Abstract
Objective To measure the impact of a computerized guideline for glucose regulation in an ICU.
Design A randomized, controlled trial with an off-on-off design.
Methods We implemented a glucose regulation guideline in an intensive care unit in paper form during the first study period. During the second period, the guideline was randomly applied in either paper or computerized form. In the third period, the guideline was available only in paper form.
Measurements and results We analyzed data for 484 patients. During the intervention period, the control group included 54 patients and the computerized intervention group included 66 patients. The two guideline-related outcome measures consisted of compliance with: (a) glucose measurement timing recommendations and (b) insulin dose advice. We measured clinical impact as the proportion of time that glucose levels fell within target range. In the first (paper-based) study period, 29.0% of samples occurred with optimal timing; during the second period, this increased to 35.5% for paper-based and to 40.2% for computerized protocols. The third study period timeliness scores reverted to the first period rates. Late (suboptimal) sampling occurred for 66% of glucose measurements in the first study period, for 42% of paper-based and 28% of computer-based protocol samples in the second period, and for 50.0% of samples in the third study period. In the first study period, insulin-dosing guideline compliance was 56.3%; in the second period, it was 64.2% for paper-based and 77.3% for computer-based protocols, and it fell to 42.4% in the third period. For the second study period, the time that a patient's glucose values fell within target range improved for both the control (52.9%) and the computerized groups (54.2%) compared with the first study period (44.3%) and the third period (42.3%).
Conclusion Implementing a computerized version of a guideline significantly improved timeliness of measurements and glucose level regulation for critically ill patients compared with implementing a paper-based version of the guideline.
Introduction
Until the late 1990s, clinicians viewed stress hyperglycemia as a beneficial defense mechanism of the human body.1 Recent scientific publications have underscored the importance of strict glycemic control in hospitalized patients.2,3,4,5,6,7,8 However, in our institution's intensive care unit (ICU), translation of these recommendations into daily clinical practice proved difficult using conventional means. In response, we developed a guideline for strictly regulating patients' glucose levels. The literature on clinical guidelines shows that adherence to guidelines is often poor.9,10,11,12 Therefore, one must develop and implement mechanisms to improve guideline adherence.9 After other groups tested a number of approaches, computer-based guideline implementation appears most promising.13,14,15,16,17,18,19 Nevertheless, outcome studies on this topic remain inconclusive.20
The goal of improved glucose regulation provided an opportunity to study the impact of computer-based guideline implementation. This problem matched Tierney's criteria21 for when medical informatics approaches can improve guideline compliance—a common clinical problem with sufficiently available data to apply an algorithm that calls for an explicit clinical action that has a measurable outcome and an intervention that does not require additional time from clinicians.
We implemented the guideline using Event Manager, an integrated decision support module within our institution's clinical information system (CIS) (iMD-Soft; MetaVision, Tel Aviv, Israel). Event Manager notifies clinicians when a set of predefined conditions are met. It is fully customizable and can utilize multiple data set parameters to reduce the frequency of false-positive alerts. Researchers hypothesized that the computer-based guideline would improve nursing staff's responsiveness in measuring patient glucose levels and improve the care delivered (correct insulin doses) in response to the glucose levels. These results would cause patients' blood glucose levels to fall within the target range (4.0–7.0 mmol/L) more often.
Methods
Setting
We conducted the study in an 18-bed medical/surgical ICU in a teaching hospital (Onze Lieve Vrouwe Gasthuis Hospital, Amsterdam, the Netherlands). This unit admits approximately 1,900 patients annually; approximately 1,100 of these patients had recent cardiothoracic surgery. The medical staff has sole direct supervisory responsibility for patients admitted to the unit. This staff includes five permanent intensivists and six intensive care fellows (who work for as long as two years in the unit). A physician is always present on the unit. The nursing staff consists of 93 nurses working in eight-hour shifts. The patient-to-nurse ratio is normally 1:2 but varies with the severity of the patient's illness.
The hospital unit achieved full CIS implementation on April 1, 2001. The staff uses the CIS to complete all patient charting and documentation, such that no information has paper as its primary storage mechanism. The system is connected to the bedside monitor (Siemens SC8000; Siemens-Elema, Solna, Sweden), the ventilator (Siemens Servo 300, Dräger Evita II, Dräger Evita IV; Dräger, Lübeck, Germany), the medication pumps (P1000; Alaris Medical, San Diego, CA), and the Hospital Information System (HIS). Each bed is equipped with a CIS workstation. Every patient room (containing two beds) has an additional CIS workstation. The central desk and the physicians' room also contain workstations.
