Summary
Red blood cells (RBCs) degrade progressively during the weeks of refrigerated storage. No universally accepted definition of “fresh” or “old” RBCs exists. While practices vary from country to country, preservative solutions permitting shelf life as long as 7 weeks have been licensed. Transfusion of stored RBCs, particularly those at the end of the approved shelf life, has been implicated in adverse clinical outcomes. The results of observational analyses, animal models and studies in volunteers have proved provocative, controversial and contradictory. A recently completed randomized controlled trial (RCT) in premature infants exemplifies the difficulties with moderately sized clinical studies. Several other RCTs are in progress. The effect of RBC storage may well vary according to the clinical setting. Resolution of the importance of the storage lesion may require large pragmatic clinical trials. In the meantime, institutions involved in blood collection and transfusion should explore strategies that assure blood availability, while limiting the use of the oldest RBCs currently approved by regulation.
Keywords: Red cell units, red blood cells, storage, therapy, adverse effects
Introduction
An estimated 90 million units of red blood cells (RBCs) are transfused worldwide annually. Transfusion of RBCs saves lives and enables many medical therapies. RBCs meet the definition of an essential medicine, although use became widespread before and without the rigorous evaluation of randomized clinical trials (RCTs) (Klein, 2013). Despite well-publicized, immunological and rare infectious adverse events (Vamvakas, 2007; Klein, 1999), in developed countries RBC transfusion has a therapeutic index exceeding that of many common medications (Klein et al, 2007). However, RBC shelf life is determined and strictly regulated not on the basis of clinical studies, but based on in vitro haemolysis and in vivo radiochromium label recovery and survival studies (Dumont & AuBuchon, 2008). The acceptable limits were originally derived from expert opinion, not through correlation with clinical outcome (Hess, 2012).
For almost 20 years, retrospective and prospective observational studies have hinted at a harmful effect of RBC storage (Aubron et al, 2013; Lelubre & Vincent, 2013). A large retrospective single-centre study comprising 6002 patients (Koch et al, 2008) raised both interest and concern about the quality of stored RBCs and their potential toxicity. A formal meta-analysis of observational studies and RCTs focusing on patient survival rates concluded that regardless of clinical situation, trial size or volume of blood transfused, older stored blood was associated with a significant increase in mortality (Wang et al, 2012). This article will review and analyse current RBC preservation issues, animal data, volunteer studies, the observational database and the several ongoing RCTs that are designed to correlate clinical outcomes with RBC storage time.
RBC shelf life in current clinical practice
For most of the 20th century, the goal of red cell preservative solution research was to extend RBC shelf life in order to enhance inventory control and reduce outdating. Most blood providers extended the RBC shelf life from 21 to 28 days with the addition of phosphate to the solution, to 35 days with the addition of adenine, and to 42 days when additive solutions became widely introduced (Hogman et al, 1978; Beutler & West, 1979; Hogman, 1985; Moroff et al, 1990; Hess, 2006). A brief survey (Table I) documents the variability in the approved versus the practiced RBC shelf life in different healthcare systems (Sparrow, 2012). The longest shelf life currently in clinical use is 49 days (Zehnder et al, 2008). A new additive solution has been recently Conformité Européenne (CE)-certified for 56-day storage in Europe, and Food and Drug Administration (FDA) licensed for 42-day storage in the US (Hess et al, 2006). Some blood centres have opted to voluntarily retain 35 or 42 days and forgo the opportunity to increase shelf life, although their national regulations allow extending the shelf life to 42 or 49 days. Japan has elected to restrict the “42-day RBC” to a 21-day shelf life since 1995; the shorter storage period reduced the risk of septic reactions from slow-growing bacteria and permitted universal irradiation of RBC to prevent transfusion-associated graft-versus-host disease (GvHD) (Sakakibara & Juji, 1986; Otani et al, 2012).
Table I.
Examples for RBC storage times currently approved and applied depending on the additive solution
| Additive solution* and shelf life (days)
|
Country | |
|---|---|---|
| Approved | Applied in clinical service | |
| 49 days | 49 days | Germany and Switzerland1 |
| 49 days | 42 days | Germany1 |
| 42 days | 42 days | Canada, China, Malaysia, and USA;2 Thailand;2,3 Argentina, Australia, Austria, Brazil, France, Germany, India, Italy, Saudi-Arabia, Switzerland, Spain, and Tunisia3 |
| 42 days | 35 days | China;2,3,4 Netherlands and United Kingdom (uniform for the countries);3 Germany (large blood services for >20 years)3 |
| 42 days | 21 days | Japan4 |
| 35 days | 35 days | Brazil, China, Ethiopia, India, Malaysia, Oman, Pakistan, and Tunisia5 |
| 21 days | 21 days | China and Saudi-Arabia (few blood services)6 |
1PAGGS-M (phosphate-adenine-glucose-guanosin-saline-mannitol, 285 mOsm/l isotonic): approved for a shelf life of 49 days; 2AS-3 (saline-adenine-glucose-citrate-phosphate): 42 days; 3SAGM (saline-adenine-glucose-mannitol, 376 mOsm/L hypertonic): 42 days; 4MAP (mannitol-adenine-phosphate): 42 days;;; 5CPDA-1 ( citrate-phosphate-dextrose-adenine): 35 days; 6CPD (citrate-phosphate-dextrose): 21 days.
Some national blood systems have shortened the RBC storage interval in the expectation that the shortened shelf life improves RBC quality. The UK restricts RBC shelf life to 35 days by policy (UK Blood Transfusion & Tissue Transplantation Services, 2013) despite the use of additive solutions deemed suitable for 42 day storage by law (UK Legislation, 2005). The Netherlands elected to apply 35-day storage even with the availability of additive solutions, because inventory management is satisfactory with this shorter outdate and discard rates remain less than 2%. Other health care systems have instituted a policy of discarding RBCs, once storage has reached the last 7 days of the longest acceptable shelf life for their additive solutions. Large regions and entire nations have determined that a 35-day shelf life can be safe and cost-effective for providing RBC support.
Retrospective, prospective observational and case-control clinical studies
Meta-analysis of studies on mortality
We conducted a formal meta-analysis of studies comparing patient survival rates associated with the transfusion of fresh versus older RBCs (Wang et al, 2012). As no universal definition of “fresh” or “old” RBCs exists, we accepted the definitions used in the various studies. Seventeen to 387,130 patients were enrolled between 1991 and 2009 in 3 RCTs (Schulman et al, 2002; Fernandes da Cunha et al, 2005; Hebert et al, 2005), 6 prospective observational and 12 retrospective studies. The effects were similar across these 21 studies and overall mortality was increased significantly for patients receiving older RBCs. Based on these predominantly retrospective data, published mortality rates and odds ratios (Wang et al, 2012), one would have to transfuse between 97 and 69,428 patients with exclusively fresh RBCs to save one life.
