Abstract
Organization of membrane micro-domains by Ypt/Rab GTPases is key for all membrane trafficking events in eukaryotic cells. Since autophagy is a membrane trafficking process, it was expected that these GTPases would play a role in autophagy as well. While evidence about participation of Ypt/Rabs in autophagy is beginning to emerge, the mechanisms by which they act in this process are still not clear. Moreover, it is still questionable if and how Ypt/Rabs coordinate autophagy with other cellular trafficking processes. Yeast Ypt1 and its mammalian homolog Rab1 are required for both endoplasmic reticulum (ER)-to-Golgi transport and autophagy, suggesting that they coordinate these two processes. In our recent paper, we identify Atg11, a bona fide phagophore assembly site (PAS) component, as a downstream effector of Ypt1. Moreover, we show that three components of a GTPase module—the Ypt1 activator, Trs85-containing TRAPP complex, Ypt1, and the Atg11 effector—interact on the PAS and are required for PAS formation during selective autophagy. We propose that Ypt/Rabs coordinate the secretory and the autophagic pathways by recruiting process-specific effectors.
Keywords: Selective Autophagy, Ypt/Rab GTPases, Ypt1, Trs85, Atg11, PAS
Until recently, much of the progress done in the autophagy field was in the identification of autophagy-specific machinery components. One important lesson learned was that these components are conserved from yeast to human cells. In addition, because autophagy is a membrane-bound process, it was expected that it would utilize machinery components of intracellular trafficking as well, including the Ypt/Rab GTPases. When on membranes in the GTP-bound form, Ypt/Rabs recruit their multiple downstream effectors. These effectors mediate vesicular transport in the different steps of the exocytic and endocytic pathways. Indeed, Ypt/Rabs were implicated in autophagy. However, because of their key role in all membrane trafficking processes and the need of membrane for formation of the autophagosome, it was not clear whether their involvement in autophagy is direct or indirect. Moreover, it is not known whether autophagy is coordinated with other membrane trafficking events and, if so, how. Our recent paper provides evidence that Ypt1 is directly involved in the first step of autophagy and suggests that it coordinates the exocytic pathway with autophagy. We reported the following three major findings.
The role of Ypt1 in endoplasmic reticulum (ER)-to-Golgi transport is well documented. In addition, Ypt1 plays a role in selective and nonselective autophagy. The key finding that provides evidence for a direct role for Ypt1 in autophagy is the identification of Atg11 as its downstream effector. Atg11 is a component of the PAS that assembles during the cytoplasm-to-vacuole targeting (Cvt) pathway. In yeast, the Cvt pathway is one of several selective autophagy processes, which is required for delivery of biosynthetic enzymes, e.g., Ape1, to the vacuole (the yeast lysosome). The finding that Ypt1 interacts with Atg11 directly in its GTP-bound form and that this interaction is required for Atg11 localization to the PAS indicates that Atg11 is a Ypt1 effector. Importantly, it establishes a direct role of Ypt1 in PAS assembly.
Ypt/Rabs act in modules of an activator, a GTPase and an effector. Ypt/Rab activators stimulate the exchange of guanine nucleotide by the Ypt/Rab and are termed GEFs. The proposed GEF for Ypt1 in autophagy is the TRAPPIII complex, which includes an autophagy-specific subunit, Trs85. We showed that a module, which consists of Trs85, Ypt1 and Atg11, localizes to the PAS and is required for PAS assembly. This is the first Ypt/Rab module shown to function at the onset of autophagy.
Ypt/Rabs function on membranes and their GEFs are required for maintaining GTP-bound Rabs on membranes. Atg9 is the only core PAS component required for all autophagy processes that contains a transmembrane domain. There are multiple Atg9 puncta in each cell and one of these puncta coincides with the PAS. Therefore, Atg9-containing membranes are considered the source for the PAS membrane. We showed that Trs85 interaction with Ypt1 coincides with Atg9, identifying the membrane on which Ypt1 and Trs85 interact as Atg9-containing membranes and suggesting this as a step that precedes PAS assembly (Fig. 1).