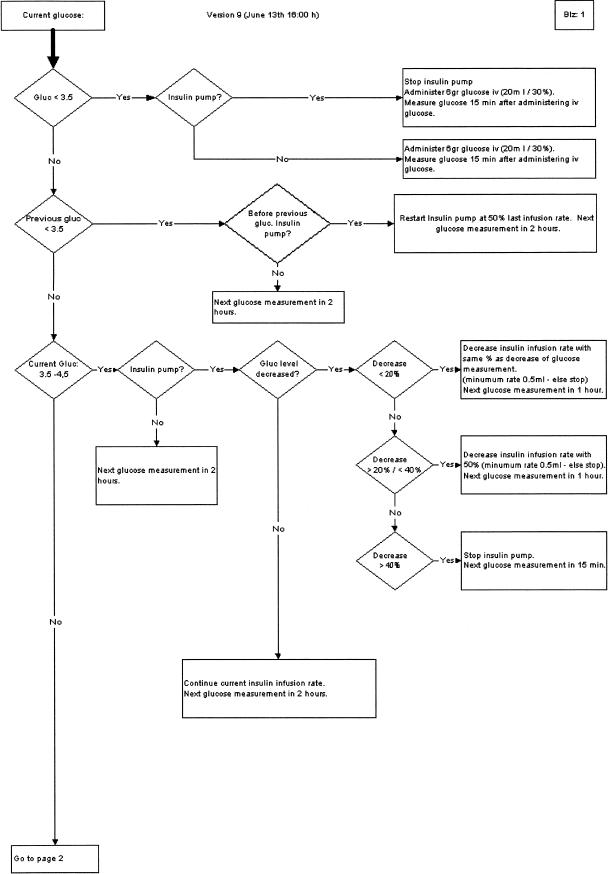
Prior to the study, health care workers performed glucose regulation ad hoc; “acceptable” glucose levels were between 10 and 12 mmol/L. The compelling and growing body of evidence in the literature regarding the adverse effects of this practice made it imperative to develop a guideline for strictly regulating patient glucose levels. We analyzed existing guidelines, consensus methods, and published evidence to develop the guideline. The guideline recommends the timing between glucose measurements and the administration of insulin doses. The guideline does so uniformly in both diabetic and nondiabetic patients. Guideline activation occurs for any patient with an expected length of stay (LOS) longer than 24 hours and for all patients with a preexisting diagnosis of diabetes mellitus. Exclusion (or deactivation) criteria for guideline usage included induced hypothermia, administration of glucose-insulin-potassium infusions, and normal eating. The guideline could be reactivated when exclusion criteria no longer applied. The nursing staff has responsibility for executing the guideline. Further details on the guideline and its development are beyond the scope of this article (▶ shows the first page of the guideline).
Figure 1.
Sample guideline segment (page 1 of 4).
To prevent disparities caused by different glucose measuring methods (laboratory versus handheld devices, whole blood sampling versus plasma sampling), clinical staff performed all measurements with the Accu-check, a handheld glucose measurement device. The laboratory computer interface conveys Accu-check results to the CIS. Consequently, the CIS can display and process all values directly after their measurement with a maximum delay, introduced by the interface, of 1 minute. The Institutional Ethics Committee reviewed the study protocol. The committee waived requirements for informed consent because the study involved different methods of introducing the same guideline for the same treatment in all study groups.
Study Design
The study included three distinct periods with an off-on-off design. The design allows for estimation of bias through crossover and learning effects. After extensive training and the initial introduction of the guideline, there was a two-week prestudy period (with no data collection). During this period, researchers fine-tuned the guideline, and staff continued to familiarize themselves with the new guideline. The actual first study period then lasted six weeks and involved continued use of the paper-based guideline (paper implementation group [group A]). A ten-week second study period followed during which patients were randomly assigned to either the computerized- or the paper-based version of the guideline (computerized [B1] and control [B2] groups, respectively). During the third and final four-week study period, treatment reverted to paper-based guideline utilization (postintervention group [group C]). Additionally, we collected data over an eight-week period directly prior to the guideline introduction. These data provided baseline characteristics for the level of glucose measurements (preimplementation period [group R]). The duration of the third (postintervention) study period was limited because the study was performed as part of a master's thesis project.
Study Population
During the second study (intervention) period, all patients admitted to the ICU were randomly assigned to a study group based on their “internal” patient number. The CIS automatically generates the number, which is not visible to the user. Researchers did not reveal the randomization method to the staff. Only after a patient was admitted to the CIS would the study group (paper-based vs. computerized guideline utilization) be displayed. Authors only used data from those patients and periods when the guideline was activated for study analyses (see activation and exclusion criteria in the Setting section).