Six studies enrolled trauma patients (Schulman et al, 2002; Murrell et al, 2005; Weinberg et al, 2008a; Weinberg et al, 2008b; Spinella et al, 2009; Weinberg et al, 2010), 6 cardiac surgery patients (Hebert et al, 2005; van de Watering et al, 2006; Yap et al, 2008; Koch et al, 2008; Robinson et al, 2010; van Straten et al, 2011) and 9 a mix of varied patient populations (Mynster & Nielsen, 2001; Fernandes da Cunha et al, 2005; Leal-Noval et al, 2008; van Buskirk et al, 2009; Gauvin et al, 2010; Karam et al, 2010; Eikelboom et al, 2010; Edgren et al, 2010; Pettila et al, 2011). The results of each of these 3 subgroups were consistent with an overall increase of mortality associated with older RBCs, as they were in small and large studies (<500 versus > 500 patients) and in studies of patients receiving on average 3 RBCs or less versus more than 3 RBCs per patient. Seven studies reported serious adverse events; 3 studies an overall significant increase of pneumonia and 3 others showed an overall increase in multiple-organ-dysfunction associated with transfusion of older blood (Wang et al, 2012), suggesting an increased risk of death from old RBCs.
Current reviews
A plethora of clinical data, primarily from retrospective studies, were recently published in reviews (Aubron et al, 2013; Lelubre & Vincent, 2013; van de Watering, 2013). Additional retrospective studies in different clinical situations appear almost monthly (Janz et al, 2013; Middelburg et al, 2013; Saager et al, 2013). Despite the numerous studies, practitioners are left with divergent results for clinically important outcomes.
Among the 32 studies tabulated by one review (Aubron et al, 2013), 18 reported a harmful effect of prolonged RBC storage, while 14 did not. The 7 prospective observational or case-controlled studies were evenly distributed (4 with and 3 without significant harmful effect). Of note, the 4 prospective randomized studies in this tabulation showed no significant harmful effect (Wasser et al, 1989; Schulman et al, 2002; Hebert et al, 2005; Kor et al, 2012).
One report tabulated 55 studies including 8 small RCTs (Lelubre & Vincent, 2013). The 47 non-RCT studies addressed clinical outcomes, including mortality (22 studies); occurrence of infection (18); organ failure (12); tissue oxygenation and microcirculation (11); length of hospital stay (9) and other outcomes (8). The considerable heterogeneity and methodological flaws of the included studies prevented the authors from combining the data or trying to determine the reasons for the different effects (Lelubre & Vincent, 2013).
The largest retrospective cohort comprised 364,037 patients, and concluded that the excess mortality, if any, caused by older RBC is probably less than 5% (Edgren et al, 2010). Among the 4 next largest studies, 2 studies comprising 6002 (Koch et al, 2008) and 4933 patients (Eikelboom et al, 2010) reported a harmful effect, while the 2 other studies, comprising 3475 (van Straten et al, 2011) and 2732 patients (van de Watering et al, 2006), did not. Possible confounders, such as variations in leucocyte depleted RBC blood products or ABO matching, are of concern and not always controlled or even documented in detail.
The retrospective and small prospective studies raise concerns about the efficacy and toxicity of older stored RBCs. However important as they are in generating hypotheses, the data are not sufficiently robust to warrant changes in national blood policies. In retrospective studies, transfusions may represent no more than a marker of illness severity independent of other parameters, like the APACHE II (Acute Physiology and Chronic Health Evaluation II) score. The known confounding factors are difficult to adjust, no matter how much effort authors put into statistical analysis. Furthermore, publication bias favouring positive studies is an unfortunate fact. RCTs are the current “gold standard” in evidence-based medicine.
Randomized clinical trials (RCTs)
There is a dearth of reliable prospective controlled clinical data regarding the safety and effectiveness of RBC transfusion and there are surprising gaps, despite the 142 related RCTs published up to 2009 (Wilkinson et al, 2011). Eleven RCTs evaluated RBC storage times with a median of 35 patients (range 10 – 237) and only 2 RCTs addressed clinical outcome, such as postoperative bleeding (Wasser et al, 1989) and mortality in a trial published subsequently (Fergusson et al, 2012). Physiological parameters, rather than mortality, were the primary outcomes in most of the completed RCTs (Table II). None of these RCTs detected any effect of RBC storage time on clinical outcomes.
Table II.
Completed randomized controlled trials without an effect of red blood cell storage time on outcome in different clinical settings
| Reference | Patients
|
Primary outcome parameters | Trial registration and acronym | ||
|---|---|---|---|---|---|
| Treatment arm (n) | Control arm (n) | Clinical setting | |||
| Wasser et al, (1989) | 118 | 119 | Cardiac surgery | Postoperative bleeding | |
| Schulman et al, (2002) | 8 | 9 | Trauma | Mortality, infectious complications, respiratory failure | |
| Fernandes et al, (2001) | 10 | 5 | ICU with sepsis | Oxygen consumption, gastric mucosal pH change | |
| Walsh et al, (2004) | 10 | 12 | ICU with mechanical ventilation | gastric mucosal pH change, gastric to arterial PaCO2 gap | |
| Hebert et al, (2005) | 26 | 31 | ICU | Mortality, infections, thrombotic events, ischaemic stroke | |
| Fernandes da Cunha et al, (2005) | 26 | 26 | Premature infants <1.5 kg | Donor exposure, (not powered for) mortality | |
| 2005–2007, unpublished | 30 | 30 | Traumatic brain injury | Cerebral oxygen extraction ratio | NCT00141674 * |
| Aubron et al, (2012) | 25 | 26 | ICU | Feasibility study, (not powered for) mortality | |
| Kor et al, (2012) | 50 | 50 | ICU with mechanical ventilation | Pulmonary function, (not powered for) mortality | TRALI2 trial NCT00751322 |
| Heddle et al, (2012) | 309 | 601 | Acute care in-patients | Feasibility study, (not powered for) mortality | INFORM-P trial |
| Fergusson et al, (2012) | 188 | 189 | Premature infants <1.25 kg | Mortality, necrotizing enterocolitis, retinopathy, bronchopulmonary dysplasia, intraventricular haemorrhage | ARIPI trial NCT00326924 ISRCTN65939658 |
| Yürük et al, (2013) | 10 | 10 | Haematology, out-patients | Sublingual microcirculation | |
recruiting completed in 2007, results not published. ICU, intensive care unit; PaCO2, partial pressure of carbon dioxide in arterial blood.
The first of the larger RCTs addressing mortality as the primary outcome was recently completed (Fergusson et al, 2012). The use of fresh RBCs compared with standard blood bank practice did not improve major neonatal morbidities, such as necrotizing enterocolitis, retinopathy of prematurity, bronchopulmonary dysplasia, intraventricular haemorrhage, or death, in premature, very low birth weight infants requiring a transfusion. However, the average duration of RBC storage in the standard of care group (14.6 days) does not reflect the average RBC storage in the US (18 days) and certainly not the practice of some centres that routinely store RBCs for 21 days or longer (Fernandes da Cunha et al, 2005). The 5 large ongoing RCTs (Table III) use mortality or multiple organ dysfunction as primary outcomes and study fresh RBC versus standard of care. The patient cohorts studied will be important for the applicability of the results and include all acute care inpatients, critically ill patients in adult intensive care units (ICUs) and patients with complex cardiac surgery. The results of these large RCTs are not expected for several years. In the meantime, standards of practice must rely on evidence from other sources.
Table III.
