and APOE4 mice were randomly assigned to either a vehicle or FK506 group (n=10 for each group). Mice were treated with vehicle or FK506 for 4 days, and were euthanized by CO2 inhalation 24h after the final drug administration. PBS perfused brains were bisected and assigned to either calcineurin assay (n = 10), assigned for mRNA and protein (n= 5), or fixed for Golgi staining and dendritic spine counts (n = 5). For Golgi Counts an average of 26 neurons were counted for each animal. For spine width studies, an average of 173 individual spines were assessed per group.
Experimental Procedures
Experimental Animals
4–6 month-old male APOE targeted replacement mice express human APOE alleles in place of murine Apoe, under the control of the endogenous murine Apoe promoter.17 Levels of apoE protein and dendritic spine counts in these mice have been previously characterized.11,18
Drug Administration
FK506 (#3631, Tocris, Minneapolis, MN) was dissolved in 10% ethanol (Fisher Scientific, Hampton, NH) and suspended with 1% Tween 80 (Fisher) in phosphate buffered saline (Fisher). Mice received 10mL/kg bodyweight of a 10mg/mL FK506 solution for a final dose of 100 mg/kg. Mice received a single i.p. injection once a day for 4 days and were euthanized 24h after the final administration.19
Calcineurin Activity Assay
Calcineurin activity in the brain was measured using a Calcineurin Cellular Activity Assay Kit (#BML-AK816, Enzo Life Sciences, Farmingdale, NY). Entire cortex was isolated from lysed and free phosphates removed. Phosphatase activity was detected using a colormetric phosphopeptide substrate. A standard curve was constructed using recombinant human calcineurin. Calcineurin activity was calculated using the following formula: Phosphatase Activity(total) − Phosphatase Activity(calcium independent) = Calcineurin Activity.
Golgi Staining
For detailed characterization of dendritic spines, we stained brain hemispheres with Golgi stain using a Rapid Golgi Stain Kit (#PK401, FD NeuroTechnologies, Columbia, MD), as previously described.20,21
For the layer II/III neurons, we performed two separate counts: dendritic spines on basal shaft (BS) dendrites, and dendritic spines on the apical oblique (AO) dendrites. BS dendrites project directly off the cell soma, and our counts incorporated dendritic spines along a 20 μM section of the shaft between 30–100 μM away from the soma. AO dendrites project off the apical dendrite and our counts only incorporated primary AO dendrites at least 30μM away from the apical dendrite. Different neurons were used to quantify AO and BS segments of healthy pyramidal neurons of cortical layers II/III. We focused on Layer II/III neurons in the frontal and parietal cortex between Bregma 0.00mm to -5.60mm. Brains were coded prior to Golgi staining and dendritic spines images were captured blind to code, then counted blind to code using Image J Software (National Institute of Health, Bethesda, MD).
Calcineurin protein and mRNA analysis
Calcineurin protein detection was performed as previously described,22 using anti-calcineurin subunit A (#PA5-15579, Fisher) and secondary (anti-rabbit IgG, Jackson ImmunoResearch, West Grove, PA) antibodies.
RNA was extracted from cortex brain tissue using TRIzol® reagent (Invitrogen) and RT-QPCR was performed as described previously23 using the Prism 7900HT fast sequence detection system (Applied Biosystems). Taqman probes were used to analyse GAPDH (Mm99999915_m1), Ppp3ca (Mm01317678_m1) and Ppp3cb (Mm00920265_m1) under the following cycle conditions, 50°C for 2 minutes, 95°C for 20seconds, (95°C for 1 second, 60°C for 20 seconds)× 40 repeats. The fold change in mRNA expression was calculated using the ΔΔCt method (2-ΔΔCt).24
Statistical Analysis
Data were analyzed using either an unpaired t-test for two groups or two-way ANOVA for multiple comparisons with Bonferroni multiple comparisons test. All statistical tests were performed using GraphPad Prism software, version 5.0f (GraphPad Software, Inc., San Diego, CA), and p values of less than 0.05 were considered statistically significant.
