Abstract
The National Aeronautics and Space Administration Animal Enclosure Module (AEM) was developed as a self-contained rodent habitat for shuttle flight missions that provides inhabitants with living space, food, water, ventilation, and lighting, and this study reports whether, after minimal hardware modification, the AEM could support an extended term up to 35 days for Sprague-Dawley rats and C57BL/6 female mice for use on the International Space Station. Success was evaluated based on comparison of AEM housed animals to that of vivarium housed and to normal biological ranges through various measures of animal health and well-being, including animal health evaluations, animal growth and body masses, organ masses, rodent food bar consumption, water consumption, and analysis of blood contents. The results of this study confirmed that the AEMs could support 12 adult female C57BL/6 mice for up to 35 days with self-contained RFB and water, and the AEMs could also support 5 adult male Sprague-Dawley rats for 35 days with external replenishment of diet and water. This study has demonstrated the capability and flexibility of the AEM to operate for up to 35 days with minor hardware modification. Therefore, with modifications, it is possible to utilize this hardware on the International Space Station or other operational platforms to extend the space life science research use of mice and rats.
Introduction
Rodent models have played an integral role in the spaceflight program by permitting the collection of physiological data in support of human space exploration.1 The National Aeronautics and Space Administration (NASA) developed the Animal Enclosure Module (AEM) to conduct the rodent spaceflight research on board the Space Shuttle and since its development, the AEM has been used successfully in over 26 rodent spaceflights2–10 with the latest one on STS-135 on 8 July 2011. Because rodent models for spaceflight missions have provided critical data in support of human space exploration, great efforts have been committed to continue the rodent flight payloads beyond the Space Shuttle era. When using the shuttle as the vehicle, the flight duration for AEM was expected to be <20 days. Now new flight payload scenarios have been developed which will require the AEM to be used with minimal modification to continue the rodent flight for 30 days with an additional margin of 5 days for flight preparation for other types of flight vehicles (e.g., Russian Bion-M free flyer).
Potential operational limiting factors for the AEM, which could theoretically bar use for longer duration flights, have been outlined in previous publications11,12 and include the waste handling system, odor containing capability, and food and water supplies within the unit. With NASA’s growing push for longer duration crewed flights, there is also an increased importance placed on long-term studies of model organisms as a pre-requisite, despite existing outside the scope of the hardware originally designed for short duration travel. Therefore, a study of the durability of the hardware beyond the original design for short term Space Shuttle flights is required before application on the International Space Station and is the basis for this research, while also determining if other potential limiting factors exist when food and water can be replenished. Follow-up studies designed to test the AEM hardware life support functionality and durability are still needed beyond 35 days because these were outside of the scope of this initial investigation into the proof of concept as outlined by NASA headquarters’ science requirements (unpublished). On the basis of the food and water data from previous flight and ground studies (unpublished), the nutrient-upgraded rodent food bar (NuRFB) and water content in the unit is enough for 12 mice to reach the 35 day duration. Therefore, this study was designed to test whether the AEM, with minimal modifications, could be used for up to 35 days to support mice growth with a focus on the water handling system. Because external replenishment of NuRFB and water is easily administered to the AEM, it is outside of the current AEM hardware requirements to contain enough for a 35-day study with five rats, so we instead investigated whether the waste handling, air circulation, and lighting, and other hardware systems could support five rats for 35 days if the AEM were supplemented with the required food and water to determine which, if any, life support system existed as a limiting factor for use of the AEM for up to 35 days.
These studies exist as important ground based controls in order to demonstrate the absence of a ‘cage effect’ due to the AEM when compared with vivarium-housed animals and to establish an AEM baseline in the observed parameters after containment for 35 days, although it is still recommended (and in most cases required and supported by NASA) to house spaceflight control animals in grounded AEM.
Results
Mouse study
The body mass measurements (Figure 1) were similar between the vivarium and AEM groups at both time-points during the tests. The average initial (day 0) animal body masses for vivarium and AEM groups were 19.0±0.5 g and 19.0±0.5 g respectively. At day 35, the average animal body weights for vivarium and AEM groups were 22.3±1.2 g (n=36) and 22.4±0.8 g (n=36), respectively.
Figure 1.
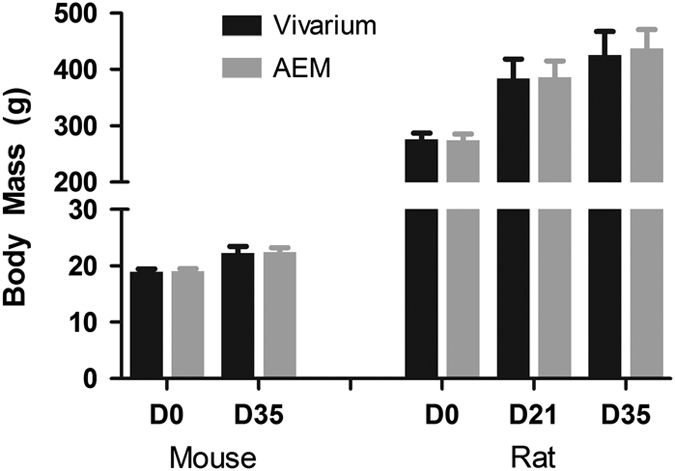
The body mass of animals contained in the vivarium or AEM cage at each time point. No statistical difference due to cage (p>0.05).