Ongoing randomized controlled trials assessing the effect of red blood cell storage time on clinical outcomes
| Official title or Acronym | Patients
|
Primary outcome | Country | Years | Trial registration | |
|---|---|---|---|---|---|---|
| Accrual goal (n) | Setting | |||||
| ABLE | 2,510 | ICU | all cause mortality at day 90 | Canada 23 locations | 2008–2013 completed* | ISRCTN44878718 |
| Red Cell Storage Duration and Outcomes in Cardiac Surgery | 2,800 | All cardiopulmonary bypass patients | mortality at day 30 post-surgery | USA single centre | 2007–2014 recruiting | NCT00458783 |
| RECESS | 1,696 | Scheduled complex cardiac surgery | MODS | USA 26 locations | 2010–2013 recruiting | NCT00991341 |
| INFORM | 24,400 | Acute care inpatients | in-hospital mortality | Canada, USA, Australia | 2012–2014 recruiting | ISRCTN08118744 |
| TRANSFUSE | 5,000 | ICU excluding cardiac surgery | mortality at day 90 | Australia, New Zealand, Finland | 2012–2016 recruiting |
NCT01638416 ACTRN12612000453886 |
recruiting completed, publication of results pending. ICU, intensive care unit; MODS, multiple organ dysfunction score.
RCT design considerations
The power of an RCT may be smaller than anticipated, if qualitative properties of RBCs differ among the participating institutions. Such variations of RBC quality, other than storage time, may be caused by differing shelf life for identical additive solutions (Table I); different leucocyte removal procedures; the lack of leucocyte removal; gamma irradiation of RBCs or other small, seemingly innocuous variances in preparation techniques and handling procedures. Confounding factors need to be evaluated, particularly for multi-centre and multi-national RCT consortia, such as INFORM and TRANSFUSE (Table III).
An informative theoretical study (Pereira, 2013) simulated the possible outcomes of hypothetical RCTs comprising 2000 patients each. The experimental (≤8, ≤10, <14 and ≤14 days) and control arms (2–42, >14, >20 and ≥21 days) of the 4 RCTs were modelled after actual study designs (Koch et al, 2008; Lacroix et al, 2011; Steiner et al, 2010); NCT00458783). Five different temporal patterns linking clinical outcomes with the RBC storage lesion were examined (Fig. 1). The power of the modelled RCTs consistently exceeded 80%, a limit often deemed desirable for RCTs, for one temporal pattern only (Fig. 1, model 1). It is sobering to note that surprisingly low study powers, often below 20%, are more the rule than the exception for the other temporal patterns (Fig. 1, models 2 to 5). While these limitations may be impossible to overcome, common pitfalls can be avoided (van de Watering, 2011).
Fig. 1.
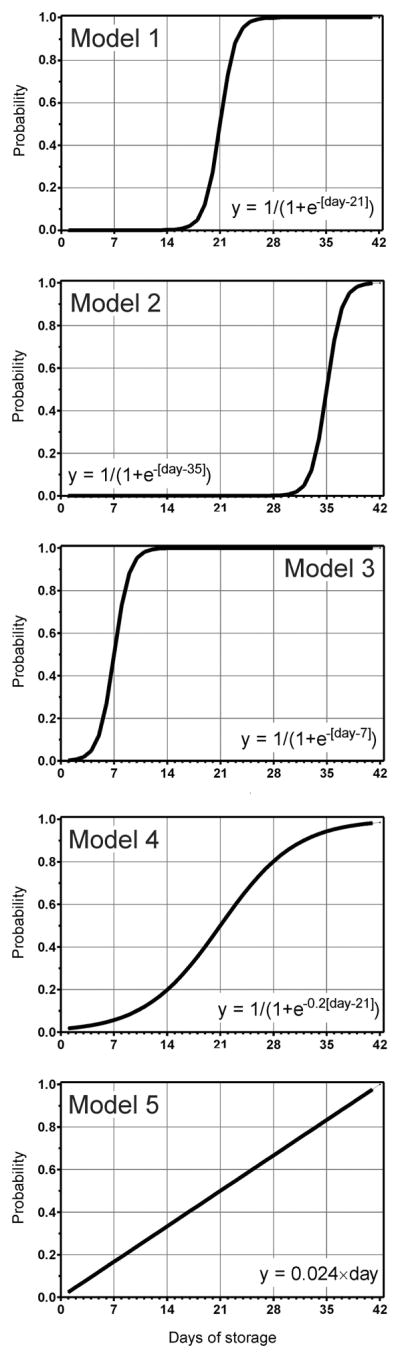
Possible temporal patterns connecting red blood cell (RBC) storage time with outcome. The hypothetical models represent 5 different temporal patterns linking the RBC storage time with the probability of adverse clinical outcomes. Modified and reprinted from: Pereira, A. (2013) Will clinical studies elucidate the connection between the length of storage of transfused red blood cells and clinical outcomes? An analysis based on the simulation of randomized controlled trials. Transfusion, 53, 34–40. with permission from Wiley.
Pilot studies are conducted to assess feasibility, acceptability and blinding to aid in the design of large, pragmatic RCTs with a transfusion medicine topic, such as RBC storage (Hebert et al, 2005; Bennett-Guerrero et al, 2009; Aubron et al, 2012; Heddle et al, 2012). Patients can be randomized to a fresh RBC group, which is commonly perceived as the improved treatment arm. Patients should not be treated with old RBC by design, and the control group is typically standard-issue RBC (oldest in inventory). With the possible exception of chronic and neonatal transfusions, standard of care is “first in, first out” and the oldest available ABO identical RBC unit is transfused. This ethically imperative constraint restricts the design options and can limit the power of any RCT, because any adverse effect conveyed by the oldest RBCs only (e.g. model 2 in Fig. 1) may be missed, when such RBCs are hardly occurring in the standard of care arm.
An accepted frequent deviation from standard of care is the use of the oldest ABO compatible RBCs, which is ABO antigen compatible, but not identical, with the recipient’s ABO blood group. This deviation may affect recipients of blood group B and AB more often than those of blood group A, while recipients of blood group O, of course, can be transfused only with ABO identical RBCs. These discrepancies are not trivial and ABO blood group differences among the study arms should be carefully evaluated (Frenzel et al, 2008; Dzik, 2008; Middelburg et al, 2013) if they are unavoidable. The storage times of the standard inventory practice units would vary, depending on the ABO and Rh type and the vagaries of the blood supply (Steiner et al, 2010; Cheng et al, 2010; Middelburg et al, 2013).
If the combined results of RCTs should not favour fresh RBCs (or old RBCs), clinical differences may exist that are too small to be discerned within the scope of the clinical studies but still important enough to be of public health concern, for example an excess mortality of less than 5% (Edgren et al, 2010). The decision on an acceptable RBC storage period is unlikely to rest on RCTs alone and should include other clinical and non-clinical evidence. A comparative effectiveness research (CER) approach could be pursued, particularly if the ongoing RCTs end inconclusively (Klein, 2012; Blajchman et al, 2012; Klein & Natanson, 2012). In contrast to double-blinded RCTs of efficacy, CER compare two different but accepted standard practices. CER studies are often referred to as large, simple pragmatic trials and should allow enrollment of large cohorts (>10,000 patients) within a short time. Our decisions should also draw on basic principles and the understanding derived from pre-clinical studies with volunteers, small and large animal studies in vivo studies, and in vitro experiments.