Intervention
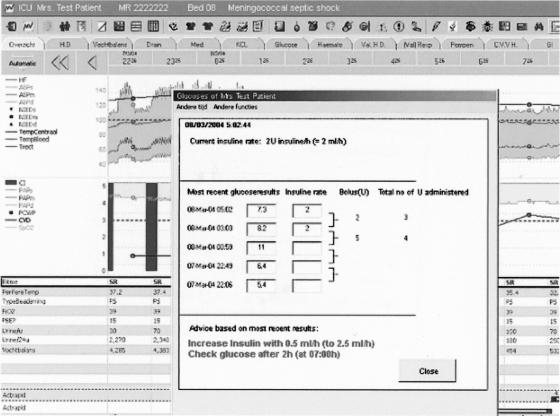
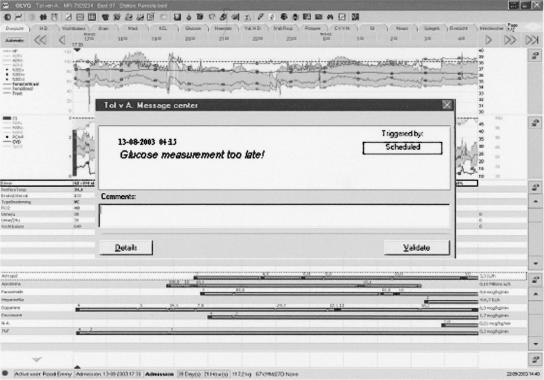
During the second study period, clinicians treating patients randomized to the computerized group (B1) received guideline-based advice via the CIS decision support software module (Event Manager) and a custom-made Visual Basic application integrated within the CIS. The application displayed glucose and insulin data and suggested current treatment and the interval to the next glucose measurement (▶). During this study period, for the intervention group (B1) the Event Manager continuously ensured that guideline activation occurred for eligible patients (based on physiological data within the system). These data included LOS, ventilation status, and body temperature (LOS was monitored because this was the main inclusion criterion; other exclusion criteria were ventilation status to exclude that patients were eating and body temperature to exclude hypothermia). The Event Manager also monitored the time interval between glucose measurements and alerted the staff if a measurement was overdue. Furthermore, the Event Manager checked whether guideline-based insulin infusion rate changes occurred properly. For group B1, each noncompliant event triggered a pop-up window to appear on top of the active CIS screen, alerting clinical staff members. This window appeared on bedside workstations and at any workstation where the patient's record was activated. (▶). If the staff took no action, the alert would again pop up within a few minutes. For patients in the control group (B2), treatment was based solely on the paper-based version of the guideline, a four-page flowchart that directs the nurse to the relevant guideline advice. No automated support from the CIS was given in any form. A copy of the guideline was available at every workstation and on the unit's Intranet.
Figure 2.
Automated guideline advice.
Figure 3.
Event alerting the staff to the late glucose measurement.
The CIS automatically collected and processed all study data. To do so, the CIS utilized interfaces with the laboratory (as part of the HIS), the vital signs monitor, mechanical ventilator data, and medication infusion pump data.
For the third study period (postintervention) group (C), computerized implementation of the guideline was discontinued, and we blocked end-user access to computer-based guideline-related data. Clinical staff treated all patients in this group using the paper guideline in a manner similar to that of the first study period.
Data Collection and Analysis
After the third study period, an automated script retrieved all relevant data from the MetaVision database. To determine adherence to the time (of glucose measurement) advice of the guideline, we verified the time at which a sample was taken, calculated the guideline-recommended time interval to the next measurement, and then compared this interval with the actual time interval.
The recommended interval between measurements could range from 15 to 180 minutes depending on the patient's measured glucose level and its rate of change since the last measurement. Therefore, the study calculated the deviation between advised and actual measurement times as the percentage and minutes of elapsed time between measurements compared with the advice. A deviation of 5% was allowed, with a minimum of 2 minutes, before a measurement was considered to be taken too early or too late. We determined adherence to the advised insulin infusion rate by retrieving the actual insulin infusion rates along with the rate recommended by the guideline.
To assess the guideline's impact on glucose regulation, we calculated the amount of time that patients' glucose levels fell within predefined ranges using the trapezoidal rule, assuming a linear progression between two measurements.22 Based on clinical relevance, we chose to measure the amount of time glucose measurements fell within the target range and not the number of measurements (data points) that fell within the target range. The time between measurements differed significantly (as determined by the guideline). The recommended time between measurements in the lowest glucose range (hypoglycemia) was 15 minutes; in the normal and high ranges, it was two or three hours. The guideline calls for oversampling when lower glucose values occur (for patient safety because hypoglycemia is potentially harmful), so that any analysis of “glucose control” based on data points being weighted equally (independent of timing) would skew the results because more low than normal or high values would be obtained per protocol.