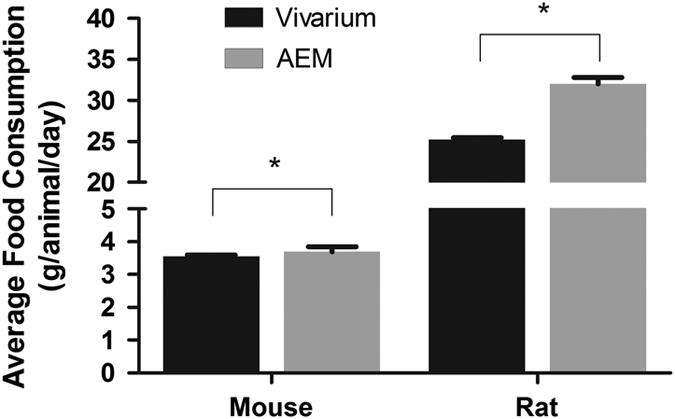
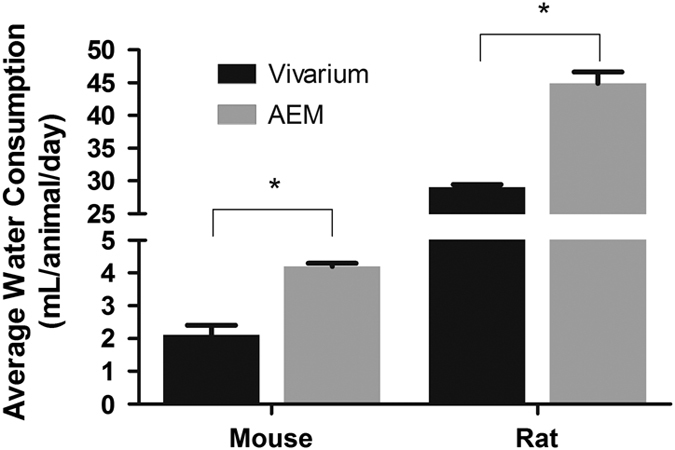
The NuRFB consumption among the vivarium controls and AEM-caged animals were similar (Figure 2). There was no significant difference between the average NuRFB consumption for mice in vivarium (3.55±0.06 g per mouse per day) and AEM (3.69±0.15 g per mouse per day). The average water consumption for mice in the vivarium (2.2±0.4 ml per animal per day) was lower than the average water consumption in the AEMs (4.2±0.1 ml per animal per day) by over 90% (Figure 3).
Figure 2.
Water consumption throughout study as expressed as an average per animal per day for all animals in each compartment of AEM. Mass adjusted for water loss in AEM group. *indicates p>0.05.
Figure 3.
Food consumption throughout study as expressed as an average per animal per day for all animals in each compartment of AEM. Mass adjusted for water loss in AEM group. *indicates p>0.05.
CO2 concentration in the AEMs was<0.1% throughout the test which is well under the acceptable limit (NASA Internal Document #6341 from 11 August 2013: Rodent Research Science Requirements Envelope Document) of 0.7% and ammonia (NH3) concentration was detectable only towards the end of the test at <2 p.p.m. for most readings. On a single occasion, the NH3 level in one of the AEM was measured as 8 p.p.m., but these readings were also well within the acceptable limit of 25 p.p.m. The filters contained all animal waste throughout the test without any break in containment. All fans functioned normally throughout the test.
The pressure gradient across the exhaust filter was measured throughout the experiment to provide an indication of airflow across the filter. Initial pressure gradient for all three AEMs was between 0.33 and 0.35 inches of water, and the pressure changed by no more than 0.02 inches of water over the duration of the test. This indicates that no significant airflow restriction developed due to occlusion of the filters by animal waste or food. Notably, on test day 20 of the AEM Test, NuRFB mounted on the food plates in one cage began detaching from the food plates, but the airflow across the filter did not change.
Rat study
The rat body mass measurements are shown in Figure 1. There were no statistical differences between the AEM and vivarium groups at the beginning (day 0), interim (day 21), or in the end (day 35) of the study. The average body weights (n=15) for rats in vivarium and AEM at day 0 were 275.8±11.4 g and 274.2±11.3 g, respectively, 384.0±34.4 g and 386.0±28.6 g, respectively, at day 21, and 425.6±41.7 g and 437.6±32.8 g, respectively, at day 35.
The average daily food consumption is shown alongside the data of that of the mice in Figure 2 and we observed that the AEM values (32.0±0.8 g per rat per day) were higher than the average food consumption in the vivarium cage (25.2±0.3 g per rat per day) groups. A trend similar to the one observed in mice average water consumption is shown in Figure 3, where this parameter was also elevated in the AEMs (45.0±1.7 ml per rat per day) compared with the vivarium control (25.2±0.3 ml per rat per day).
Immediately prior to euthanasia, blood samples were collected for a standard panel of clinical chemistry tests including quantification of blood glucose, albumin, aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine total protein. AEM and vivarium control group values were within the reference range or within 10% of the vivarium controls. Also, there was no significant difference in organ mass per gram total body mass of the thymus, adrenals, spleen, heart, or lungs between the AEM group and the vivarium controls (Figure 4).
Figure 4.