Pre-clinical studies with volunteers
The effect of fresh-versus-old RBCs on physiological parameters can be assessed reliably and safely by transfusing autologous RBCs to healthy volunteers (Table IV). Such studies, unlike clinical trials, permit maximal separation of the age of stored RBCs. No differences were found for cognitive and pulmonary function or hyperaemia in three different volunteer studies. The informative study by Hod et al, (2011) documented highly significant increases of serum iron and transferrin saturation at 4 h after transfusion of older RBCs. Ferritin concentrations increased from baseline only after transfusion of older RBCs. While non-transferrin-bound iron concentration was not significantly increased after fresh RBCs, it progressively increased until 4 h after transfusion of older RBCs. The ongoing haemolysis during RBC storage may explain the significantly increased serum total bilirubin peaking at 4 h, which correlated with a peak of unconjugated bilirubin for some volunteers and a small, but significant, rise in serum conjugated bilirubin. While the results from volunteers raise few concerns, the volumes transfused are small and the recipients healthy so the effects may be quite different in critically ill patients. The exact study details, such as RBC preparation and volumes transfused, need to be considered when extrapolating from these convincing data.
Table IV.
Assessment of the effects of RBC storage time on various physiological parameters in healthy volunteers
| Reference | Autologous RBC in crossover design
|
RBC characteristics
|
Outcome parameters | Summary of results | Country | Trial registration | ||
|---|---|---|---|---|---|---|---|---|
| Volun-teers (n) | RBC (n) | RBC storage time | Leuco-reduction | |||||
| Weiskopf et al, (2006) | 9 | 2 | 3.4 h versus 23 days (median) | No | Anaemia-induced cognitive dysfunction | No difference in reversal of dysfunction | USA | |
| Hod et al, (2011) | 14 | 2 | 3 to 7 days versus 40 to 42 days | Yes | Iron, extravascular haemolysis, metabolism parameters, inflammation, bacterial growth in vitro | Significant changes in iron and extravasular haemolysis parameters only | USA | NCT01319552 |
| Berra et al, (2012) | 9 | 1 | 3 days versus 40 days | Yes | Reactive hyperaemia index (reactive hyperperfusion) | No difference in hyperaemia index | USA | |
| Weiskopf et al, (2012) | 35 | 2 | 1.7 h versus 24.5 days | Yes | Pulmonary function (gas exchange variables) | Equivalent slight declines in variables | USA | |
RBC, red blood cell.
Small and large animal in vivo studies
Animal studies can be designed to address specific physiological questions and mechanisms that could not be done using volunteers. Such studies are particularly important for understanding the underlying mechanism, unlikely to be attained by RCTs, and eventually result in the formulation of improved RBC units. The results of these studies may contribute to the design of RCTs as much as they complement the results of RCTs. There are benefits and limitations for RBC transfusion in models (Simonova et al, 2013) of small animals, such as mice (Gilson et al, 2009), rat (d’Almeida et al, 2000), guinea pig (Baek et al, 2012) and cat (Wardrop, 1995), as well as larger animals, such as dog (Wardrop et al, 1997) and sheep (Simonova et al, 2013). Animal studies may be the only way to test the hypothesis that old RBCs affect outcome by a “late” temporal pattern (Fig. 1, model 2) or in few critically ill patients, such as those with severe bacterial pneumonia.
Murine
Mouse and human RBCs show a progressive decline in survival depending on storage time (Gilson et al, 2009), unlike the precipitous loss of viability reported for rat RBC (d’Almeida et al, 2000). Non-transferrin-bound iron concentration and acute tissue iron deposition were increased after transfusion of old mouse RBCs, which may initiate inflammation not seen after fresh mouse RBCs (Hod et al, 2010). The increased non-transferrin-bound iron was implicated in enhancing bacterial growth in vitro (Hod et al, 2010), which could not be confirmed subsequently in a prospective human study (Hod et al, 2011). Old mouse RBCs induced more cytokines and alloimmunization than did fresh RBCs, which may introduce another variable in RBC storage studies (Hendrickson et al, 2011). In a rat model, 28- and 35-day-old rat RBCs promoted lung oedema (Nicholson et al, 2011); no effect of rat RBC storage on survival was tested. In an ex vivo model with aortic rings from rats, older RBCs inhibited NO-induced vasodilation (Alexander et al, 2013).
Guinea pig
Transfusion of 28-day-old guinea pig RBCs led to intravascular haemolysis, hypertension, vascular injury and kidney dysfunction (Baek et al, 2012). This plasma haemoglobin-driven toxicity could be attenuated by infusion of haptoglobin. The precise mechanism of injury is unknown but may involve the direct toxicity of cell free haemoglobin, iron, scavenging of nitric oxide (NO) or a combination of such mechanisms (Schaer et al, 2013).
Dogs
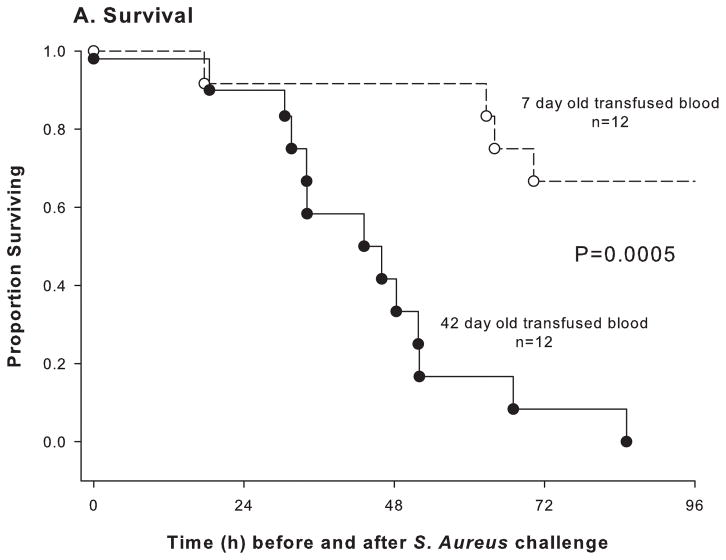
The most striking results of any animal study on old RBCs so far have been observed in canines (Fig. 2): 42-day-old dog RBCs dramatically increased mortality in dogs with experimental Staphylococcus aureus pneumonia (Solomon et al, 2013). This first RCT used standard blood bank techniques and evaluated if RBCs transfused at the end of the storage period increase mortality. Canines with pneumonia were treated like critically ill patients with bacterial pneumonia in an intensive care unit. RBCs were collected and prepared by an FDA-licensed canine blood bank mimicking procedures and technologies used for human RBCs (Wardrop, 1995; Steiner et al, 2010). The bacterial challenge dose was optimized in this validated blinded canine model to account for the effects of multiple transfusions on mortality.
Fig. 2.
Survival curves in Staphylococcus aureus-challenged dogs. Kaplan-Meier plots over the 96 h course of 12 dogs exchange transfused with 42-day-old red blood cells (RBCs; solid circle, solid line) and 12 dogs exchange transfused with 7-day-old RBC (open circle, dashed line). Reprinted from Solomon et al, (2013).