We used Student's t-test, nonparametric tests, and analysis of proportions and their differences. Because the data for the glucose measurements were not normally distributed, we performed log transformation before statistical analysis. Results include 95% confidence intervals (CIs) for the values.
Results
During the study, all patients were treated by the same group of clinicians. No changes in staff composition occurred during the study. We analyzed data for 484 patients. Of these, 120 patients were enrolled during the second study period (intervention period), with 66 in the computerized group (B1) and 54 in the control group (B2). Basic patient characteristics appear in ▶, including patients' Acute Physiology, Age, and Chronic Health Evaluation II and Simplified Acute Physiology II23,24 scores (taken as indicators of severity of illness and the degree of physiological derangements). We found no significant differences among the groups, except for the prevalence of preexisting diabetes mellitus (see Discussion).
Table 1.
Description of Study Population
| Intervention Period Group B |
||||||
|---|---|---|---|---|---|---|
| Total Group | Pre-impl. Group R | Paper impl. Group A | Computerized (B1) | Control (B2) | Postintervention Group C | |
| No. of patients | 484 | 225 | 116 | 66 | 54 | 23 |
| Mean age, yr (SD) | 67 ± 13 | 67 ± 12 | 68 ± 12 | 65 ± 13 | 65 ± 14 | 66 ± 13 |
| Gender male (%) | 298 (62) | 132 (59) | 71 (61) | 45 (68) | 36 (67) | 14 (60) |
| APACHE II (SD) | 20.3 ± 7.7 | 20 ± 7.8 | 20.7 ± 7.2 | 19.5 ± 7.4 | 21.6 ± 8.8 | 20.3 ± 6 |
| SAPS II (SD) | 41.7 ± 17 | 40.2 ± 16.1 | 43.9 ± 16.1 | 41.4 ± 18.9 | 43.3 ± 19.5 | 43 ± 18.6 |
| Preexisting DM, % | 25.2 | 18.0 | 19.8 | 42.4 | 38.8 | 39.0 |
| LOS (SD) | 4.4 ± 7 | 4.8 ± 8.5 | 4.6 ± 6.1 | 3.6 ± 5.1 | 3.7 ± 5.1 | 3.7 ± 3.9 |
| Surgical patients (%) | 321 (66.3) | 148 (65.7) | 72 (62.1) | 47 (71.2) | 39 (72.2) | 15 (65.2) |
Pre-impl. = preimplementation period; Paper impl. = period in which the guideline was only available in its paper form; APACHE II = average Acute Physiology, Age, and Chronic Health Evaluation II score; SAPS II = average Simplified Acute Physiology Score II; Preexisting DM = percentage of admitted patients with preexisting diabetes, irrespective of type; LOS = length of stay in Intensive Care Unit (days).
Adherence to Instructed Time between Glucose Measurements
The frequency of “compliant” glucose measurement timing (measurement taken within time frame dictated by the guideline) improved significantly between the first study (paper implementation) period (A) and the second study (intervention) period (B) (29% vs. 38% observed difference of 8.8 with a 95% CI of 6.9–11) (▶). During the second (intervention) study period, the computerized group (B1) showed a significantly better timing adherence than the control group (B2) (40% vs. 36% observed difference of 4.6 with a 95% CI of 2.0–7.4). During the third (postintervention) study period (C), the number of samples taken on time remained at the level of the computerized group (B1) (41% vs. 40%).
Table 2.
Adherence to the Guideline for the Percentage of Samples Taken Too Early/On Time/Too Late
| No. of Samples |
||||
|---|---|---|---|---|
| Total No. of Samples | Too Early (%) | On Time (%) | Too Late (%) | |
| A. Paper implementation | 4,634 | 36.36 | 28.98 | 34.66 |
| B. Intervention period | 4,949 | 33.87 | 37.75 | 28.39 |
| B1. Computerized | 2,352 | 34.31 | 40.18 | 25.51 |
| B2. Control | 2,597 | 33.46 | 35.54 | 31.00 |
| C. Postintervention | 1,154 | 30.07 | 40.99 | 28.94 |
The number of samples taken too late decreased from 35% in the first study (paper implementation) period (A) to 28% during the second (intervention) period (B) (observed difference of 6.3 with a 95% CI of 4.4–8.1). Within the second (intervention) period, the computerized group showed a significantly better rate compared with the control group (26% vs. 31% observed difference of 5.5 with a 95% CI of 3.0–8.0). During both the third (postintervention) period (C) and the second (intervention) period (B), comparable numbers of samples were taken too late (29% and 28%, respectively). The computerized group's (B1) performance was slightly lower than the 29%, at 26% (observed difference of 3.4 with a 95% CI of 0.28–6.6). When samples were taken too late, the deviation from the advised time interval dropped from 67% in the first study (paper) group (A) to 36% in the second study (intervention) period (B) (difference between sample means of 31 with a 95% CI of 29–33). The computerized group (B1) did significantly better than the control group (B2) (28% vs. 42% observed difference between sample means of 14 with a 95% CI of 11–16). During the third (postintervention) period (C), results deviated from the advised time interval more (50%) compared with the 36% rate for the second (intervention) period (B) (▶).