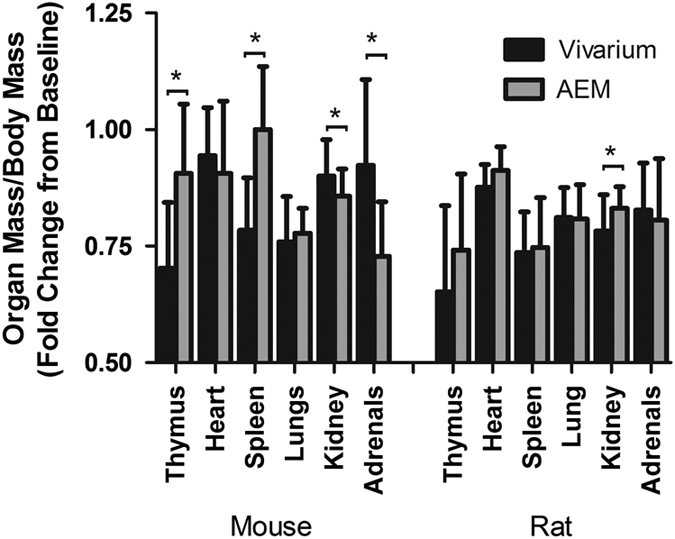
The observed change in organ mass to body mass ratio for VIV and AEM cages, normalized as fold change to mean of baseline (T0) group. Bars marked with *indicate a statistical difference (p>0.05).
No mortalities or noticeable health problems occurred during the study. In the present study, gross morphological evaluations of the stomach, small intestine, and large intestines showed no signs of discoloration or gastric lesions suggestive of digestive problems or allergies during the study. In addition, CO2 concentration in the AEMs was <0.07% throughout the test (well within the acceptable limit of 0.7%) and ammonia (NH3) concentration was undetectable throughout the test (well below the acceptable limit of 25 p.p.m.).
With the rats occupying the AEM, the filters were again able to contain all waste without any break in containment throughout the experiment. Odors were barely detectable upon opening of the AEMs as determined by a group of untrained personnel who were asked to indicate the presence/absence, and acceptable range of any odor at day 21 when the food was replenished or at day 35 at the conclusion of the study. Again, all fans functioned normally throughout the test. Interior pressure dropped only slightly over the course of the test but the airflow did not appear to be impaired. Initial readings were between 0.32 inches of water and 0.34 inches of water for all AEMs, at day 21 the readings dropped by 0.02 inches of water in each AEM, and final readings at day 35 were between 0.25 inches of water and 0.28 inches of water.
Discussion
Several organs were observed to have slight changes in mass, and the following discussion speculates on these potential variations. Although no further tests were conducted to validate these speculations, they are offered as areas of potential further study and as cautions to researchers which may utilize the AEM hardware in spaceflight experiments to study a particular organ system.
The AEM unit induced a decreased organ mass (normalized to baseline and total body mass) in both kidneys (−4.8%) and adrenals of mice (−21.1%; Figure 4) after 35 days of habitation in the AEM. Enlargement of the kidneys is commonly associated clinically with increased water retention as a result of an obstruction of urine flow, such as by kidney stones. Speculatively, the observed decrease in kidney size could be a result of the observed increase in water consumption that eliminated blockages in the kidneys in the AEM mice, which consumed more water. The observed decrease in mouse adrenal mass can potentially be explained by a decrease in stress in the AEM cages that results in a decrease in cortisol production, a major function of the adrenals.
Conversely, there was an observed increase in normalized mass of the mouse thymus (+28.8%) and spleen (+27.6%) as a result of habitation for 35 days in the AEM. Thymic involution, or the shrinking of the thymus with age, is consistent in almost all vertebrates,13 and was again observed in our study, although the shrinkage in relation to baseline size was diminished in AEM mice (−29.7% in vivarium versus −9.4% in AEM). Involution could have a major impact on the immune system’s ability to mount a strong response to new threats due to the decreased ability to produce naive T-cells. Thus, the AEM cage, which evidently prevents some degree of thymic involution, may be a benefit to the inhabitant’s immune system. This effect was lost with the rats, which although they showed the same trend as the mice, they were not statistically significant. Reported literature claims that enlarged thymus in humans shows negligible occurrences of significant thymic disease,14 so the mitigated thymic shrinkage when compared with vivarium controls after the 35 day stay in the AEM unit potentially demonstrates no significant impact on thymic health.
Analysis of the mouse blood (Table 1), however, indicates that all analytes studied exist within the normal ranges within the blood provided by Charles River Laboratory, although there are minor signs of increased stress or infection in AEM mice when compared with vivarium controls, such as the 38% decreased absolute lymphocyte levels (lymphocytopenia), 35% increased monocyte composition (monocytosis), 79% decreased absolute basophil levels (basopenia), 43% decreased absolute eosinophil levels (eosinopenia), and 27% decreased absolute neutrophil count (neutropenia). Further blood analysis also illustrated several statistically significant, but too slight to be considered biologically relevant, differences in the AEM group such as a 6.8% elevation in hematocrit (HCT%), which could be a sign of minor dehydration or even slight chronic hypoxia, a 5.2% increase in mean corpuscular volume (MCV) commonly indicative of a slight vitamin B12 and/or folic acid deficiency, and a 2.2% decrease in mean corpuscular hemoglobin (MCH) and 7.8% decrease in its concentration (MCHC), which both could be indicative of minor iron deficiency.