Increased systemic and pulmonary artery pressures indicated that old RBCs were more vasoactive than fresh RBCs (McLaughlin et al, 2013; Badesch et al, 2009). Pulmonary hypertension caused right ventricular dilatation and, by adversely affecting left ventricular filling, resulted in marked tachycardia to maintain cardiac output. Prolonged haemolysis of old RBCs resulted in increased cell-free haemoglobin and decreased haptoglobin. Old RBCs caused a steady rise in NO consumption capability of plasma for days (Jeffers et al, 2006), indicating the presence of oxyhaemoglobin, the vasoactive reduced form of haemoglobin, known to scavenge NO (Reiter et al, 2002; Minneci et al, 2005; Rother et al, 2005; Yu et al, 2008; Hu et al, 2010; Donadee et al, 2011). Prolonged exposure to oxyhaemoglobin resulted in ischaemic vascular damage at the site of tissue injury in the lung, causing gas exchange abnormalities, pulmonary arterial hypertension and an increased risk of death.
Non-transferrin-bound and labile iron, the toxic iron moiety, were elevated only during transfusion, but not associated with survival (Solomon et al, 2013). NO scavenging and in vivo haemolysis were augmented after transfusion of old RBCs, which appeared to result in an excess of non-transferrin-bound and labile iron, but worsened outcome only in the presence of an established infection (Wang et al, 2013). The availability of iron, circulating 8 to 12 h after bacterial challenge, may have promoted bacterial growth contributing to mortality. Washing RBCs before transfusion had a significantly different effect on canine survival, multiple organ injury and plasma iron and cell-free haemoglobin concentrations, depending on the age of stored RBCs. Washing old RBCs improved canine survival, whereas washing seemed to damage fresh RBCs and increased canine mortality (Cortes-Puch et al, 2014). Studies in human volunteers, mouse, guinea pig and dog hinted consistently to significant increases in cell-free haemoglobin and iron, plausibly caused by an increased haemolysis of the old, more fragile RBCs.
Sheep
Similar to the TRALI2 study in humans (Kor et al, 2012), which examined transfusion-related acute lung injury (TRALI) and was expanded to study RBC storage effects, a TRALI study prompted the development of a sheep model (Tung et al, 2011; Simonova et al, 2013). Transfusion of 40-day-old autologous RBCs increased pulmonary vascular resistance and pressure, which was worsened by an NO synthase inhibitor and ameliorated by NO inhalation (Baron et al, 2012). These observations were compatible with the NO and free iron findings in dogs. As TRALI is thought to be caused predominantly by plasma and not by cellular components, initially the effect of the supernatant from stored human RBCs was tested in sheep (Tung et al, 2012). Such human supernatants decreased arterial pressure and cardiac output in lipopolysaccharide-primed sheep more than did supernatants from stored human platelet components (Tung et al, 2012). Ovine RBCs stored for 35 to 42 days induced pulmonary arterial hypertension but not TRALI (Fung et al, 2013).
It is not clear to what extent rodent, canine, sheep or, for that matter, any non-primate models can mimic the variety of human clinical circumstances, and whether canine or other RBCs are equivalent to similarly stored human RBCs (McCullough, 2013). Even if transferable, the current canine study results may apply only to severely ill patients with infections and RBCs at the very end of the shelf life permitted by the additive solutions used.
RBC storage
RBC storage lesion
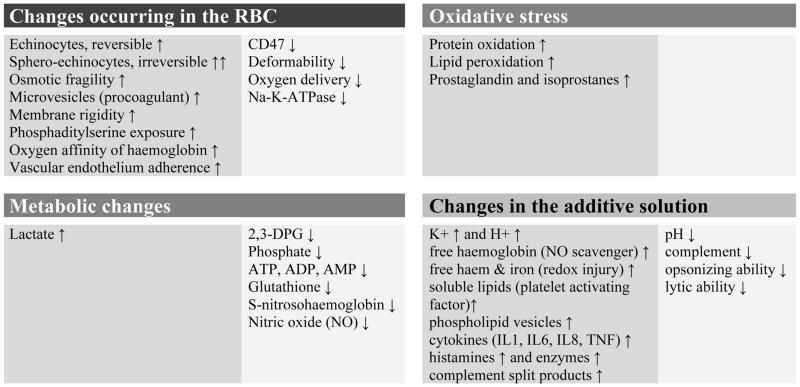
The original concern regarding RBC storage time and maximal acceptable shelf life for patient care evolved from observations of the RBC storage lesion(s) (Fig. 3) (Dzik, 2008; Zubair, 2010; Blajchman et al, 2010; Zimring et al, 2011; Pavenski et al, 2012; Koch et al, 2013). Although the RBCs rest at +4 °C and are not agitated for the duration of their shelf life, RBCs are exposed to oxidative stress and undergo metabolic changes. The accumulating metabolites, altered proteins and particles, such as microvesicles, may be released into the supernatant (Klein et al, 2007). The damage affecting the membrane and cytoskeleton results in altered deformability and shape and compromises RBC integrity (Frank et al, 2013).
Fig. 3.
The RBC storage lesion. The changes affect the RBC cytoplasm and more so the membrane, parts of which are shed into the supernatant as microvesicles.
RBC, red blood cell; 2,3-DPG, 2,3-diphosphoglycerate; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; IL, interleukin; TNF, tumour necrosis factor.
The dynamics of the processes vary widely: Some effects occur within a few hours of blood donation, whereas other effects represent the accumulation of changes that occur over days and weeks. As a consequence, it is conceivable that there may be examples of determinants for the RBC storage lesion, which are represented by any of the temporal patterns discussed previously. Further, donor-related biological variability should be expected in the kinetics of change as schematically shown for the 5 models (Fig. 1). The temporal pattern linking clinical outcomes with the critical determinant of the RBC storage lesion may also vary among patients and their different clinical conditions.
Data from dogs, sheep and mice support the hypotheses that NO depletion and free iron release, occurring as a consequence of the RBC storage lesion, contribute to the detrimental effects of old RBCs (Solomon et al, 2013; Wang et al, 2013; Baron et al, 2012; Hod et al, 2010). However given the extent of the changes in the RBC, other mechanisms may well be involved.
Ex vivo and in vitro studies
51Cr-labelling, widely used to study RBC survival in vivo and a measure generally required by licensing authorities, has intrinsic variability in addition to the donor-to-donor biological variability (Dumont & AuBuchon, 2008). Flow cytometry allows demonstration of a nearly identical long-term RBC survival in vivo for fresh and old RBCs, once a removal-prone fraction in the older RBCs is eliminated (Luten et al, 2008). Haemoglobin increments at 48 h did not differ between fresh and old RBCs in an RCT involving 10 patients (Wallis et al, 2005), while 2,3-diphosphoglycerate (2,3-DPG) was significantly lower up to 48 h after transfusion of the old RBCs. No clinical efficacy studies are required or recommended for assessing RBC quality in the US and European Union (EU).
Types of RBC preparation and shelf life
Many RBC preparation and storage conditions have been studied to enhance RBC survival in the storage container, as in vitro changes are considered to predict clinical outcomes (Hess, 2012; Orlina & Josephson, 1969). Even within the past decade, RBC preparation and storage solutions have been modified (Greening et al, 2010). Although the general approach becomes standardized, many small technical variations add to the inherent complexity of the RBC blood product (Hess et al, 2009). As a biological product from human volunteer blood donors, its source cannot be standardized. Unlike small molecule drugs, each RBC unit is considered a “lot” or a “batch.” A substantial donor-to-donor variability in RBC storage, haemolysis and survival has been recognized since the 1960s with non-normal distribution, in that the RBCs of approximately two-thirds of the donors store better than the mean; there are donors whose RBC store exceptionally well (Hess, 2012; Dumont & AuBuchon, 2008). Biomarkers to predict suboptimal storage and to identify “poor storers” do not currently exist, but would be extremely valuable (Francis et al, 2013).