Table 3.
Deviation from the Suggested Time Interval between Measurements, the Quantity of Time a Measurement Was Taken Too Early/Late Expressed as Percentage
| Quantity of Time |
||||
|---|---|---|---|---|
| Total No. of Samples | Too Early % (SD) | Too Late % (SD) | Too Late Quantity of Time Average in Minutes (SD) | |
| A. Paper implementation | 4,634 | 36.90 (32.7) | 66.60 (152.5) | 71.46 (164.7) |
| B. Intervention period | 4,949 | 28.39 (29.1) | 35.76 (101.2) | 36.06 (128.9) |
| B1. Computerized | 2,352 | 27.80 (28.8) | 28.10 (103.3) | 27.95 (118.3) |
| B2. Control | 2,597 | 28.90 (29.3) | 41.90 (99.1) | 42.49 (139.5) |
| C. Postintervention | 1,154 | 33.20 (31.2) | 50.00 (87.9) | 52.95 (95.2) |
Adherence to Dose Advice
During the second (intervention) period (B), adherence to the insulin advice increased significantly to 70% as compared with the first (paper) implementation period (A) at 56% (▶). The computerized group (B1) followed the recommended insulin dose recommendations more closely than the paper-based control group (B2) (77% vs. 64% observed difference of 13.10 with a 95% CI of 11–16). During the third study (postintervention) period (C), compliance with advice fell to 42%.
Table 4.
Adherence to the Insulin Advice
| Total No. of Samples | Insulin Advice Followed (%) | |
|---|---|---|
| A. Paper implementation | 4,634 | 56.3 |
| B. Intervention period | 4,949 | 70.4 |
| B1. Computerized | 2,352 | 77.3 |
| B2. Control | 2,597 | 64.2 |
| C. Postintervention | 1,154 | 42.4 |
Effect of Guideline on Patient Glucose Homeostasis
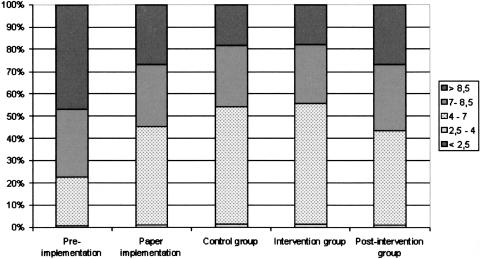
The linear percentage of time that patients' glucose levels fell within the target range (4.0–7.0 mmol/L) before guideline implementation (R) was 22% (▶ and ▶). After guideline implementation, this increased to 44% during the first (paper implementation) study period (A) (observed difference of 22.2 with a 95% CI of 22.1–22.3). The second (intervention) period (B) also improved to 53% (compared with group A, observed difference of 10.3 with a 95% CI of 10.1–10.5). The difference between the computerized (B1) and control (B2) groups, although still significant, was small (54.2% vs. 52.9% observed difference of 1.3 with a 95% CI of 1.0–1.56). For the third period (postintervention) group (C), the time spent in the target range dropped significantly to the lowest level of the three study periods (42%) (compared with group B, with 53% in the target range, an observed difference of 12.3 with a 95% CI of 12.0–12.5).
Table 5.
Time Spent within Each Range per Period in Percentage of Time
| Total Time | <2.5 | 2.5–4 | 4–7 | 7–8.5 | >8.5 | |
|---|---|---|---|---|---|---|
| R. Preimplementation | 1,273,751 | 0.07% | 0.54% | 22.04% | 30.35% | 47.00% |
| A. Paper implementation | 835,414 | 0.05% | 1.06% | 44.25% | 27.91% | 26.73% |
| B. Intervention period | 591,342 | 0.07% | 1.30% | 54.55% | 27.09% | 18.00% |
| B1. Computerized | 272,939 | 0.09% | 1.28% | 54.20% | 26.64% | 17.79% |
| B2. Control | 318,403 | 0.05% | 1.32% | 52.90% | 27.53% | 18.21% |
| C. Post intervention | 142,861 | 0.10% | 1.23% | 42.29% | 29.42% | 26.95% |
<2.5 = cumulative time during each study period with glucose <2.5 mmol/L; 2.5–4 = cumulative time during each study period with glucose between 2.5 and 4 mmol/L; 4–7 = cumulative time during each study period with glucose between 4 and 7 mmol/L; 7–8.5 = cumulative time during each study period with glucose between 7 and 8.5 mmol/L; >8.5 = cumulative time during each study period with glucose >8.5 mmol/L.