RESULTS
Peripheral administration of FK506 inhibits brain calcineurin activity and increases dendritic spine number in C57Bl/6 mice
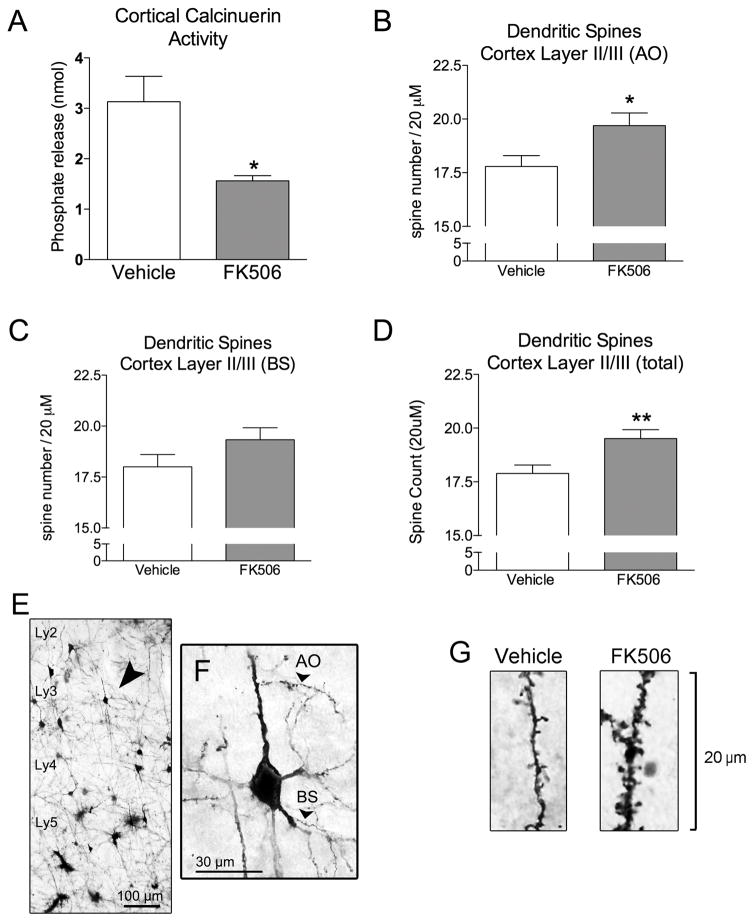
Peripheral administration of FK506 for 4 days resulted in a 50.1% reduction in calcineurin activity in C57Bl/6 mice compared to vehicle-treated mice (P < 0.05; Fig 1a). Dendritic spine analysis revealed that FK506 caused a significant increase in the number of apical oblique spines (10.7% increase; P < 0.05; Fig 1B). We found a 7.4% increase in spine density on basal shaft dendrites however this did not attain significance (Fig 1C). Overall these changes resulted in a 9.1% increase in total spine density (P < 0.01; Fig 1D).
Figure 1. Calcineurin inhibition by FK506 causes dendritic spine growth in C57Bl/6 mice.
A) Four day peripheral administration of 100 mg/kg FK506 results in a decrease in calcineurin activity in the mouse cortex. B) Spine density quantification in the apical oblique (AO) dendrites, C) basal shaft (BS) dendrites, and D) total spine counts following four day vehicle or FK506 administration in mice. E–F) Golgi stained cortical section showing the layers of the cortex, and the areas of the dendrites where spines were quantified. G) Representative images of the Golgi stained AO dendrites spines following vehicle or FK506 treatment. Unpaired t-test. * = P < 0.05; ** = P < 0.01.