Whole blood is typically collected into a sterile plastic bag and anticoagulated with a defined concentration of citrate, for example a CPD (citrate, phosphate, dextrose) solution. The packed RBC are then transferred into a second bag with an “additive solution”, for which SAGM (sodium, adenine, glucose, mannitol, 376 mOsm/l) is commonly used and approved for a 42-day RBC shelf life (Hess, 2006). Approval of additive solutions depends on in vivo RBC recovery and survival as well as on measurement of analytes thought critical for RBC survival. The most important RBC analytes are adenosine 5′-triphosphate (ATP) concentration and 2,3-DPG recovery rate (Dumont & AuBuchon, 2008). RBCs are regulated by drug acts, biological medicine regulations (UK Legislation, 2005), pharmaceutical affairs laws and national standards (UK Blood Transfusion & Tissue Transplantation Services, 2013), which vary by country. In the UK, the evaluation of new procedures to produce RBCs is detailed (chapter 8.2, UK Blood Transfusion & Tissue Transplantation Services, 2013). Commonly accepted RBC recovery and haemolysis rates after 12 weeks storage may be achieved with non-standard solutions, which are not yet approved (Hess, 2006; Hess et al, 2003).
Leucocyte removal during RBC preparation is commonplace in Europe and Canada, but not standard in the US. RBC preparations differ in many regards that can be easily delineated in vitro and may be of clinical relevance (Bordin et al, 1994; Willy et al, 2000). Irradiation, which is used for specific indications in some institutions or universally for other hospitals and national blood supplies, is well documented to damage the RBC membrane. The plasticizer diethylhexyl phthalate (DEHP), which leaches from the polyvinyl chloride (PVC) bags that are widely used for RBCs, affects the RBC membrane stability and limits membrane loss by microvesiculation beyond 3 weeks storage (Rock et al, 1984; AuBuchon et al, 1988; Hess, 2006). While plasticizer reduces haemolysis, a critical parameter for approval of new blood container devices (Hill et al, 2001), possible DEHP toxicity remains a concern, particularly for premature infants (Luban et al, 2006).
The overall quality of a RBC blood product may actually improve during the first few days of storage. Bacterial contamination during the donation process may be cleared within the first few hours of storage of whole blood or RBC fraction, before the leucocytes are removed. Storage at +4 °C for several days may reduce the risk of spirochetes, some cell-associated viruses and mononuclear cells responsible for GvHD (Sakakibara & Juji, 1986). Whereas no claim has been made that RBC quality improves during storage beyond the first few days, an abundance of in vitro evidence implicates refrigerated storage as increasingly detrimental to RBC quality (Chin-Yee et al, 1997; Tinmouth & Chin-Yee, 2001; Solheim et al, 2004).
Novel RBC preservatives
Evaluation of additive solutions in vitro by studying the proteome indicated an aging process that was not addressed by either of the two additive solutions commonly used in North America and Europe (D’Amici et al, 2012). Anaerobic storage, which was shown to improve in vivo RBC survival in a crossover RCT involving 8 volunteers has been proposed as a novel approach (Dumont et al, 2009). RBC stored under argon are not exposed to the oxidative processes thought to induce structural damage; molecular iron released from haem could not catalyse reactions with oxygen as its substrate (Yoshida & Shevkoplyas, 2010). Research opportunities in optimizing RBC storage, such as new additive solution, anaerobic storage or addition of free radical scavengers, have recently been summarized (Wagner et al, 2014).
Clinical guidelines
Guidelines in some healthcare facilities require transfusing fresher RBCs to vulnerable patient groups, including fetuses for intrauterine transfusion, premature infants, all newborns, or patients undergoing cardiac surgery. Some chronically transfused patients, such as those with thalassaemia and sickle cell disease or haematological malignancies, receive fresh RBCs to extend transfusion intervals and reduce the total RBC volume transfused with its concomitant iron burden.
In the US, the guidance by the American Association of Blood Banks (AABB) Standards (Carson, 2012) and recommendation by the AABB Technical Manual do not restrict RBC storage time for any adult patient group (Nester & AuBuchon, 2011); RBCs less than 7 days old are recommended for intrauterine transfusions (Kennedy, 2011). In the UK, RBCs must be used by the end of day 5 for intrauterine (chapter 7.22) and exchange transfusions (chapter 7.24), and for large-volume transfusion of neonates and infants under 1 year (chapter 7.26, UK Blood Transfusion & Tissue Transplantation Services, 2013). In Germany, RBCs should be as fresh as possible, but less than 7 days old, for intrauterine transfusion and exchange transfusion of preterm babies and neonates, and must not be older than 28 days for large-volume transfusion of neonates (chapter 4.4.2) (Bundesärztekammer & Paul-Ehrlich-Institut, 2010). Although these guidelines have long traditions, seem to be intuitive and could prove to be beneficial for patients, evidence has been lacking or inconclusive that outcome is affected by the RBC storage time in any patient group.
Besides perinatal patients, no cohorts of vulnerable, critically ill patients are defined at the national levels to preferably receive fresher RBCs.
Inadvertent consequences of using fresher RBCs
A transfusion policy with fresher RBCs could curtail the blood supply in critical situations for some patients. Adverse effects of transfusion are strictly monitored, but adverse effects caused by an inadequate blood supply are notoriously difficult to gauge at the aggregate level (Flegel, 2012). Patients in critical need could be harmed by either the lack of RBCs or by availability of blood that is not ideally matched by blood group. Such a circumstance would reverse any benefit gained from slightly “fresher” RBCs for the majority of patients.
Transfusing fresher RBCs might benefit some patient cohorts, such as severely ill patients in intensive care units or patients with infections, while offering a limited or no benefit for the majority of patients. If fresher RBCs were generally transfused without restricting the RBC shelf life, older RBCs might be sequestered to certain patients, such as those with trauma and extensive surgical procedures requiring large amounts of RBCs; paradoxically, these patients might benefit most from fresher RBCs. Some neonatologists avoid transfusing the freshest RBCs by using aliquots drawn from a single donor when limiting donor and infectious disease exposure is considered the superior objective; fresh RBCs and limiting donor exposure may not be conflicting goals, if the shelf life is 28 days or shorter (Fernandes da Cunha, 2005).
A review of the reports on heavily transfused patients concluded that several studies indicate that fresh RBC might be associated with adverse outcomes (van de Watering, 2013). In a retrospective study, the same group reported an almost 2-fold increase in mortality rate after transfusion of fresh compared to older RBC (Middelburg et al, 2013). The proposition that RBCs with a shelf life of 5 to 21 days may have any negative effect, worse than older RBCs, is not intuitive and has not been widely shared.
Implementing and maintaining any policy of fresher RBCs may require major operational changes to the current practice of collection, storage and transfusion. Recruiting additional blood donors, increasing RBC outdating or more complex inventory management will increase costs. For changes that involve major practical and organizational consequences, such as reduction of the RBC storage time, a multi-tiered, stepwise and carefully monitored approach seems prudent.