Table 1. Analysis by colorimetric enzyme assays of mouse blood at completion (day 35) of study.
| Lipid profile |
Vivarium
|
AEM
|
||||
|---|---|---|---|---|---|---|
| Mean | s.d. | N | Mean | s.d. | N | |
| Cholesterol (mg/dl) | 54.9 | 31.8 | 35 | 76.1 | 12.0 | 36 |
| Triglyceride (mg/dl) | 91.2 | 28.2 | 36 | 119.9 | 37.3 | 36 |
|
Chemical profile
| ||||||
| ALK phasphatase (IU/l) | 103.5 | 18.6 | 35 | 87.4 | 19.1 | 35 |
| ALT (SGPT, IU/l) | 25.9 | 36.7 | 36 | 26.0 | 9.8 | 36 |
| AST (SGOT, IU/l) | 96.9 | 51.3 | 35 | 115.9 | 52.2 | 36 |
| CPK (IU/l) | 293.0 | 235.5 | 36 | 313.2 | 202.9 | 36 |
| Albumin (g/dl) | 2.8 | 0.3 | 35 | 2.7 | 0.1 | 36 |
| Total protein (g/dl) | 4.5 | 0.5 | 35 | 4.5 | 0.2 | 36 |
| Globulin (g/dl) | 1.7 | 0.2 | 35 | 1.8 | 0.1 | 36 |
| Total bilirubin (mg/dl) | 0.2 | 0.1 | 36 | 0.2 | 0.1 | 36 |
| Direct bilirubin (mg/dl) | 0.2 | 0.0 | 36 | 0.2 | 0.0 | 36 |
| BUN (mg/dl) | 24.4 | 5.7 | 36 | 25.4 | 3.5 | 36 |
| Creatinine | 0.0 | 0.0 | 35 | 0.0 | 0.0 | 36 |
| Glucose (mg/dl) | 229.8 | 37.2 | 36 | 231.1 | 31.3 | 36 |
| Calcium (mg/dl) | 7.5 | 1.0 | 35 | 7.7 | 0.5 | 36 |
| Phosphorus (mg/dl) | 5.2 | 1.1 | 35 | 5.1 | 0.6 | 36 |
| TCO2 (bicarbonate) | 12.5 | 3.3 | 35 | 10.2 | 2.8 | 36 |
| A/G ratio | 1.6 | 0.1 | 35 | 1.5 | 0.1 | 36 |
| Indirect bilirubin | 0.0 | 0.0 | 36 | 0.0 | 0.0 | 0 |
|
Basic CBC
| ||||||
| WBC (×103/μl) | 3.8 | 1.1 | 35 | 2.5 | 0.8 | 35 |
| RBC (×106/μl) | 9.0 | 0.4 | 35 | 9.1 | 0.3 | 35 |
| HGB (g/dl) | 14.7 | 0.4 | 35 | 14.6 | 0.5 | 35 |
| HCT (%) | 41.8 | 1.8 | 35 | 44.7 | 1.6 | 35 |
| MCV (fl) | 46.5 | 0.7 | 35 | 48.9 | 0.9 | 35 |
| MCH (pg) | 16.3 | 0.4 | 35 | 15.9 | 0.3 | 35 |
| MCHC (g/dl) | 35.2 | 1.0 | 35 | 32.6 | 0.4 | 35 |
| NRBC (/100 WBC) | 0.1 | 0.7 | 35 | 0.3 | 0.7 | 35 |
| Neutrophil SEG (%) | 12.3 | 4.6 | 35 | 13.8 | 6.3 | 35 |
| Lymphocyte (%) | 81.7 | 5.5 | 35 | 78.7 | 7.3 | 35 |
| Monocyte (%) | 4.7 | 1.6 | 35 | 6.4 | 2.9 | 35 |
| Eosinophil (%) | 1.2 | 0.7 | 35 | 1.1 | 0.6 | 35 |
| Basophil (%) | 0.4 | 0.5 | 35 | 0.2 | 0.4 | 35 |
| ABS neutrophil SEG (1/μl) | 443.7 | 143.3 | 35 | 323.6 | 140.8 | 35 |
| ABS lymphocyte (1/μl) | 3,154.3 | 987.8 | 35 | 1,944.7 | 724.5 | 35 |
| ABS monocyte (1/μl) | 177.5 | 73.4 | 35 | 159.0 | 100.9 | 35 |
| ABS eosinophil (1/μl) | 45.4 | 36.1 | 35 | 25.9 | 13.9 | 35 |
| ABS basophil | 15.9 | 19.8 | 35 | 3.3 | 7.8 | 35 |
Abbreviations: ABS, absolute; AEM, Animal Enclosure Module; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; CPK, creatine phosphokinase; HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; MCV, mean corpuscular volume; NRBC, nucleated red blooc cells; RBC, red blood corpuscles; SEG, segmented neutrophils; SGPT, serum glutamic-pyruvic transaminase; SGOT, serum glutamic-oxaloacetic transaminase; WBC, white blood corpuscles.
Mouse blood analysis also showed statistically significant differences in the AEM compared to the vivarium animals for several parameters such as a 38.7% increase in cholesterol, which may be stress induced, a 16% decrease in alkaline phosphatase which may illustrate malnutrition or vitamin B12 deficiency, an 18.1% decrease in bicarbonate which could be a sign of increased fluid loss, and a 6.4% decrease in albumin to globulin ratio which may be indicative of a slight kidney disorder which removes more albumin than in the vivarium animals.
The rat blood analysis (Table 2) also demonstrated a 5.4% decrease in hematocrit, a 3.4% increase in mean corpuscular hemoglobin concentration, a 45% increase in monocytes, a 47% decrease in creatine phosphokinase (CPK), an 88% increase in total bilirubin, and 71% increase in direct bilirubin which may indicate liver problems due to increased hemolysis. Also present were signs that may indicate a slight kidney disorder: a 14.5% increase in phosphate, the 2.4% increase in chloride, 8.1% decrease in potassium, a 1.6% increase in sodium, and a 9.7% increase in sodium/potassium ratio.
