Abstract
Most pathogenic bacteria express surface carbohydrates called capsular polysaccharides (CPSs). CPSs are important vaccine targets since they are easily accessible and recognizable by the immune system. However, CPS-specific adaptive humoral immune responses can only be achieved by the covalent conjugation of CPSs with carrier proteins to produce glycoconjugate vaccines. We previously described a mechanism by which a model glycoconjugate vaccine can activate the adaptive immune system and demonstrated that the mammalian CD4+ T cell repertoire contains a population of carbohydrate-specific T cells (i.e., Tcarbs). In this study, we employ glycoconjugates of type 3 Streptococcus pneumoniae CPS (Pn3P) to assess whether the carbohydrate-specific adaptive immune response exemplified in our previous study can be applied to the conjugates of this lethal pathogen. Here, we provide evidence for the functional roles of Pn3P-specific CD4+ T cells utilizing mouse immunization schemes that induce Pn3P-specific immunoglobulin G (IgG) responses in a Tcarb-dependent manner.
Introduction
Exploiting their high antigenicity, capsular polysaccharides (CPSs) have been used as main components of glycoconjugate vaccines in clinical practice worldwide in the past three decades (1). Immunizations with glycoconjugates containing CPSs from H. influenzae, S. pneumoniae, and N. meningitidis have been utilized in preventing/controlling infectious diseases caused by these bacterial pathogens (2, 3). While glycoconjugate vaccines have provided great health benefits in controlling bacterial diseases, glycoconjugate construction has often been a random process of empirically linking two molecules (carbohydrate and protein) with minimum consideration of their mechanism of action (4), resulting in poorly characterized, heterogeneous and variably immunogenic glycoconjugate vaccines (1, 5). Demystifying T cell activation mechanisms of glycoconjugate vaccines is a key step towards designing new-generation vaccines. We recently demonstrated a mechanism through which uptake of a glycoconjugate vaccine by antigen presenting cells (APCs) results in the presentation of a carbohydrate epitope by the major histocompatibility class II complex (MHCII), thus stimulating carbohydrate-specific CD4+ T cells (Tcarbs) (6–8).
In the present study, we employ model glycoconjugates of type 3 Streptococcus pneumoniae CPS (Pn3P) to examine whether the carbohydrate-specific adaptive immune responses exemplified in our previous findings apply to other carbohydrate antigens used in glycoconjugate vaccines. The Gram-positive pathogen Streptococcus pneumoniae can be sorted into over 90 capsular serotypes (9). Multiple studies have shown the ability of CPS-specific antibodies to provide protection from pneumococcal challenges (10, 11). However, most CPSs are poorly immunogenic, since they cannot, in their pure form, induce T cell dependent immune responses (4, 12). Immunization with glycoconjugates, as opposed to pure glycans, elicits T cell help for B cells that produce high-affinity IgG antibodies to the CPS component of the vaccine and induces memory B and T cell development (4, 12). Since the introduction of the first pneumococcal conjugate vaccine, PCV7, the incidence rate of pneumococcal disease has been reduced significantly (11). The current pneumococcal conjugate vaccine is the 13-valent Prevnar13®, encompassing CPSs from thirteen of the most prevalent serotypes of S. pneumoniae (11). The model conjugate vaccine used in this study is in fact a component of the existing 13-valent pneumococcal conjugate vaccine. S. pneumoniae remains among the most lethal infectious agents despite the availability of global vaccination programs (11, 13). The type 3 strain in particular is among the most virulent strains. Despite current vaccination programs, morbidity of the type 3 strain remains high, raising questions regarding the efficacy of this vaccine (14). The knowledge gained in the present study may have implications in producing a highly protective knowledge-based pneumococcal vaccine.
Materials and Methods
Mice
Eight-week-old female BALB/c mice were obtained from Taconic Biosciences (Hudson, NY) and housed in the Coverdell Rodent Vivarium at the University of Georgia. Mice were kept in microisolator cages and handled under a laminar flow hood. All mouse experiments were in compliance with the University of Georgia Institutional Animal Care and Use Committee under the approved animal use protocol # A2013 12-011-Y1-A0.