Figure 1.
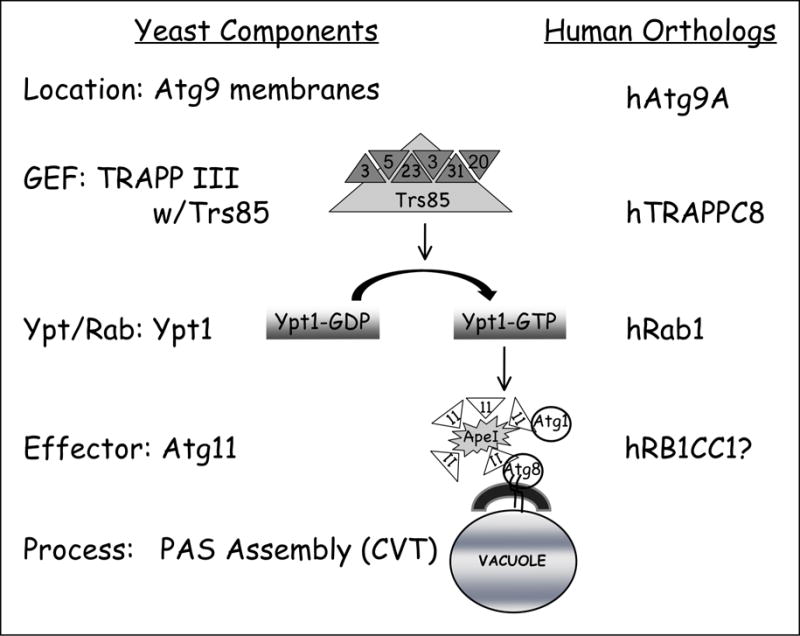
A model of the mechanism by which the Ypt1 GTPase module regulates PAS assembly during the selective Cvt pathway. In yeast, TRAPPIII, which contains the essential TRAPPI subunits, Bet3, Bet5, Trs23, Trs31 and Trs20, as well as the autophagy-specific subunit Trs85, acts as a GEF for Ypt1 during selective autophagy. Activated Ypt1 interacts with Atg11, an adaptor for Atg19, the Ape1 cargo receptor. This interaction is required for assembly of PAS components during the Cvt pathway, e.g., Atg11, Atg8 and Atg1. Human orthologs of the yeast machinery components are shown on the right.
Based on these findings, we propose that Ypt/Rabs coordinate autophagy with other membrane trafficking processes by recruiting process-specific effectors. For example, Ypt1 coordinates ER-to-Golgi transport in the exocytic pathway with PAS formation during autophagy by recruiting different effectors, Atg11 being an autophagy-specific Ypt1 effector. Moreover, we propose that Ypt/Rabs can regulate more than one process because they function in modules in which both the GEF and the effector are process-specific. The Ypt1 module that regulates selective autophagy includes TRAPPIII as a GEF and Atg11 as an effector.
Our model raises multiple questions. First, in addition to the Cvt pathway, Ypt1 is required for other selective and nonselective autophagy processes. What might be the mechanism that underlies this versatility? We expect that in addition to Atg11, other autophagy-specific Ypt1 effectors exist. Second, is the use of autophagy-specific effectors conserved from Ypt1 to Rab1? Atgs, Ypt/Rabs and their GEFs are conserved. The known human orthologs of Ypt1 and Trs85 are RAB1 and TRAPPC8, respectively. However, Atg11 does not have a clear human ortholog and the existence of the Cvt pathway was not shown in mammalian cells. Interestingly, Atg11 has a yeast paralog, Atg17, which functions in nonselective autophagy. A human protein, RB1CC1, was suggested as an ortholog for either Atg17 or Atg11 (Fig. 1). We speculate that even if Atg11 and the Cvt pathway are not conserved from yeast to humans, the principle of autophagy-specific RAB1 effectors is conserved. Lastly, it would be interesting to elucidate how Ypt/Rabs are regulated to interact with the right effector at the right place and time. Specifically, it is a mystery how Ypt/Rabs are diverted from the exocytic pathway to autophagy.