Table 2. Analysis by colorimetric enzyme assays of rat blood at completion (day 35) of study.
| Lipid profile |
Vivarium
|
AEM
|
||||
|---|---|---|---|---|---|---|
| Mean | s.d. | N | Mean | s.d. | N | |
| Cholesterol (mg/dl) | 74.070 | 18.590 | 15 | 76.070 | 10.400 | 15 |
| Triglyceride (mg/dl) | 181.100 | 76.820 | 15 | 148.900 | 47.510 | 15 |
|
Chemical profile
| ||||||
| ALK phasphatase (IU/l) | 184.700 | 28.320 | 15 | 200.400 | 35.820 | 15 |
| ALT (SGPT, IU/l) | 31.930 | 10.040 | 15 | 31.670 | 4.776 | 15 |
| AST (SGOT, IU/l) | 75.070 | 24.060 | 15 | 71.270 | 11.310 | 15 |
| CPK (IU/l) | 213.400 | 160.000 | 15 | 112.100 | 44.160 | 15 |
| Albumin (g/dl) | 3.173 | 0.088 | 15 | 3.200 | 0.146 | 15 |
| Total protein (g/dl) | 6.153 | 0.304 | 15 | 6.280 | 0.221 | 15 |
| Globulin (g/dl) | 2.980 | 0.248 | 15 | 3.080 | 0.137 | 15 |
| Total bilirubin (mg/dl) | 0.113 | 0.035 | 15 | 0.213 | 0.035 | 15 |
| Direct bilirubin (mg/dl) | 0.113 | 0.035 | 15 | 0.193 | 0.059 | 15 |
| BUN (mg/dl) | 21.930 | 2.549 | 15 | 22.070 | 2.658 | 15 |
| Creatinine | 0.293 | 0.059 | 15 | 0.247 | 0.099 | 15 |
| Glucose (mg/dl) | 168.300 | 11.980 | 15 | 173.100 | 13.310 | 15 |
| Calcium (mg/dl) | 9.960 | 0.392 | 15 | 9.940 | 0.304 | 15 |
| Phosphorus (mg/dl) | 4.360 | 0.861 | 15 | 4.993 | 0.846 | 15 |
| TCO2 (Bicarbonate) | 17.200 | 4.724 | 15 | 17.200 | 3.342 | 15 |
| Chloride (mEq/l) | 103.100 | 1.817 | 14 | 105.500 | 1.293 | 11 |
| Potassium | 4.650 | 0.228 | 14 | 4.300 | 0.303 | 11 |
| Sodium | 139.400 | 1.336 | 14 | 141.500 | 1.214 | 11 |
| A/G ratio | 1.053 | 0.092 | 15 | 1.040 | 0.063 | 15 |
| B/C ratio | 80.730 | 34.260 | 15 | 95.600 | 47.150 | 14 |
| NA/K ratio | 30.000 | 1.301 | 14 | 32.910 | 2.587 | 11 |
|
Basic CBC
| ||||||
| WBC (×103/μl) | 7.421 | 2.227 | 14 | 7.240 | 1.934 | 15 |
| RBC (×106/μl) | 7.815 | 0.332 | 14 | 7.569 | 0.384 | 15 |
| HGB (g/dl) | 14.990 | 0.529 | 14 | 14.680 | 0.524 | 15 |
| HCT (%) | 43.790 | 1.265 | 14 | 41.530 | 1.279 | 15 |
| MCV (fl) | 56.210 | 1.718 | 14 | 55.000 | 2.507 | 15 |
| MCH (pg) | 19.180 | 0.687 | 14 | 19.410 | 0.573 | 15 |
| MCHC (g/dl) | 34.190 | 0.403 | 14 | 35.360 | 1.211 | 15 |
| NRBC (/100 WBC) | 0.000 | 0.000 | 14 | 0.000 | 0.000 | 15 |
| Neutrophil SEG (%) | 20.640 | 5.300 | 14 | 22.530 | 5.854 | 15 |
| lymphocyte (%) | 75.710 | 5.567 | 14 | 73.200 | 5.672 | 15 |
| Monocyte (%) | 1.786 | 0.579 | 14 | 2.600 | 1.121 | 15 |
| Eosinophil (%) | 1.643 | 0.842 | 14 | 2.000 | 0.926 | 15 |
| ABS neutrophil SEG (1/μl) | 1,496.000 | 480.300 | 14 | 1,603.000 | 508.100 | 15 |
| ABS lymphocyte (1/μl) | 5,655.000 | 1,871.000 | 14 | 5,329.000 | 1,583.000 | 15 |
| ABS monocyte (1/μl) | 133.400 | 62.240 | 14 | 1,86.100 | 89.150 | 15 |
| ABS eosinophil (1/μl) | 117.900 | 61.150 | 14 | 143.900 | 71.110 | 15 |
Abbreviations: ABS, absolute; AEM, Animal Enclosure Module; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; CPK, creatine phosphokinase; HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; MCV, mean corpuscular volume; NRBC, nucleated red blooc cells; RBC, red blood corpuscles; SEG, segmented neutrophils; SGPT, serum glutamic-pyruvic transaminase; SGOT, serum glutamic-oxaloacetic transaminase; WBC, white blood corpuscles.