Antigens
Purified Pn3P powder was obtained from American Type Cell Collection (Cat. #172-X). Pn3P was reduced to an average molecular weight of 100kDa by hydrolysis with 0.3M trifluoroacetic acid. Pn3P was conjugated to either ovalbumin (Sigma A7641) or keyhole limpet hemocyanin (Calbiochem 374805) through reductive amination as previously described (6, 7). Pn3P conjugates were isolated from unconjugated components by size exclusion chromatography (Suppl. Fig. 1). A combination of phenol sulfuric acid and BCA assays using Pn3P and carrier proteins for standard curve generation confirmed that the conjugates consisted of 45–55% protein and 45–55% Pn3P (7).
Immunizations
Groups of BALB/c mice were immunized intraperitoneally on days 0 and 14 with 5 μg of antigen in phosphate buffered saline (PBS) mixed with 2% alhydrogel (Invivogen #vac-alu-50) in a 3:1 ratio. For T cell assays, groups of BALB/c mice were immunized with antigens emulsified in Freund’s adjuvant (Thermo Scientific) by subcutaneous injection. Where indicated, mixtures consisted of quantities consistent with conjugate ratios.
Adoptive transfers
Groups of donor BALB/c mice were primed and boosted with 5 μg Pn3P or 10 μg of Pn3P-KLH subcutaneously at 3-week intervals. Mice were sacrificed 5 days after boost. CD4+ T cells were isolated from spleen and lymph nodes of Pn3P and Pn3P-KLH primed mice were negatively selected using mouse CD4+ T lymphocyte enrichment magnetic beads (BD Biosciences 558131), and splenic B cells from Pn3P-KLH primed mice were isolated using mouse B lymphocyte enrichment magnetic beads (BD Biosciences 557792). Isolation of a given cell type was confirmed by flow cytometry. CD4+ T cells (1×107) from either Pn3P or Pn3P-KLH immunized donors and B cells (1×107) from Pn3P-KLH immunized mice were adoptively transferred to recipient mice through tail vein injections in PBS. The recipient mice were then immunized with Pn3P-KLH or Pn3P-OVA 1 day after adoptive transfer.
Detection of Pn3P-specific serum antibodies
Mice were bled from the tail vein on days 14 and 21 during the prime-boost immunization experiments. Mice used in the adoptive transfer experiment were bled 3 days after booster immunization (4 days after transfer). Pn3P-specific antibodies in serum were detected by ELISA in 96 well plates coated with 2.5 μg/ml of Pn3P-HSA conjugate. Four immune sera per group were used in all ELISA experiments.
Opsonophagocytic killing assay
Opsonophagocytic killing assay was performed as previously described (15). Briefly, Type 3 Streptococcus pneumoniae WU2 strain (approximately 800 CFU/10 μL/well) was incubated with mouse sera samples (20 μL each at 1:30 dilutions) in duplicate wells in a 96-well round-bottom plate for 30 minutes with shaking at room temperature in Opsonization Buffer B (15). HL60 cells (ATCC CCL-240, generously provided by Gerald Pier) were cultured in RPMI with 10% heat-inactivated FetalClone (HyClone, SH30080.03) and 1% L-glutamine. HL60 cells were differentiated as previously described (15). Baby rabbit complement (Pel-Freez, 31061) was added to HL60 cells at 20% final volume, with a final cell concentration of 1×107 cells/mL. The HL60/complement mixture was added to the serum/bacteria at 5×105 cells/well. The reactions were incubated at 37°C for 1 hour with shaking. The reactions were stopped by incubating the plate(s) on ice for approximately 20 minutes. 10 μL of each reaction was diluted into a final volume of 50 μL and plated onto blood agar plates. Plates were incubated overnight at 30°C in anaerobic conditions and counted. Percent viability was calculated as each duplicate reaction subtracted from the mean values obtained for control samples (naïve serum, 100% survival).
Antibodies and flow Cytometry
The following fluorophore-conjugated antibodies used in flow cytometry detection were purchased from Biolegend: anti-mouse CD3ε (clone145-2C11), anti-mouse CD4 (clone GK1.5), anti-mouse CD69 (clone H1.2F3). 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE) was purchased from Sigma. Following surface staining of cell suspensions at 4˚C, samples were analyzed on CyAn (Beckman Coulter). Data were analyzed using FlowJo software (Tree Star).