APOE4 mice have elevated basal brain calcineurin activity compared to APOE3 mice, and fewer cortical dendritic spines
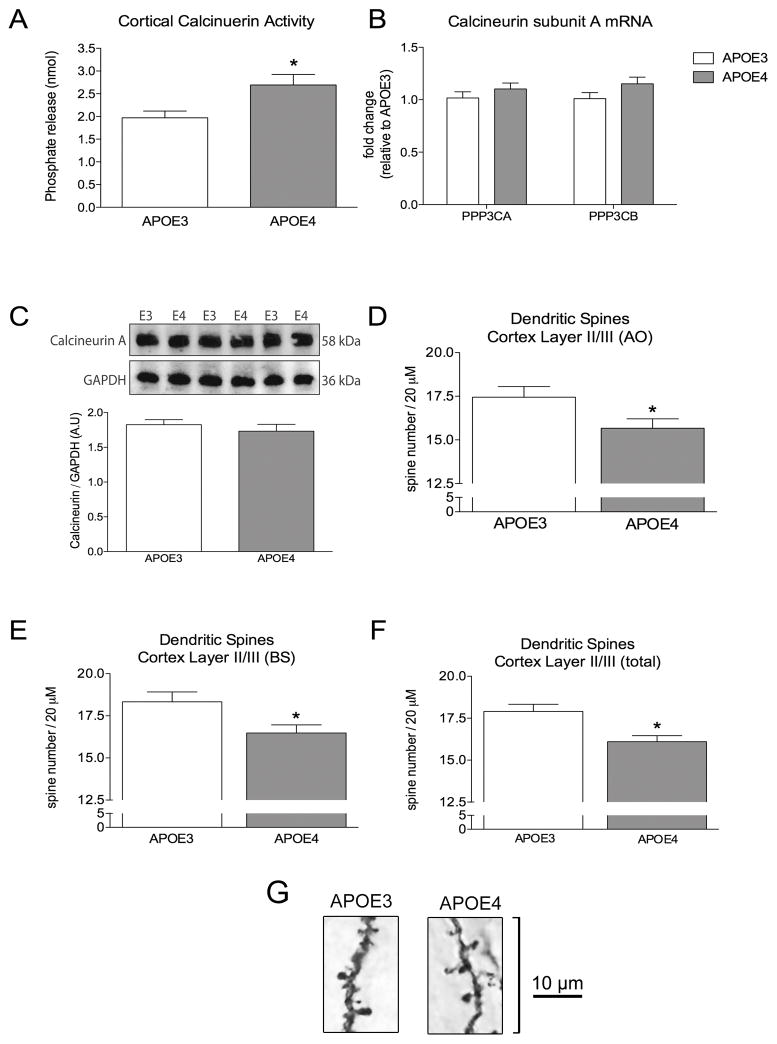
Calcineurin activity was measured in isolated cortices from APOE3 and APOE4 mice. We found that basal levels of brain calcineurin activity in APOE4 mice was increased by 36.7% compared to APOE3 mice (P < 0.05; Fig 2A). This increase in activity was not due to an increase in mRNA expression of the catalytic A subunit (encoded by PP3CA or PP3CB; Fig 2B) or an increase in protein levels of calcineurin A (Fig 2C). The elevated calcineurin activity was associated with decreased dendritic spine number on apical oblique dendrites (10.3% decrease, P < 0.05; Fig 2B) and basal shaft dendrites (10.0%, P < 0.05; Fig 2C) in APOE4 mice. Total spine number decreased by 10.1% (P < 0.05; Fig 2D).
Figure 2. APOE4 targeted replacement mice have elevated calcineurin activity and reduced cortical dendritic spine density.
A) Calcineurin activity is increased in the cortex of naïve APOE4 mice compared to APOE3 mice. B) QT-PCR determined the relative levels of PPP3CA and PPP3CB, two of the genes responsible for producing isoenzymes of the catalytic calcineurin-A subunit. C) Protein levels of the catalytic calcineurin-A subunit were quantified by western blot. D) Spine density quantification of apical oblique (AO) dendrites, E) basal shaft (BS) dendrites, and F) total spine counts. G) Representative images of the Golgi stained AO dendrites in APOE3 and APOE4 mice. Unpaired t-test. * = P < 0.05.