Further analysis is required to confirm or refute given causes and potential diagnoses above based on blood and organ results, and they are provided merely as a suggested explanation for future studies and to prepare researchers utilizing the AEM hardware on spaceflight projects for potential limitations in studies which focus on these organ systems. The reported values were within the normal physiological range reported for rats,15 so we conclude that although the AEM cage seemed to cause variations in several parameters, these fluctuations are not biologically significant and support the proof of concept for 35-day habitation in the AEM on a diet of the NuRFB, providing a basis for expanding the AEM hardware originally designed for short duration space Space Shuttle into 35-day missions aboard the International Space Station and other spaceflight vehicles. The nutritional adequacy of the NuRFB for short-term (18 to 20 day) rodent spaceflight and ground-based spaceflight simulation experiments has been verified.16 However, the nutritional adequacy of the NuRFB for use in longer spaceflight and on-ground studies has not been evaluated.
The results obtained from the mouse study have demonstrated for the first time that the AEM with its current configurations can be used to house up to 12 female C57BL/6 mice for up to 35 days of extended operations without any observable system failures. In addition, data obtained from the rat study have demonstrated that AEM can provide proper life supports for up to 5 adult Sprague–Dawley male rats for 35 days if the food is replenished as needed throughout the experiment duration. This research provides NASA with an essential lessoned learned, namely that the limiting factor to 35 day housing of rats and mice within the AEM will be successful replenishment to food and water, as supported by the absence of any hardware failures in life support systems and the blood and organ analysis of subjects which fell within the normal ranges of animals not exposed to AEM cages. This opens the doors to similar ground based and flight missions of longer duration of up to 90 days, the desired range of the next generation of NASA long-term rodent studies aboard the International Space Station.
Materials and methods
AEM hardware
Three ground government support equipment AEM units (Figure 5) were used for the feeding studies of rats and mice. AEM units were built according to flight configurations which, for mouse studies, delineates that each AEM is loaded with two water bags in the water box container with approximately 2 liters of heat-sterilized distilled water per bag. Water consumption was derived as a difference in the water bag mass before and after the test. Each AEM cage was fitted with a cage divider that provided two separate and equal compartments for mice. In each half of the AEM cage, two food plates were provided with one attached to the water box side, and one attached to the cage side. For the rat study, water was provided with two external graduated cylinders connected with plastic tubes through the water box to lixits. Control animals were handled and provided the same environment and provisions as flight configuration. Water consumption was recorded daily and fresh heat-sterilized water was added as needed. No divider was used in the cage for the rat study due to the size of the animals, therefore only five rats were housed in each AEM. Each AEM was instrumented with humidity (Vernier Software and Technology Type RH-BTA) and temperature probes (Vernier Software and Technology Type TMP-BHA) and a gas port for periodic sampling for ammonia (NH3) and carbon dioxide (CO2). NH3 and CO2 were measured using appropriate Draeger tubes and calibrated SKC Personal Sampling Pumps (model 222). Pressure drops across the exhaust filter were also measured at the gas port using an inclined Dwyer manometer accurate to 0.1 inches of water. The temperature and room humidity data were logged using LabView (National Instruments, Austin, TX, USA) software running on two laptop computers. The sensor to laptop connections used Vernier Software and Technology ‘SensorDAQ’ (Vernier Software & Technology, Beaverton, OR, USA) interfaces. During the test period, the AEMs were placed on vertical stands and covered with shrouds at all times except for daily health checks and any lighting was provided by the internal AEM light fixtures.
Figure 5.
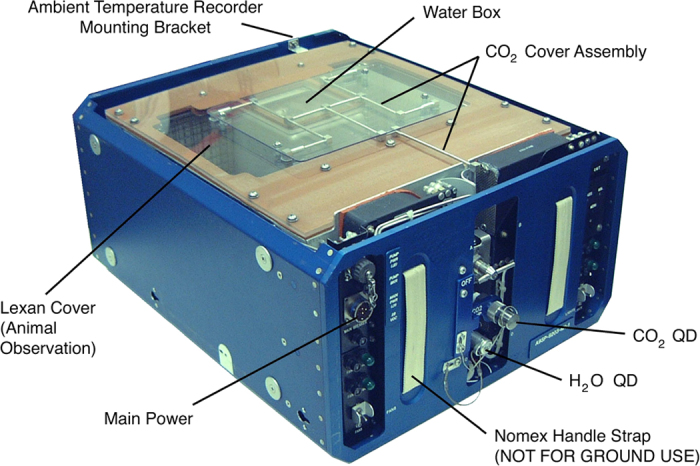
Schematic outlining the Animal Enclosure Module key features.
Diets
The NASA Type 12 NuRFB, the official approved rodent flight diet, were used for the reported studies and a detailed description of the NASA NuRFB has previously been reported.17–19 Diet requirements for the 35-day study were estimated based on the AEM flight payload preparation formula, which states that the average daily NuRFB and water consumption requirement are 4.0 g and 4.5 ml, respectively, per mouse per day, and 40 g and 50 ml per rat per day. In the shuttle flight payload operations, an additional 20% mass of NuRFB and water were loaded as waste allowance for both the mice and rats.