Figure 4.
Time patients' glucose levels spend in predefined ranges (mmol/L).
Discussion
Unfortunately, adherence to guidelines is low,9,13 despite evidence that guidelines can improve the quality of care.25,26,27 A number of reasons for this have been documented, such as poor dissemination of guidelines,28 a lack of agreement concerning the content of a guideline,29 and a low outcome expectancy by clinicians.30 Computerizing the guidelines seems to be one of the most promising methods,31,32 but studies on this subject are still inconclusive.20
The increasing evidence that stress hyperglycemia is harmful for critically ill patients has created an interest in glucose management in intensive care medicine.1,2,3,4,5,6,7,8 The current study demonstrates that guideline adherence can be considerably improved over no guidelines or paper-based guidelines through using a computerized version of the guideline, even in complex environments such as the ICU. Clinical staff using the computer-based version of the guideline showed significantly improved adherence with respect to the timing of glucose measurements and with dosing of insulin; consequently, they improved the glucose regulation in their ICU patients. Implementation of the paper guideline significantly improved the linear time that the patients' glucose levels fell within the normal range, from 22% to 44%. Both the control (B2) and the computerized (B1) groups improved, with the time spent in the normal range increasing to 53% and 54%, respectively. Although this difference between the control (B2) and intervention (B1) groups is statistically significant, it is too small to be clinically significant.
Limitations of the Current Study
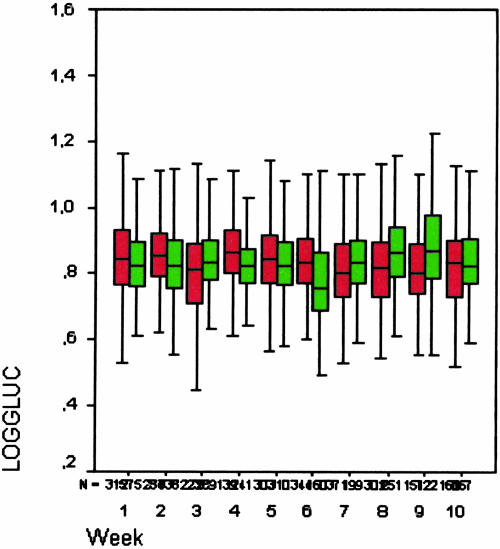
Because both the control and computerized groups in the second study period displayed substantial and similar improvements, we hypothesized that a learning effect might have occurred. However, analysis of the data for the ten weeks revealed comparable glucose levels throughout the study period with very similar glucose levels between the groups (▶). This is not compatible with a learning effect in which an initial difference between the groups would be followed by convergence of the curves combined with improved glucose levels. The immediate drop in time spent within the normal range for the postintervention group (C) to 42% indicates that the effect seen in the control group is most likely a crossover effect. Although the data on the adherence to the timing of the measurements do not support this assumption, the adherence to the insulin advice does support it. We did not expect the crossover effect from the computerized to the control groups, and the exact reasons for this effect are not clear. A partial explanation might be the combination of the randomization method and the method of work assignment for the nursing staff. As a result of randomization, the patients in the intervention group were spread over the unit. When a nurse was assigned to take care of two patients in one room, one within the computerized group and one in the control group, the reminders provided to the nurse for the patient in the computerized group are likely to have had an effect on the glucose regulation for the patient in the control group. A different method of randomization, e.g., based on the bed and room where patients were admitted, might have prevented this effect but would have created a serious bias. In such a situation, the nursing staff might have developed preferences for the rooms where they worked, resulting in a select group of staff members working with either the control or computerized tool.
Figure 5.
Mean value of log10 glucose during intervention period (green: B1, computerized group; red: B2, control group).
Of note, more patients in groups B and C had a preexisting diagnosis of diabetes mellitus; this might have occurred because diabetes mellitus was an inclusion criterion for guideline utilization. It is not clear why this was not the case in group A, nor can it be explained why the adherence to the insulin advice in the postintervention period (C) dropped even below the results for the paper implementation period (A).