Dendritic spine loss in APOE4 mice can be rescued with FK506 treatment
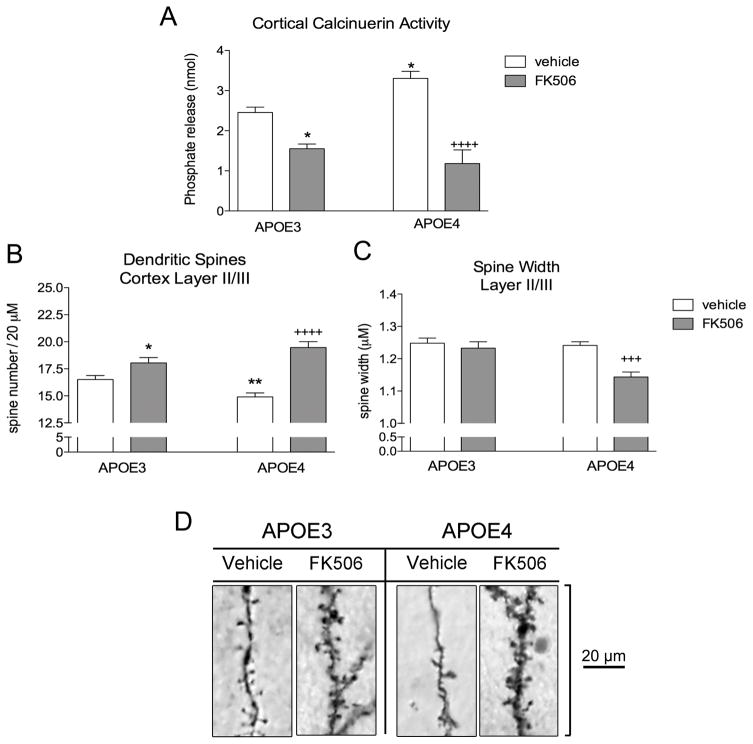
We treated APOE3 and APOE4 mice with either vehicle or FK506 for 4 days, and euthanized the mice for analysis 24h after the final dose. Again, we found that sham APOE4 mice had elevated levels of brain calcineurin activity compared to sham APOE3 mice (34.9% increase; P < 0.05; Fig 3A). FK506 administration reduced calcineurin activity by 36.6% in APOE3 mice (P < 0.05; Fig 3A) and by 64.2% in APOE4 mice (P < 0.0001; Fig 3A).
Figure 3. Calcineurin inhibition by FK506 reverses spine loss in APOE4 targeted replacement mice.
A) Four day peripheral administration of 100 mg/kg FK506 results in a decreased calcineurin activity in both APOE3 and APOE4 mouse cortex. B) Spine density quantification of apical oblique dendrites. C) Spine head width of apical oblique dendritic spines. D) Representative images of the Golgi stained AO dendrites following vehicle or FK506 treatment. Two way ANOVA with Bonferroni multiple comparisons. * = P < 0.05 vs APOE3 vehicle; ** = P < 0.01 vs APOE3 vehicle; +++ = P < 0.001 vs APOE4 vehicle; ++++ = P < 0.0001 vs APOE4 vehicle.
Lastly, Golgi staining revealed that vehicle-treated APOE4 mice had less apical oblique dendritic spines compared to vehicle-treated APOE3 mice (9.8% reduction; P < 0.01; Fig 3B). FK506 treatment resulted in a 9.3% increase in spine number in APOE3 mice (P < 0.05; Fig 3B), and a 30.6% increase in spine number in APOE4 mice (P < 0.0001; Fig 3B).
Apical oblique spine length was not impacted in FK506-treated APOE3 mice, but FK506 caused a 7.8% decrease in the width of the dendritic spine head (P < 0.05 vs APOE4 vehicle; Fig 3C).
DISCUSSION
In this study we investigated potential mechanisms to explain why APOE4 mice have reduced dendritic spine numbers compared to APOE3 mice. We first validated previously published work to show that peripheral administration of the calcineurin inhibitor FK506 could successfully reduce calcineurin activity in the brain, resulting in increased spine density in the cortex. Here, we report that APOE4 mice have elevated basal calcineurin activity and that this corresponds to decreased spine density in the cortex of these mice. Moreover, we demonstrate that calcineurin in APOE4 mice remains responsive to FK506 treatment, which normalized dendritic spine numbers in APOE4 mice.
Calcineurin activation results in a dephosphorylation of the ser(845) site on the GluR1 subunit of the AMPA receptor. This leads to AMPA receptor endocytosis and an overall reduction in total number AMPA receptors, and ultimately a decrease in dendritic spines.16 FK506 is an immunosuppressant that binds to a family of proteins called the FK506 binding proteins (FKBP), with the resultant FKBP-FK506 complex directly inhibiting calcineurin activity.25 Previous studies have found that seven day administration of FK506 in mice results in an increase in the number of dendritic spines in Layer II/III of the cortex.26 Our data in C57Bl/6 mice confirmed that peripherally administered FK506 can reduce brain calcineurin activity and subsequently increase cortical dendritic spine density. It is an important distinction that FK506 itself does not cause spine growth, but rather prevents the degradation of spines leading to a greater overall spine number.