In the mouse study, each AEM cage was fitted with a cage divider, providing two separate areas for the mice. In each half of the AEM cage, two food plates were provided: one attached to the water box and the other to the cage side. Each water box diet plate accommodated four pieces of NuRFB, and each cage diet plate accommodated five pieces of NuRFB, for a total of 9×Type 12 M NuRFB (20.32 cm×2.54 cm×3.05 cm) per half cage, or ~2.24 kg per half cage (4.48 kg per AEM). In the rat study no cage divider was utilized in the AEM and the remaining NuRFB on the food plates were replaced with new food plates on day 21.
Animal study
All procedures used in this study conformed to the NRC Guide for the Care and Use of Laboratory Animals20 and Title 14 of the Code of Federal Regulations.21 The animal protocols for mouse and rat studies were reviewed and approved by the Institutional Animal Care and Use Committee at the NASA Ames Research Center prior to the experiments.
For the mouse study, female C57BL/6 mice (~35 days old) were acquired from Charles River (Wilmington, MA, USA) 4 weeks prior to the start of the test. Female C57BL/6 mice (body mass 15.2±0.8 g) were used in this study because they are a strain commonly used in the NASA flight experiments.2–10 For the rat portion of the study, male Sprague–Dawley rats aged 12 weeks (body weight 159±8.6 g) were obtained from Charles River (Wilmington, MA, USA). Young male Sprague–Dawley rats were used in the study because many spaceflight and ground-based spaceflight simulation studies have been conducted using young male rats and they are the strain likely to be used in future NASA rat flight experiments.
At the NASA Ames Research Center Animal Care Facility, animals were housed in standard polypropylene ‘shoebox’-style cages in a room maintained at a temperature of 24±1 °C, relative humidity of 40–70% and lighting which allows for a 12 h light/dark cycle. The control animal lighting cycle in the vivarium cages was synchronized with the AEM hardware operations.
Upon receipt, the animals were randomly assigned to groupings of six mice or five rats, housed in vivarium cages with microisolator tops, and provided distilled water via standard water bottles and sipper tubes. The animals were continued on the same food diet as at the vendor’s facility. After 3 days of acclimation, the animals were implanted subcutaneously with microchips (Biomedic Data Systems IMITM 1000, Biomedic Data Systems, Inc., Seaford, DE, USA) under anesthesia for identification purposes, a standard practice in AEM flight payload operations. Then, animals were provided distilled water via water bottles with stoppers fitted with AEM-like lixits that were refurbished prior to the test. The diet was transitioned slowly from the standard rodent diet over the course of 5 days until the animal diet consisted entirely of the NuRFB, at which point all animals were fed with only NuRFB until the end of the study. Water was provided ad libitum throughout the entire study duration. Diets were provided ad libitum throughout the entire study duration but removed overnight before the end of day 35 due to blood draw taken at time of tissue collection.
In the mouse study, following a 10-day acclimation, female C57BL/6 mice at 9 weeks old were randomly assigned (n=36 per group) to AEM units or vivarium cages. There were no significant differences in the initial body masses of mice in vivarium (19.0±0.5 g) or AEM cages (19.0±0.5 g).
In the rat study, following a 10-day acclimation, male Sprague–Dawley rats age 13–14 weeks old were randomly assigned (n=15 per group) to AEM units or vivarium cages with 5 rats per cage. There were no significant differences in the initial body weights of rats in vivarium (275.8±11.4 g) or AEM cages (274.2±11.3 g). The cages remained untouched during the course of the study, except for on day 21 when the AEM were opened, the NuRFB plates were removed and weighed, new NuRFB plates were added, and the animals were given a general health check-up by an on-site veterinarian while body mass measurements were recorded.
Animal water consumption was measured daily for the rats, while animal body mass and food intake were measured at day 21 and at the end of the study (day 35). CO2 and NH3 measurements were recorded on test days 1, 16, 28, and 35. NASA Animal Care Facility staff and Science team personnel performed a daily visual animal health evaluation. The attending veterinarian performed a detailed individual animal check and health evaluation on all of the animals at animal receiving, animal load at test start (day 0), during NuRFB replenishment (day 21), and animal unload at test end (day 35).
Organ mass measurements and serum clinical chemistry
One day before the end of the study (day 34), the animals were fasted overnight and then on day 35 the animals were anesthetized with isoflurane, blood was collected via cardiac puncture, and then the subjects were euthanized via isoflurane overdose paired with decapitation (rats) or cervical dislocation (mice). The kidneys, lungs, spleen, thymus, adrenals, and heart were dissected and mass measurements were recorded for each organ.
For clinical chemistry measurements, blood was collected in vacutainer tubes and serum was obtained by centrifugation at 1,500 g for 10 min at 4 °C. Serum samples were stored on ice and shipped to IDEXX Veterinary Services, Inc (West Sacramento, CA, USA). Serum triglycerides, cholesterol, fasting glucose, globulin, creatine kinase, and bicarbonates were measured by colorimetric enzyme assays using an automated Clinical Chemistry Analyzer (Olympus AU 400, Olympus America Inc, Center Valley, PA, USA). Colorimetric enzyme assays were also used to measure serum biomarkers of liver function of alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and direct and total bilirubin. Biomarkers of renal function included the measurement of albumin, creatinine, total protein, blood urea nitrogen, calcium, phosphorus, and electrolytes (potassium, sodium, and chloride) also by colorimetric assays using the Clinical Chemistry Analyzer.
A Hematology Analyzer (Bayer Advia 120, Global Medical Instruments, Ramsey, MN, USA) was also used to determine red blood cell count, mean red blood cell volume, hemoglobin, mean corpuscular hemoglobin, mean cell hemoglobin concentration, white blood cell count, percent lymphocytes, neutrophils, monocytes, eosinophils, basophils, hematocrit, prothrombin time, partial thromboplastin, and fibrinogen.