Enzyme-Linked ImmunoSpot (ELISPOT) Assay
Groups of BALB/c mice were immunized with 10ug of Pn3P-OVA emulsified in Freund’s adjuvant (Thermo Scientific) by subcutaneous injection. CD4+ T cells were isolated from lymph nodes of Pn3P-OVA immunized mice and stimulated in vitro in the presence of irradiated APCs pulsed with indicated antigens. After 24hr stimulation, IL-2 production was detected by ELISPOT assay using mouse ELISPOT reagent set (Cellular Technology Ltd) according to the manufacturer’s protocol. The ELISPOT samples were analyzed by ImmunoSpot S6 analyzer using the ImmunoCapture 6.3 and ImmunoSpot 5.0 Pro DC software (Cellular Technology Ltd).
Generation of Pn3P-specific T cell Hybridomas
T-cell hybridomas were generated as previously described (16). Briefly, BALB/c mice were immunized with Pn3P-KLH emulsified in Freund’s adjuvant (Thermo Scientific). Primary CD4+ T cells obtained from lymph node of immunized mice were re-stimulated in vitro with APCs that pulsed with Pn3P-KLH. Activated CD4+ T cells were fused with BW5147 T cell lymphoma cells at a ratio of 1:1. The T cell hybrids were selected in Dulbecco’s Modified Eagle’s Medium containing hypoxanthine-aminopterin-thymidine and screened for CD3 and CD4 expression. Antigen specificity was screened by activating the T hybridoma cells with appropriate antigens in the presence of APCs. Culture supernatant was collected after 24hrs stimulation to test IL-2 production by ELISA assay using paired rat monoclonal antibodies for mouse IL-2 (Biolegend).
Results
Carrier specific T cells are unable to boost Pn3P specific IgG titers
To assess the role of carbohydrate specific CD4+ T cells on the adaptive humoral immune response to Pn3P glycoconjugate, we performed a series of immunization experiments. We first performed primary immunizations to BALB/c mice with either KLH or Pn3P-KLH conjugate and 14 days later we performed booster immunizations with the Pn3P-KLH conjugate. On day 21, we compared Pn3P-specific IgG levels in the sera of the mice receiving primary and secondary immunizations (Fig. 1A). Mice that received the Pn3P-KLH conjugate in both primary and secondary immunizations produced high IgG titers, characteristic of a booster response. However, priming with KLH alone failed to generate a booster IgG response after the secondary immunization. If the Pn3P-KLH conjugate had induced T cell stimulation via a peptide epitope, the mice primed with either carrier alone or with the conjugate should have had comparable Pn3P IgG titers after boosting with the conjugate. We compared carrier specific IgG titers in mice immunized with conjugate versus carrier alone. KLH specific IgG levels were similar in these groups (Suppl. Fig. 2). To determine whether the inability of the carrier to induce a priming response for a glycoconjugate boost is due to a lack of T-cell or B-cell priming, we immunized mice with an unconjugated mixture of Pn3P and KLH to provide T cells primed with KLH derived peptides, and B cells primed with Pn3P, and boosted these mice with the glycoconjugate (Fig.1A). Again, priming with the mixture did not support a robust secondary antibody response to the polysaccharide upon boosting with the glycoconjugate (Fig. 1A). Regardless of whether the glycan was conjugated or not, similar Pn3P IgM levels were found in the sera of both groups of immunized mice, suggesting a similar level of B cell stimulation (Fig. 1B). Additional control groups of mice primed with no antigen (PBS) or Pn3P alone (Fig. 1) showed very low levels of serum IgG and IgM.
FIGURE 1.
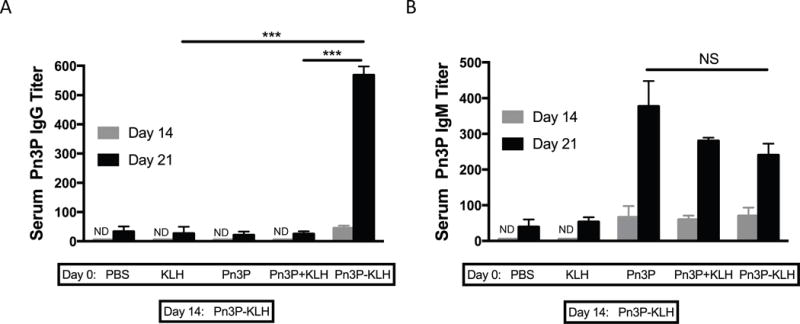
Primary immunization with the carrier protein doesn’t contribute to the Pn3P-specific secondary immune response. Pn3P IgG (A), Pn3P IgM (B) and KLH IgG (C) titers in BALB/c mice primed (day 0) and boosted (day 14) with different antigen combinations, as measured by ELISA in serum obtained on day 14 (pre-boost) and 21. n=4/group. Serum titers are reported as the reciprocal dilution that results in an OD of 0.5 at 405nm. Statistical significance was determined with the two-tailed student’s t-test. *** P<0.001. NS=not significant. ND=not detectable.