Although the guideline significantly improved glucose management, a large proportion of the patients' glucose levels still remained outside the normal range. Further analysis is required regarding how to improve the guideline. A study that compares the efficacy of a new guideline between paper and computerized forms, shortly after the development and introduction of the guideline, is threatened by several methodological weaknesses. To prevent these, we employed a strict path in relation to the research design. First, we developed and tested the new guideline. The authors postponed guideline implementation several times until the complete senior medical staff agreed with the guideline's content. In preparation for the implementation, we trained the complete medical and nursing staff. For a number of weeks, the authors gave clinical presentations on a daily basis. Additionally, we approached staff members in person to ensure that everyone was familiar with the guideline before initiating its use. During the implementation, we gave constant attention to the staff and monitored the guideline results.
Because the study involved the simultaneous comparison of two implementation methods, carryover and/or learning effects were possible. We selected an off-on-off design to allow for pre- and posttests to measure the effects of the intervention in the experimental group and the directions of any associations.33 Because the intervention's randomized allocation had been automatic, intervention removal could be conducted without informing the staff. This minimized a possible bias caused by drawing attention to the intervention's absence. Both randomization and data collection were done automatically; this minimized the amount of interference with the staff in relation to the experiment.
Because the study was performed as part of a master's thesis with a time deadline, the time for the postintervention group had to be relatively short compared with the other groups. This does, to a certain extent, limit the conclusions that can be drawn from these data.
This study shows that guideline implementation and its subsequent computerization were successful. In light of the number of unsuccessful attempts reported in other studies, it is important to reflect on why this specific attempt succeeded. To begin with, the problem targeted in this study does match the criteria as specified by Tierney summarizing when one should focus efforts on reinforcing compliance with guidelines with the help of medical informatics.19 Furthermore, we fully integrated the computerized guideline in the CIS and work flow of the unit. Previous studies report a lack of system integration in the clinical work flow as one of the main obstacles in achieving positive results.34,35,36,37
A computerized guideline as an integrated part of a commercially available CIS significantly improved the implementation of and adherence to a guideline for glucose management in a critical care setting and subsequently improved the glucose regulation for critically ill patients.
This study was performed for the dissertation of an MSc in Health Informatics at CHIME UCL. No financial support was received in relation to the study. Mrs. Rood is currently employed by iMD-Soft, though at the time of the study she was fully employed by the OLVG Hospital. She changed jobs six months after the conclusion of this study.
The authors thank R.G.H. Speekenbrink for assistance with the manuscript, H.M. Oudemans-van Straaten and J.P.J. Wester for their suggestions relating to the glucose guideline, and the nurses in the ICU for all the measurements.
References
- 1.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–51. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin treatment in critically ill patients. N Engl J Med. 2001;345:1359–67. [DOI] [PubMed] [Google Scholar]
- 3.Preiser JC, Devos P, Van den Berghe G. Tight control of glycaemia in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:533–7. [DOI] [PubMed] [Google Scholar]
- 4.Capes S, Hunt D, Malmberg K, Gerstein H. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic review. Lancet. 2000;355:773–8. [DOI] [PubMed] [Google Scholar]
- 5.Bolk J, Van der Ploeg T, Cornel JH, Arnold AD, Sepers J, Umans VA. Impaired glucose metabolism predicts mortality after a myocardial infarction. Int J Cardiol. 2001;79:207–14. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill PA, Davies I, Fullerton KJ, Bennett D. Stress hormone and blood glucose response following acute stroke in the elderly. Stroke. 1991;22:842–7. [DOI] [PubMed] [Google Scholar]
- 7.Fietsam R, Bassett J, Glover JL. Complications of coronary artery surgery in diabetic patients. Am Surg. 1991;57:551–7. [PubMed] [Google Scholar]
- 8.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycaemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–4. [DOI] [PubMed] [Google Scholar]
- 9.Brindis RG, Sennett C. Physician adherence to clinical practice guidelines: does it really matter? Am Heart J. 2003;145:13–5. [DOI] [PubMed] [Google Scholar]
- 10.Larson E. Status of practice guidelines in the United States: CDC guidelines as an example. Prev Med. 2003;36:519–24. [DOI] [PubMed] [Google Scholar]
- 11.Leape LL, Weissman JS, Schneider EC, Piana RN, Gatsonis C, Epstein AM. Adherence to practice guidelines: the role of specialty society guidelines. Am Heart J. 2003;145:19–26. [DOI] [PubMed] [Google Scholar]
- 12.Slomka J, Hoffman-Hogg L, Mion LC, Bair N, Bobek MB, Arroliga AC. Influence of clinicians' values and perceptions on use of clinical practice guidelines for sedation and neuromuscular blockade in patients receiving mechanical ventilation. Am J Crit Care. 2000;9:412–8. [PubMed] [Google Scholar]
- 13.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt JC. Practice guidelines and other support for clinical innovation. In: Clinical Knowledge and Practice in the Information Age. A Handbook for Professionals. London, UK: School of Public Policy, University College London, RSM Press, 2001.