Dendritic spines are reduced in disease states such as Alzheimer’s disease,6 where synapse number is known to correlate with cognitive decline27 Dendritic spine loss is also seen in animal models of Alzheimer’s disease, where calcineurin inhibition by FK506 has been shown to ameliorate spine loss.19 The APOE4 allele, first identified as a genetic risk factor for late onset Alzheimer’s disease over 20 years ago,7 remains one of the strongest genetic risk-factors identified to date.28 Early synapse loss and cognitive deficits are characteristic of humanized homozygous APOE4 mice11,29 and APOE transgenic mice,30 and there is a APOE4 allele-dependent loss of dendritic spines in normal aging and Alzheimer’s disease brain.30 The humanized APOE mice were used in the present study, and here we have confirmed that APOE4 mice have reduced spine density in the cortex compared to APOE3 mice. The major novel finding in this report is that we examined subcellular mechanisms that regulate dendritic spine loss and found that calcineurin activity is higher in APOE4 mice compare age-matched APOE3 mice. This change appears to be a change in enzyme kinetics, as neither mRNA nor protein levels were altered by APOE4.
To our knowledge this is the first report of a change in calcineurin activity in APOE4 mice, however published data is supportive of a role for differential regulation of this phosphatase by APOE genotype. In an in vitro study, neurons co-cultured with glial cells from humanized APOE4 mice displayed delayed spine formation and early synaptic loss due to impaired regulation of the AMPA receptor in dendritic spines.31 As discussed above, calcineurin is a key regulator of AMPA dynamics in dendritic spines.16 Multiple factors can influence how calcineurin is activated including the frequency, duration, and amplitude of calcium input following NMDA receptor stimulation.32 When rat hippocampal neurons are treated with apoE protein there is an increase in intracellular free calcium, with apoE4 protein resulting in higher levels of intracellular calcium compared to apoE3 protein.33 This elevation in intracellular calcium is due to calcium influx, and not due to intracellular calcium storage release.34 The apoE4 protein may also modulate the NMDA receptor directly as NMDA-dependent LTP induction in hippocampal APOE4 slices is lower than slices from APOE3 mice.35 These changes in intracellular calcium may be responsible for the basal increase in calcineurin activity in the APOE4 mice. Other studies indicate that apoE-containing lipoproteins can protect neurons against oxidative stress by inhibiting the activation of calcineurin.36 ApoE4-contining lipoproteins are less effective than apoE3-contining lipoproteins at inhibiting this calcineurin-dependent neuroprotection.36
To determine if the observed elevation in calcineurin was responsible for the reduced spine levels in APOE4 mice, we administered the calcineurin inhibitor FK506 and found that we could completely reverse spine loss in APOE4 mice. Indeed, APOE4 mice appeared more sensitive to FK506 treatment compared to either C57Bl/6 or APOE3 mice, given that FK506 resulted in stronger effects on both calcineurin activity and spine density in APOE4 mice. There is a strong correlation between the size of the spine and the strength of the synapse, whereas spines that are short and stubby are considered to be more mature than those that are long and thin.4 The increase in spines in FK506-treated APOE4 mice was associated with a higher proportion of immature spines, as indicated by the reduced average width of the synaptic protrusion. These data suggest that the turnover of dendritic spines is higher in APOE4 mice compared to APOE3 mice, but the newly formed immature spines caused by the suppression of calcineurin activity are not degraded as quickly as they are in vehicle-treated mice. Further studies with longer timepoints post-treatment, or longer treatments, are needed to determine if these immature spines can mature or reverse cognitive deficits in APOE4 mice.
In conclusion we find that the APOE4 gene in mice is associated with elevated calcineurin activity and fewer dendritic spine numbers compared to APOE3 mice. Importantly, calcineurin in APOE4 remains sensitive to pharmacological inhibition and spine density can be rescued by treatment with FK506.