Boosting with alternate carrier protein conjugate generates high Pn3P IgG responses
In a separate immunization experiment we performed primary immunizations to groups of BALB/c mice with Pn3P-KLH and booster immunizations with a glycoconjugate containing the heterologous carrier protein, ovalbumin (OVA), to assess whether CD4+ T-cells specifically recognizing Pn3P epitopes help to induce the secondary humoral immune response to the glycoconjugate. Serum levels of Pn3P-specific IgG were measured 7 days after secondary immunizations (Fig. 2A). Boosting of Pn3P-KLH-primed mice with Pn3P-OVA induced Pn3P-specific IgG titers consistent to those seen after priming and boosting with Pn3P-KLH or priming and boosting with Pn3P-OVA (Fig. 2A). Control groups including mice primed with Pn3P-KLH and boosted with a mixture of unconjugated Pn3P and OVA, and mice primed with Pn3P and boosted with Pn3P-OVA. Similar Pn3P IgM levels were found in the sera of all immunized mice, suggesting a similar level of B cell stimulation (Fig. 2B). To further confirm Pn3P specificity, we performed whole cell and competition ELISA experiments (Suppl. Fig. 3). Serum IgGs bound to paraformaldehyde fixed whole Pn3 cells (WU2 strain) (Suppl. Fig. 3A) whereas they failed to bind to Pn4 cells (TIGR4 strain) (data not shown). Additionally, prior incubation with soluble Pn3P inhibited IgGs from binding to the Pn3P-HSA coated plate whereas soluble Pn4P did not (Suppl. Fig. 3B). Observing a booster humoral immune response upon immunization with a heterologous carrier strongly suggests that T cell help for induction of Pn3P-specific immune responses is recruited primarily via recognition of a presented carbohydrate epitope. A key aspect of this experiment is to ensure that conjugate with OVA carrier induces similar Pn3P IgG titers upon primary and secondary immunizations as does the conjugate with KLH carrier, so alternate carrier data is comparable (Fig. 2A).
FIGURE 2.
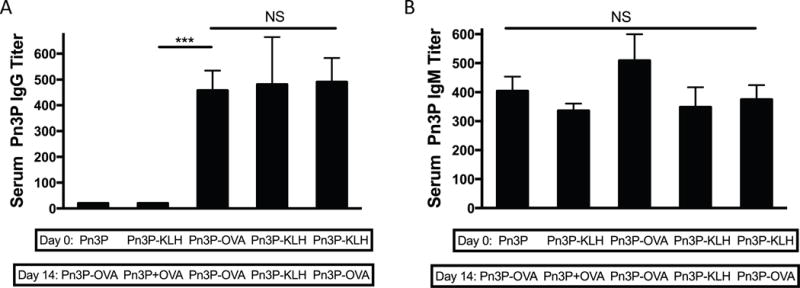
The use of a heterologous carrier in the Pn3P conjugate doesn’t interfere with the Pn3P-specific secondary immune response. Pn3P IgG (A) and IgM (B) titers in BALB/c mice primed (day 0) and boosted (day 14) with different antigen combinations, as measured by ELISA in serum obtained on day 21. n=4/group. Serum titers are reported as the reciprocal dilution that results in an OD of 0.5 at 405nm. Statistical significance was determined with the two-tailed student’s t-test. *** P<0.001. NS=not significant
CD4+ T cells adoptively transferred from Pn3P-KLH immunized mice help Pn3P IgG booster response by Pn3P-OVA in recipient mice
To directly examine the contribution of CD4+ T cells in the Pn3P-specific booster IgG responses, we performed an adoptive transfer experiment (Fig. 3). In this experiment groups of BALB/c donor mice were immunized with Pn3P or Pn3P-KLH. CD4+ T cells from each group were isolated and adoptively transferred to groups of recipient mice through tail-vein injections. Two groups of recipient mice received CD4+ T cells from mice immunized with Pn3P, and two groups of recipient mice received CD4+ T cells from mice immunized with Pn3P-KLH. All groups of recipient mice received splenic B cells obtained from Pn3P-KLH immunized mice to directly assess the functional role of CD4+ T cells in the polysaccharide specific IgG responses to glycoconjugate immunization. One day after receiving T and B cells, groups were immunized with either Pn3P-KLH, or the glycoconjugate with alternate carrier protein, Pn3P-OVA (Fig. 3A). Titers of Pn3P specific IgG were determined in serum collected three days