- 15.Grimshaw JM, Russell IT. Achieving health gain through clinical guidelines II: ensuring guidelines change medical practice. Qual Health Care. 1994;3:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized controlled trials and recommendations of clinical experts. JAMA. 1992;268:240–8. [PubMed] [Google Scholar]
- 17.Grilli R, Lomas J. Evaluating the message: the relationship between compliance rate and the subject of a practice guideline. Med Care. 1994;32:202–13. [DOI] [PubMed] [Google Scholar]
- 18.Woo B, Woo B, Cook EF, Weisberg M, Goldman L. Screening procedures in the asymptomatic adult. Comparison of physicians' recommendations, patients' desires, published guidelines, and actual practice. JAMA. 1985;254:1480–4. [DOI] [PubMed] [Google Scholar]
- 19.Tierney WM, Overhage JM, Takesue BY, et al. Computerizing guidelines to improve care and patient outcomes: the example of heart failure. J Am Med Inform Assoc. 1995;2:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA. 1998;280:1339–46. [DOI] [PubMed] [Google Scholar]
- 21.Tierney WM. Improving clinical decisions and outcomes with information: a review. Int J Med Inf. 2001;62:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Le Floch JP, Escuyer P, Baudon D, Perlemuter L. Blood glucose area under the curve. Methodological aspects. Diabetes Care. 1990;13:172–5. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 24.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalised patients. Chest. 1991;100:1619–36. [DOI] [PubMed] [Google Scholar]
- 25.Holcomb BW, Wheeler AP, Wesley Ely E. New ways to reduce unnecessary variation and improve outcomes in the intensive care unit. Curr Opin Crit Care. 2001;7:304–11. [DOI] [PubMed] [Google Scholar]
- 26.Giugliano RP, Camargo CA, Lloyd-Jones DM, et al. Elderly patients receive less aggressive medical and invasive management of unstable angina. Arch Intern Med. 1998;158:1113–20. [DOI] [PubMed] [Google Scholar]
- 27.Morris AH. Treatment algorithms and protocolized care. Curr Opin Crit Care. 2003;9:236–40. [DOI] [PubMed] [Google Scholar]
- 28.Feldman EL, Jaffe A, Galambos N, Robbins A, Kelly RB, Froom J. Clinical practice guidelines on depression: awareness, attitudes and content knowledge among family physicians in New York. Arch Fam Med. 1998;7:58–62. [DOI] [PubMed] [Google Scholar]
- 29.Wall RJ, Dittus RS, Ely EW. Protocol-driven care in the intensive care unit: a tool for quality. Crit Care. 2001;5:283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulberg HC, Block MR, Madonia MJ, Rodriquez E, Scott CP, Lave J. Applicability of clinical pharmacotherapy guidelines for major depression in primary care settings. Arch Fam Med. 1995;4:106–12. [DOI] [PubMed] [Google Scholar]
- 31.Zielstorff RD. Online practice guidelines: issues, obstacles, and future prospects. J Am Med Inform Assoc. 1998;5:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne TH. Computer decision support systems. Chest. 2000;118:47S–52S. [DOI] [PubMed] [Google Scholar]
- 33.Bowling A. Quantitative research: experiments and other analytic methods of investigation. In: Research Methods in Health. Investigating Health and Health Services. Philadelphia, PA: Open University Press, 1997, pp 190–208.
- 34.Eccles M, McColl E, Steen N, Rousseay N, Grimshaw J, Parkin D. Effect of computerized evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ. 2002;325:941–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trivedi MH, Kern JK, Marcee A, et al. Development and implementation of computerized clinical guidelines: barriers and solutions. Methods Inf Med. 2002;41:435–42. [PubMed] [Google Scholar]
- 36.Maviglia SM, Zielstorff RD, Paterno M, Teich JM, Bates DW, Kuperman GJ. Automating complex guidelines for chronic disease: lessons learned. J Am Med Inform Assoc. 2003;10:154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikulich VJ, Liu YC, Steinfeldt J, Schriger DL. Implementation of clinical guidelines through an electronic medical record: physician usage, satisfaction and assessment. Int J Med Inf. 2001;63:169–78. [DOI] [PubMed] [Google Scholar]