Statistical analysis
Animal body mass measurements, food consumption, water consumption, and organ mass data normalized to total body mass following euthanasia were analyzed using one-way analysis of variance with JMP 7.0 software (JMP, Cary, NC, USA). For all tests, P<0.05 was considered significant. Values in all figures are expressed as mean±s.d. unless specified.
Acknowledgments
We would like to express our appreciation to Dr Joanne Blum and Danielle Galindo of the NASA Ames Animal Care Facility; Dr Kevin Sato, Dr Robert Bowman, and Dr Esther Hill for support in animal processing; and Vera Vizir, Karin Perkins, David Leskovsky, and Shahn Spratt for support in hardware, logistics, and science operations. Funding for this research was provided by the NASA Space Biology program.
Footnotes
The authors declare no conflict of interest.
References
- Ballard, R. & Connolly, J. US/USSR joint research in space biology and medicine on Cosmos biosatellites. FASEB J. 4, 5–9 (1990). [DOI] [PubMed] [Google Scholar]
- Bailey, J. F. , Cheng, K. K. , Hargens, A. R. , Masuda, K. & Lotz, J. C. Effects of microgravity on the creep behavior of murine intervertebral discs. FASEB J. 25, 668.1 (2011). [Google Scholar]
- Behnke, B. J. et al. Effects of spaceflight and ground recovery on mesenteric artery and vein constrictor properties in mice. FASEB J. 27, 399–409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber, E. et al. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 13, 181–201 (2014). [DOI] [PubMed] [Google Scholar]
- Blaber, E. , Sato, K. & Almeida, E. A. Stem cell health and tissue regeneration in microgravity. Stem Cell Dev. 23, 73–78 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A. et al. (eds). Spaceflight Related Changes in Structure and Strength of Mouse Trabecular and Cortical Bone From the STS-118 Space Shuttle Mission. ASME 2013 Summer Bioengineering Conference; American Society of Mechanical Engineers, (2013). [Google Scholar]
- Taylor, C. R. et al. Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: implications for elevated cerebral perfusion and intracranial pressure. FASEB J. 27, 2282–2292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Cory, E. , Bhattacharya, R. , Sah, R. & Hargens, A. R. Fifteen days of microgravity causes growth in calvaria of mice. Bone 56, 290–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednieks, M. , Khatri, A. , Rubenstein, R. , Burleson, J. A. & Hand, A. R. Microgravity alters the expression of salivary proteins. Oral Health Dental Manage. 13, 211–216 (2014). [PubMed] [Google Scholar]
- Pecaut, M. J. et al. Genetic models in applied physiology: selected contribution: effects of spaceflight on immunity in the C57BL/6 mouse. I. Immune population distributions. J. Appl. Physiol. 94, 2085–2094 (2003). [DOI] [PubMed] [Google Scholar]
- Dalton, P. , Gould, M. , Girten, B. , Stodieck, L. S. & Bateman, T. A. Preventing annoyance from odors in spaceflight: a method for evaluating the sensory impact of rodent housing 1 November 2003. 2113–2121 p. [DOI] [PubMed]
- Naidu, S. , Winget, C. M. , Jenner, J. W. , Mele, G. & Holley, D. C. Effects of housing density on mouse physiology and behavior in the NASA Animal Enclosure Module simulators. J. Gravit. Physiol. 2, P140 (1995). [PubMed] [Google Scholar]
- Shanley, D. P. , Aw, D. , Manley, N. R. & Palmer, D. B. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 30, 374–381 (2009). [DOI] [PubMed] [Google Scholar]
- Singla, S. , Litzky, L. A. , Kaiser, L. R. & Shrager, J. B. Should asymptomatic enlarged thymus glands be resected? J. Thorac. Cardiovasc. Surg. 140, 977–983 (2010). [DOI] [PubMed] [Google Scholar]
- Weingand, K. W. , Odioso, L. W. , Dameron, G. W. , Laytart, M. J. & Stitzel, K. A. Hematology analyzer comparison: ortho ELT‐8/ds vs. Baker 9000 for healthy dogs, mice, and rats. Vet. Clin. Pathol. 21, 10–14 (1992). [DOI] [PubMed] [Google Scholar]
- Tou, J. et al. Evaluation of NASA Foodbars as a standard diet for use in short-term rodent space flight studies. Nutrition 19, 947–954 (2003). [DOI] [PubMed] [Google Scholar]
- Sun, G.-S. et al. Oxidative and nutrient stability of a standard rodent spaceflight diet during long-term storage. Lab Anim. (NY) 41, 252–259 (2012). [DOI] [PubMed] [Google Scholar]
- Sun, G.-S. , Tou, J. C. , Yu, D. , Girten, B. E. & Cohen, J. The past, present, and future of National Aeronautics and Space Administration spaceflight diet in support of microgravity rodent experiments. Nutrition 30, 125–130 (2014). [DOI] [PubMed] [Google Scholar]
- Sun, G.-S. et al. Evaluation of the nutrient-upgraded rodent food bar for rodent spaceflight experiments. Nutrition 26, 1163–1169 (2010). [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals (National Academies, 1985). [Google Scholar]
- Code of Federal Regulations. Care and Use of Animals in the Conduct of NASA Activities. 14 CFR 1232, Chapter V, Part 1232 (2012).