after immunization of recipient groups. Groups that received CD4+ T cells from Pn3P-KLH immunized donor mice displayed significantly higher Pn3P specific IgG titers than the groups that received CD4+ T cells from Pn3P immunized donor mice (Fig. 3B). Additionally, the booster IgG response in the Pn3P-KLH immunized group was comparable to the Pn3P-OVA group. Again, similar Pn3P specific IgM levels were found in the sera of all immunized mice (Fig. 3C). We then performed an opsonophagocytic killing assay to assess the functional capacity of these Pn3P specific IgGs (Fig. 3D). We observed that serum from mice that received CD4+ T cells from Pn3P-KLH immunized mice demonstrated nearly complete killing of the WU2 strain, regardless of whether the recipient groups were immunized with Pn3P-OVA (group C in Fig. 3D) or Pn3P-KLH (group D in Fig. 3D); while serum from mice that received CD4+ T cells from Pn3P immunized mice showed minor killing activity (groups A and B in Fig. 3D).
FIGURE 3.
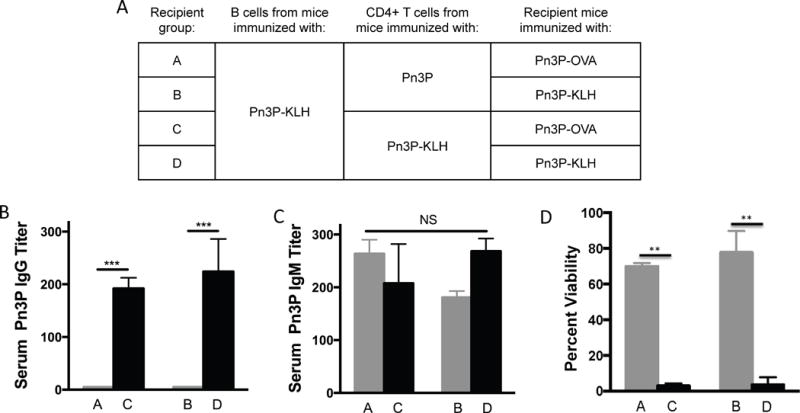
CD4+ T cells adoptively transferred from donor mice immunized with Pn3P-KLH provides the T cell help for Pn3P-specific secondary immune responses in the recipient mice upon their immunization with Pn3P-OVA (A). Pn3P IgG (B) and IgM (C) titers in BALB/c mice that received CD4+ T cells and B cells from immunized donor mice and were immunized with Pn3P-KLH or Pn3P-OVA (day 0), as measured by ELISA in serum obtained on day 3. n=4/group. Serum titers are reported as the reciprocal dilution that results in an OD of 0.5 at 405nm. Functional test of Pn3P specific antibodies in serum using opsonophagocytic killing assay, displaying percent viability of bacterial cells after incubation with HL-60 cells in the presence of serum and complement (D). Statistical significance was determined with the two-tailed student’s t-test. *** P<0.001. NS= not significant
In vitro stimulation of primary CD4+ T cells display carbohydrate specificity
To further investigate the CD4+ T cell responses to glycoconjugates, we isolated CD4+ T cells from lymph nodes of Pn3P-OVA immunized mice. These cells were stimulated in vitro with polysaccharide alone, the carrier protein ovalbumin, or the conjugate Pn3P-OVA in the presence of irradiated splenic APCs. T cell activation by these antigens was examined by detecting the early activation marker CD69 expression by flow cytometry (Fig. 4A–B). After 3 days of in vitro stimulation, a significantly higher percentage of T cells were activated by the conjugate in vitro than by the carrier protein or polysaccharide alone, an evidence for carbohydrate dependent recognition in this T cell population. Furthermore, we looked at T cell proliferation by CFSE staining of the CD4+ T cells after in vitro stimulation. The T cell proliferation rate was measured by CFSE dilution after 5 days of culture (Fig. 4C–D). Again, a higher percentage of these cells proliferate upon stimulation with Pn3P-OVA conjugate compared to carrier protein or polysaccharide alone. In an additional immunization scheme, in which mice were primed with Pn3P-OVA, and then boosted with Pn3P-KLH (Fig. 4E), we observed that in vitro stimulation by both conjugates stimulated a higher percentage of CD4+ T cell response than their carriers alone or polysaccharide alone. Further evidence was obtained by stimulating CD4+ T cells from Pn3P-OVA immunized mice with a heterologous (Pn3P-TT) conjugate compared to its carrier protein. Higher T cell activation was observed in response to the Pn3P-TT conjugate than the TT alone by measuring IL-2 production in an ELISPOT assay (Suppl. Fig. 4).