Acknowledgments
Funding: This work was supported by the National Institute for Neurological Disorders and Stroke by the following funding sources: R01 NS067417 to MPB, R03 NS095038 to SV, through a supplement to Promote Diversity in Health-Related Research (R01NS067417S1), and a T32NS041218 to Georgetown University’s Neural Injury and Plasticity Training Program (CNW). Fellowship support for BSM was supplied by a Department of Health and Human Services-funded Advanced Rehabilitation Research and Training Program (90AR5005). Funding was supplied by Georgetown Undergraduate Research Opportunities Program (AN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lee KF, Soares C, Beique JC. Examining form and function of dendritic spines. Neural Plast. 2012;2012:704103. doi: 10.1155/2012/704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420(6917):812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 3.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16(1):95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gertz HJ, Cervos-Navarro J, Ewald V. The septo-hippocampal pathway in patients suffering from senile dementia of Alzheimer’s type. Evidence for neuronal plasticity? Neurosci Lett. 1987;76(2):228–232. doi: 10.1016/0304-3940(87)90720-8. [DOI] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60(4):559–569. doi: 10.1002/glia.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trommer BL, Shah C, Yun SH, Gamkrelidze G, Pasternak ES, Ye GL, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15(17):2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 11.Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29(48):15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoe HS, Pocivavsek A, Chakraborty G, Fu Z, Vicini S, Ehlers MD, et al. Apolipoprotein E receptor 2 interactions with the N-methyl-D-aspartate receptor. J Biol Chem. 2006;281(6):3425–3431. doi: 10.1074/jbc.M509380200. [DOI] [PubMed] [Google Scholar]
- 14.May P, Rohlmann A, Bock HH, Zurhove K, Marth JD, Schomburg ED, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol Cell Biol. 2004;24(20):8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7(11):850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 16.Kam AY, Liao D, Loh HH, Law PY. Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci. 2010;30(45):15304–15316. doi: 10.1523/JNEUROSCI.4255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 18.Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28(45):11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozkalne A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41(3):650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winston CN, Chellappa D, Wilkins T, Barton DJ, Washington PM, Loane DJ, et al. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. Journal of neurotrauma. 2013;30(23):1966–1972. doi: 10.1089/neu.2013.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winston CN, Noel A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D, et al. Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am J Pathol. 2016;186(3):552–567. doi: 10.1016/j.ajpath.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Washington PM, Morffy N, Parsadanian M, Zapple DN, Burns MP. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J Neurotrauma. 2014;31(1):125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Main BS, Zhang M, Brody KM, Ayton S, Frugier T, Steer D, et al. Type-1 interferons contribute to the neuroinflammatory response and disease progression of the MPTP mouse model of Parkinson’s disease. Glia. 2016;64(9):1590–1604. doi: 10.1002/glia.23028. [DOI] [PubMed] [Google Scholar]
- 24.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270(1):41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 26.Spires-Jones TL, Kay K, Matsouka R, Rozkalne A, Betensky RA, Hyman BT. Calcineurin inhibition with systemic FK506 treatment increases dendritic branching and dendritic spine density in healthy adult mouse brain. Neurosci Lett. 2011;487(3):260–263. doi: 10.1016/j.neulet.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 28.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn Mem. 2013;20(5):256–266. doi: 10.1101/lm.030031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122(2):305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Nwabuisi-Heath E, Rebeck GW, Ladu MJ, Yu C. ApoE4 delays dendritic spine formation during neuron development and accelerates loss of mature spines in vitro. ASN Neuro. 2014;6(1):e00134. doi: 10.1042/AN20130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Stefan MI, Le Novere N. Calcium input frequency, duration and amplitude differentially modulate the relative activation of calcineurin and CaMKII. PLoS One. 2012;7(9):e43810. doi: 10.1371/journal.pone.0043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller W, Meske V, Berlin K, Scharnagl H, Marz W, Ohm TG. Apolipoprotein E isoforms increase intracellular Ca2+ differentially through a omega-agatoxin IVa-sensitive Ca2+-channel. Brain Pathol. 1998;8(4):641–653. doi: 10.1111/j.1750-3639.1998.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res. 2002;67(3):379–387. doi: 10.1002/jnr.10138. [DOI] [PubMed] [Google Scholar]
- 35.Dolejsi E, Liraz O, Rudajev V, Zimcik P, Dolezal V, Michaelson DM. Apolipoprotein E4 reduces evoked hippocampal acetylcholine release in adult mice. J Neurochem. 2016;136(3):503–509. doi: 10.1111/jnc.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi H, Campenot RB, Vance DE, Vance JE. Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cgamma1 and inhibition of calcineurin. J Biol Chem. 2009;284(43):29605–29613. doi: 10.1074/jbc.M109.039560. [DOI] [PMC free article] [PubMed] [Google Scholar]