Figure 4.
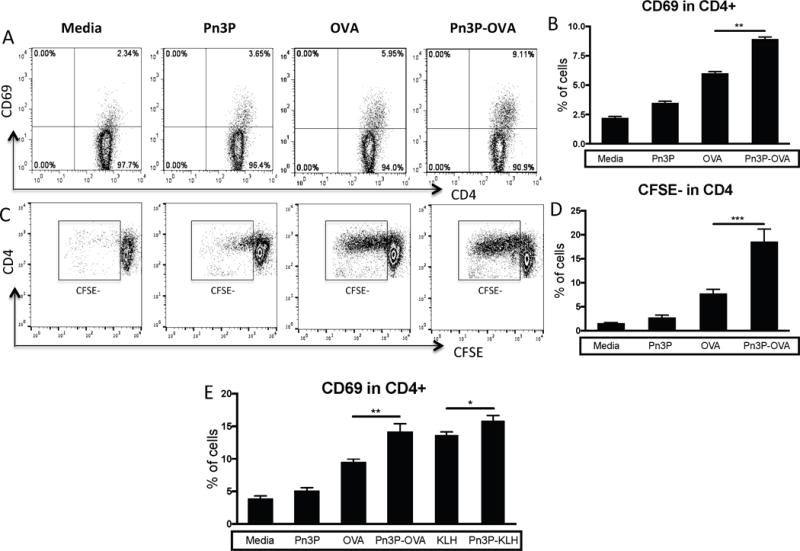
Evidence for Pn3P specific CD4+ T cell response after Pn3P conjugates immunization. (A-D) CD4+ T cells were isolated from lymph nodes of Pn3P-OVA immunized mice and stimulated in vitro in the presence of irradiated APCs (CD3 depleted mouse splenic mononuclear cells) pulsed with indicated antigens. T cell activation and proliferation were detected by flow cytometry. Representative FACS profiles (A) and the percentile (B) of CD69 expression on CD4+ T cells after 3 days of stimulation were shown. (C, D) CD4+ T cells were labeled with CFSE fluorescence before culture. CFSE dilution as T cell proliferation rate was measured after 5 days of stimulation. (E) CD4+ T cells from mice primed with Pn3P-OVA and boosted with Pn3P-KLH incubated with the indicated antigens in the presence of iAPCs and CD69 expression was measured. Representative results are shown from one of three independent experiments performed. Data were shown as mean+SD. *P<0.05, **P<0.01, ***P<0.001.
Pn3P specific CD4+ T cell hybridoma generation
To further assess the T cell specificity, we generated T cell hybridomas by fusing primary CD4+ T cells from Pn3P-KLH immunized mice with BW5147 T cell lymphoma cells. CD3+CD4+ hybridomas were then screened by incubation with Pn3P-KLH in vitro in the presence of irradiated APCs. Numerous T cell hybridomas were stimulated in vitro by Pn3P-KLH as measured by the production of Interleukin 2 (IL-2) using ELISA (Fig. 5A). Hybridomas that secreted high concentrations of IL-2 compared to controls were tested for their carbohydrate specificity by incubation with Pn3P-OVA, Pn3P-KLH, and the carrier proteins alone. Four select hybridomas shown here (Fig. 5B) consistently secreted more IL-2 when stimulated by glycoconjugates, no matter which carrier protein, indicating a carbohydrate dependent recognition by these cells. Along with the immunization and adoptive transfer experiments described above, these T cell assays further demonstrate that a population of carbohydrate-specific CD4+ T cell play an important role in helping anti-Pn3P antibody generation.
Figure 5.
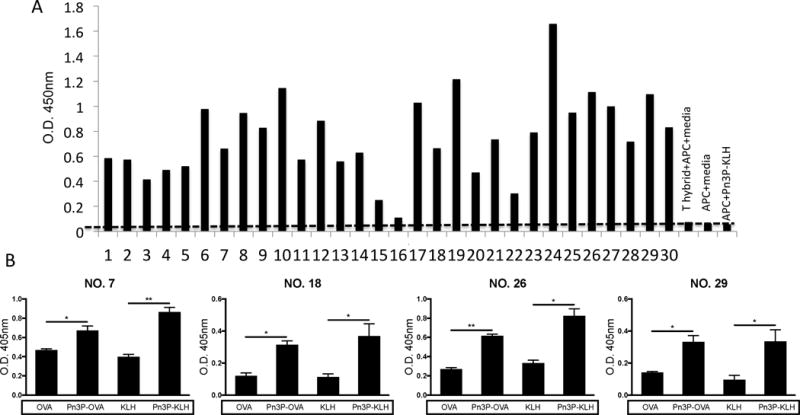
Generation of Pn3P specific CD4+ T cell hybridomas. Pn3P conjugate specific T cell hybridomas were generated by fusion of primary CD4+ T cells isolated from Pn3P-KLH immunized mice with BW5147 T cell lymphoma cells. T hybridoma cell lines positive for both CD3 and CD4 expression were selected to investigate their response to Pn3P-KLH antigen. (A) Antigen specificity of T hybridoma cell lines was determined by their ability to produce IL-2 using ELISA assays in the presence of irradiated APCs and Pn3P-KLH antigen. (B) Four representative CD4+ T hybridoma cell lines were selected and tested their ability to produce IL-2 using ELISA assays in the presence of APCs pulsed with Pn3P conjugates or carrier proteins. Data were shown as mean+SD. *P<0.05, **P<0.01
Discussion
Despite the availability of a glycoconjugate vaccine against S. pneumoniae, it remains one of the world’s most deadly pathogens. The current 13-valent vaccine induces variable immune responses to each serotype and is not as effective against serotype 3, an exceptionally virulent serotype (17, 18). Studies have shown that multivalent glycoconjugate vaccines encompassing serotype 3 CPS have impaired booster responses, and fail to protect from otitis media infections from serotype 3 (19). In addition, individuals vaccinated with PCV13 require higher opsonophagocytosis assay serum titers for serotype 3 in comparison with other serotypes (20).
T cell responses described here are consistent with our previous findings (6, 7). Upon immunization, a very small percentage of T cells that recognize carbohydrate epitopes are expanded. Through in vitro expansion, this T cell population could be enriched. In addition to our own studies, multiple past and present studies demonstrate the presence of T cell populations that recognize carbohydrate epitopes of glycoconjugate vaccines (21–24). This work contributes to the broader application of Tcarb mediated immune responses and next-generation vaccine development strategies against bacterial pathogens. Future studies on cloning the T cell populations described here will shed light on functional properties of Tcarb populations and structural requirements for their T cell receptor engagement with carbohydrate epitopes and MHCII complexes. Further identification of Tcarb epitopes generated through processing and presentation of pneumococcal glycoconjugate vaccines by the antigen presenting cells will have direct implications in producing future knowledge-based vaccines that are target-specific, structurally designed, highly immunogenic and protective, and produced at much lower cost, thus allowing a much wider use on a global scale than current vaccines to control or eliminate invasive pneumococcal diseases (13).
Supplementary Material
Acknowledgments
We thank Ms. Karen Howard for her editorial assistance and Drs. Eric Harvill and Jeff Hogan for their valuable suggestions.
This work was supported by funding from the National Institute of Health grants: AI-115451-01, 1R01AI123383-01A1.
References
- 1.Avci F. Novel Strategies for Development of Next-Generation Glycoconjugate Vaccines. Current Topics in Medicinal Chemistry. 2013;13:2535–2540. doi: 10.2174/15680266113136660180. [DOI] [PubMed] [Google Scholar]
- 2.Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, Hombach J, Kieny MP. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26:4434–4445. doi: 10.1016/j.vaccine.2008.05.073. [DOI] [PubMed] [Google Scholar]
- 3.Weintraub A. Immunology of bacterial polysaccharide antigens. Carbohydr Res. 2003;338:2539–2547. doi: 10.1016/j.carres.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Avci FY, Kasper DL. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annual Review of Immunology. 2010;28:107–130. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 5.Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov. 2011;6:1045–1066. doi: 10.1517/17460441.2011.609554. [DOI] [PubMed] [Google Scholar]
- 6.Avci FY, Li XM, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature Medicine. 2011;17:1602–1610. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avci FY, Li XM, Tsuji M, Kasper DL. Isolation of carbohydrate-specific CD4(+) T cell clones from mice after stimulation by two model glycoconjugate vaccines. Nature Protocols. 2012;7:2180–2192. doi: 10.1038/nprot.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avci FY, Li XM, Tsuji M, Kasper DL. Carbohydrates and T cells: A sweet twosome. Seminars in Immunology. 2013;25:146–151. doi: 10.1016/j.smim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clinical Microbiology Reviews. 2015;28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLeod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. [PMC free article] [PubMed] [Google Scholar]
- 11.Torres A, Bonanni P, Hryniewicz W, Moutschen M, Reinert RR, Welte T. Pneumococcal vaccination: what have we learnt so far and what can we expect in the future? Eur J Clin Microbiol Infect Dis. 2015;34:19–31. doi: 10.1007/s10096-014-2208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchison NA. T-cell-B-cell cooperation. Nat Rev Immunol. 2004;4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 13.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 14.Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, Tyrrell GJ, Desai S, Sherrard L, Adam H, Gilmour M, Zhanel GG, N. Toronto Bacterial Diseases, and N. Canadian Public Health Laboratory Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol. 2013;59:778–788. doi: 10.1139/cjm-2013-0614. [DOI] [PubMed] [Google Scholar]
- 15.Burton RL, Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol. 2012;19:835–841. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandakumar S, Kannanganat S, Dobos KM, Lucas M, Spencer JS, Fang S, McDonald MA, Pohl J, Birkness K, Chamcha V, Ramirez MV, Plikaytis BB, Posey JE, Amara RR, Sable SB. O-mannosylation of the Mycobacterium tuberculosis adhesin Apa is crucial for T cell antigenicity during infection but is expendable for protection. PLoS Pathog. 2013;9:e1003705. doi: 10.1371/journal.ppat.1003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 18.Gruber WC, Scott DA, Emini EA. Development and clinical evaluation of Prevnar 13, a 13-valent pneumocococcal CRM197 conjugate vaccine. Ann N Y Acad Sci. 2012;1263:15–26. doi: 10.1111/j.1749-6632.2012.06673.x. [DOI] [PubMed] [Google Scholar]
- 19.Hausdorff WP, Dagan R, Beckers F, Schuerman L. Estimating the direct impact of new conjugate vaccines against invasive pneumococcal disease. Vaccine. 2009;27:7257–7269. doi: 10.1016/j.vaccine.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 20.Kieninger DM, Kueper K, Steul K, Juergens C, Ahlers N, Baker S, Jansen KU, Devlin C, Gruber WC, Emini EA, Scott DA, s group Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28:4192–4203. doi: 10.1016/j.vaccine.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Polonskaya Z, Deng S, Sarkar A, Kain L, Comellas-Aragones M, McKay CS, Kaczanowska K, Holt M, McBride R, Palomo V, Self KM, Taylor S, Irimia A, Mehta SR, Dan JM, Brigger M, Crotty S, Schoenberger SP, Paulson JC, Wilson IA, Savage PB, Finn MG, Teyton L. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J Clin Invest. 2017;127:1491–1504. doi: 10.1172/JCI91192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Middleton DR, Wantuch PL, Ozdilek A, Avci FY. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology. 2016 doi: 10.1093/glycob/cww062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Z, Schreiber JR. Antigen processing of glycoconjugate vaccines; the polysaccharide portion of the pneumococcal CRM(197) conjugate vaccine co-localizes with MHC II on the antigen processing cell surface. Vaccine. 2009;27:3137–3144. doi: 10.1016/j.vaccine.2009.03.064. [DOI] [PubMed] [Google Scholar]
- 24.Muthukkumar S, Stein KE. Immunization with meningococcal polysaccharide-tetanus toxoid conjugate induces polysaccharide-reactive T cells in mice. Vaccine. 2004;22:1290–1299. doi: 10.1016/j.vaccine.2003.08.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