Conclusion
Retrospective and prospective observational studies and a meta-analysis of 21 studies reporting on mortality have suggested that fresher RBCs may benefit defined patient groups or all patients, whereas the only completed RCT failed to show improved outcomes in premature, very low-birth weight infants transfused with fresher RBCs (Fergusson et al, 2012). By one calculation, between 97 and 69,428 patients needed to be treated with exclusively fresh RBC to save one life (Wang et al, 2012). Studies in several animal models report evidence of organ toxicity and increased mortality when older RBCs are transfused. The several ongoing RCTs should allow narrowing the range of the actual risk, which would determine the need for changes, if any, in current transfusion practice. The effect of optimal RBCs storage may well vary according to different clinical settings.
If transfusion of old RBCs is shown to pose clinically significant risks, current systems of blood collection, storage and transfusion would need to be revised and would probably entail considerable operational and financial impacts. It would be premature to jeopardize the currently available blood supply based on retrospective clinical studies with marginal results or equivocal evidence (Dzik, 2008). Neither should fresher blood be withheld from small, well defined, particularly vulnerable patient groups based on negative RCTs that are not designed to discriminate among clinically relevant and important difference in outcome (Flegel, 2012). However, at this time blood collectors can address the storage lesion and clinicians can model systems and investigate strategies to use fresher blood in order to prepare for the possibility of a shortened RBC shelf life.
The decision regarding appropriate RBC storage time at transfusion currently relies on clinical judgment derived by combining the evidence from in vitro, in vivo, pre-clinical and prospective observational studies while several RCTs are in progress. A safe and practical approach might be to resort to the oldest RBCs, such as those within the last 7 days of the approved shelf life, only if there are national shortages. Resolution of the importance of the storage lesion may require large pragmatic clinical trials. In the meantime, it seems prudent to make plans and test models for using fresher RBCs without disrupting blood availability while the evidence for or against is being gathered.
Acknowledgments
We thank Arturo Pereira for sharing graphic data files used in Fig. 1; Stephen Thomas, Pieter van der Meer, Dirk de Korte, Thomas Schulzki, Yoshihiko Tani, Qing Chen, Noorah Salman Almarry, Judith Chapman, Joan Vidal Cid, Gregory A Denomme, Beat Frey, Catherine Hyland, Sanmukh Joshi, Wolfgang R Mayr, Kenneth E Nollet, France Noizat-Pirenne, Mouna Ouchari, Pairaya Rujirojindakul, Addisalem Taye-Makuria, Claudio Velati, Christof Weinstock, and Silvano Wendel for communicating RBC shelf life data.
All authors performed literature research and designed the review format; WAF analysed literature and data; WAF wrote and CN and HGK edited the manuscript.
This work was supported by the Intramural Research Program of the NIH Clinical Center.
Footnotes
Conflict of interest disclosure: The author declares no competing interests relevant to this article.
Statement of Disclaimer: The opinions expressed in this review are those of the authors and do not necessarily represent the views or policies of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
References
- Alexander JT, Eli-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, Sutliff RL, Roback JD. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53:2619–2628. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubron C, Syres G, Nichol A, Bailey M, Board J, Magrin G, Murray L, Presneill J, Sutton J, Vallance S, Morrison S, Bellomo R, Cooper DJ. A pilot feasibility trial of allocation of freshest available red blood cells versus standard care in critically ill patients. Transfusion. 2012;52:1196–1202. doi: 10.1111/j.1537-2995.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- Aubron C, Nichol A, Cooper DJ, Bellomo R. Age of red blood cells and transfusion in critically ill patients. Ann Intensive Care. 2013;3:2–3. doi: 10.1186/2110-5820-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AuBuchon JP, Estep TN, Davey RJ. The effect of the plasticizer di-2-ethylhexyl phthalate on the survival of stored RBCs. Blood. 1988;71:448–452. [PubMed] [Google Scholar]
- Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. Journal of Clinical Investigation. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DM, Yu B, Lei C, Bagchi A, Beloiartsev A, Stowell CP, Steinbicker AU, Malhotra R, Bloch KD, Zapol WM. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116:637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Guerrero E, Stafford-Smith M, Waweru PM, Bredehoeft SJ, Campbell ML, Haley NR, Phillips-Bute B, Newman MF, Bandarenko N. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49:1375–1383. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- Berra L, Coppadoro A, Yu B, Lei C, Spagnolli E, Steinbicker AU, Bloch KD, Lin T, Sammy FY, Warren HS, Fernandez BO, Feelisch M, Dzik WH, Stowell CP, Zapol WM. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, West C. The storage of hard-packed red blood cells in citrate-phosphate-dextrose (CPD) and CPD-adenine (CPDA-1) Blood. 1979;54:280–284. [PubMed] [Google Scholar]
- Blajchman MA, Glynn SA, Josephson CD, Kleinman SH. Clinical trial opportunities in Transfusion Medicine: proceedings of a National Heart, Lung, and Blood Institute State-of-the-Science Symposium. Transfusion Medicine Reviews. 2010;24:259–285. doi: 10.1016/j.tmrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Blajchman MA, Carson JL, Eikelboom JW, Heddle NM, Lacroix J, Lauer MS, Platt R, Tilley B, Triulzi D, Vickers AJ, Yusuf S, Glynn S, Mondoro TH, Wagner E. The role of comparative effectiveness research in transfusion medicine clinical trials: proceedings of a National Heart, Lung, and Blood Institute workshop. Transfusion. 2012;52:1363–1378. doi: 10.1111/j.1537-2995.2012.03640.x. [DOI] [PubMed] [Google Scholar]
- Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–1721. [PubMed] [Google Scholar]
- Bundesärztekammer & Paul-Ehrlich-Institut. Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie) gemäß §§ 12 und 18 des Transfusionsgesetzes (TFG) Bundesanzeiger. 2010;62:3–36. [Google Scholar]
- Carson TH. Standards for Blood Banks and Transfusion Services. 28. AABB; Bethesda MD: 2012. [Google Scholar]
- Cheng CK, Trethewey D, Sadek I. Comprehensive survey of red blood cell unit life cycle at a large teaching institution in eastern Canada. Transfusion. 2010;50:160–165. doi: 10.1111/j.1537-2995.2009.02375.x. [DOI] [PubMed] [Google Scholar]
- Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfusion Sciences. 1997;18:447–458. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- Cortes-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, Feng J, Kania T, Bellavia L, Sinchar D, Perlegas A, Solomon MA, Kelley WE, Popovsky MA, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanon C. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014 doi: 10.1182/blood-2013-11-539353. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfusion Medicine. 2000;10:291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- D’Amici GM, Mirasole C, D’Alessandro A, Yoshida T, Dumont LJ, Zolla L. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10:s46–s54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49:458–464. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzik W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med. 2008;18:260–265. doi: 10.1111/j.1365-3148.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Edgren G, Kamper-Jorgensen M, Eloranta S, Rostgaard K, Custer B, Ullum H, Murphy EL, Busch MP, Reilly M, Melbye M, Hjalgrim H, Nyren O. Duration of red blood cell storage and survival of transfused patients (CME) Transfusion. 2010;50:1185–1195. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelboom JW, Cook RJ, Liu Y, Heddle NM. Duration of red cell storage before transfusion and in-hospital mortality. American Heart Journal. 2010;159:737–743. doi: 10.1016/j.ahj.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Fergusson DA, Hebert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. Journal of the American Medical Association. 2012:1–9. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- Fernandes CJ, Jr, Akamine N, De Marco FV, De Souza JA, Lagudis S, Knobel E. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Crit Care. 2001;5:362–367. doi: 10.1186/cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes da Cunha DH, Nunes Dos Santos AM, Kopelman BI, Areco KN, Guinsburg R, de Araujo PC, Chiba AK, Kuwano ST, Terzian CC, Bordin JO. Transfusions of CPDA-1 red blood cells stored for up to 28 days decrease donor exposures in very low-birth-weight premature infants. Transfusion Medicine. 2005;15:467–473. doi: 10.1111/j.1365-3148.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- Flegel WA. Fresh blood for transfusion: how old is too old for red blood cell units? [Editorial] Blood Transfusion. 2012;10:247–251. doi: 10.2450/2012.0105-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, Spitalnik SL. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sanguinis. 2013;105:271–282. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SM, Abazyan B, Ono M, Hogue CW, Cohen DB, Berkowitz DE, Ness PM, Barodka VM. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116:975–981. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenzel T, Sibrowski W, Westphal M. Red-cell storage and complications of cardiac surgery. The New England Journal of Medicine. 2008;358:2841–2842. [PubMed] [Google Scholar]
- Fung YL, Tung JP, Foley SR, Simonova G, Thom O, Staib A, Collier J, Dunster KR, Solano C, Shekar K, Chew MS, Fraser JF. Stored blood transfusion induces transient pulmonary arterial hypertension without impairing coagulation in an ovine model of nontraumatic haemorrhage. Vox Sanguinis. 2013;105:150–158. doi: 10.1111/vox.12032. [DOI] [PubMed] [Google Scholar]
- Gauvin F, Spinella PC, Lacroix J, Choker G, Ducruet T, Karam O, Hebert PC, Hutchison JS, Hume HA, Tucci M. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion. 2010;50:1902–1913. doi: 10.1111/j.1537-2995.2010.02661.x. [DOI] [PubMed] [Google Scholar]
- Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening DW, Glenister KM, Sparrow RL, Simpson RJ. International blood collection and storage: clinical use of blood products. J Proteomics. 2010;73:386–395. doi: 10.1016/j.jprot.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Hebert PC, Chin-Yee I, Fergusson D, Blajchman M, Martineau R, Clinch J, Olberg B. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. table. [DOI] [PubMed] [Google Scholar]
- Heddle NM, Cook RJ, Arnold DM, Crowther MA, Warkentin TE, Webert KE, Hirsh J, Barty RL, Liu Y, Lester C, Eikelboom JW. The effect of blood storage duration on in-hospital mortality: a randomized controlled pilot feasibility trial. Transfusion. 2012;52:1203–1212. doi: 10.1111/j.1537-2995.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson JE, Hod EA, Hudson KE, Spitalnik SL, Zimring JC. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–2702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JR. An update on solutions for red cell storage. Vox Sanguinis. 2006;91:13–19. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- Hess JR. Scientific problems in the regulation of red blood cell products. Transfusion. 2012;52:1827–1835. doi: 10.1111/j.1537-2995.2011.03511.x. [DOI] [PubMed] [Google Scholar]
- Hess JR, Hill HR, Oliver CK, Lippert LE, Rugg N, Joines AD, Gormas JF, Pratt PG, Silverstein EB, Greenwalt TJ. Twelve-week RBC storage. Transfusion. 2003;43:867–872. doi: 10.1046/j.1537-2995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Hess JR, Rugg N, Joines AD, Gormas JF, Pratt PG, Silberstein EB, Greenwalt TJ. Buffering and dilution in red blood cell storage. Transfusion. 2006;46:50–54. doi: 10.1111/j.1537-2995.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- Hess JR, Sparrow RL, van der Meer PF, Acker JP, Cardigan RA, Devine DV. Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion. 2009;49:2599–2603. doi: 10.1111/j.1537-2995.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- Hill HR, Oliver CK, Lippert LE, Greenwalt TJ, Hess JR. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sanguinis. 2001;81:161–166. doi: 10.1046/j.1423-0410.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, la-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hod EA, Brittenham GM, Billote GB, Francis RO, Ginzburg YZ, Hendrickson JE, Jhang J, Schwartz J, Sharma S, Sheth S, Sireci AN, Stephens HL, Stotler BA, Wojczyk BS, Zimring JC, Spitalnik SL. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogman CF. In vitro haemolysis of SAG-M and CPD-A blood units. Vox Sanguinis. 1985;48:126–127. doi: 10.1111/j.1423-0410.1985.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Hogman CF, Hedlund K, Zetterstrom H. Clinical usefulness of red cells preserved in protein-poor mediums. The New England Journal of Medicine. 1978;299:1377–1382. doi: 10.1056/NEJM197812212992502. [DOI] [PubMed] [Google Scholar]
- Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, Bronson RT, Halperin JA, Loscalzo J, Qin X. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116:1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz DR, Zhao Z, Koyama T, May AK, Bernard GR, Bastarache JA, Young PP, Ware LB. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care. 2013;3:33. doi: 10.1186/2110-5820-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam O, Tucci M, Bateman ST, Ducruet T, Spinella PC, Randolph AG, Lacroix J. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care. 2010;14:R57. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MS. Perinatal issues in transfusion practice. In: Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical Manual. AABB; Bethesda: 2011. pp. 631–644. [Google Scholar]
- Klein HG. Immunomodulatory aspects of transfusion: a once and future risk? Anesthesiology. 1999;91:861–865. doi: 10.1097/00000542-199909000-00040. [DOI] [PubMed] [Google Scholar]
- Klein HG. Comparative effectiveness research: welcome to the real world. Transfusion. 2012;52:1162–1164. doi: 10.1111/j.1537-2995.2012.03692.x. [DOI] [PubMed] [Google Scholar]
- Klein HG. Should blood be an essential medicine? The New England Journal of Medicine. 2013;368:199–201. doi: 10.1056/NEJMp1213134. [DOI] [PubMed] [Google Scholar]
- Klein HG, Natanson C. Red blood cell transfusion. Annals of Internal Medicine. 2012;157:753–754. doi: 10.7326/0003-4819-157-10-201211200-00019. [DOI] [PubMed] [Google Scholar]
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–426. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. The New England Journal of Medicine. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Koch CG, Figueroa PI, Li L, Sabik JF, III, Mihaljevic T, Blackstone EH. Red Blood Cell Storage: How Long Is Too Long? Annals of Thoracical Surgery. 2013;96:1894–1899. doi: 10.1016/j.athoracsur.2013.05.116. [DOI] [PubMed] [Google Scholar]
- Kor DJ, Kashyap R, Weiskopf RB, Wilson GA, van Buskirk CM, Winters JL, Malinchoc M, Hubmayr RD, Gajic O. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: a randomized clinical trial. Am J Respir Crit Care Med. 2012;185:842–850. doi: 10.1164/rccm.201107-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfusion Medicine Reviews. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Leal-Noval SR, Munoz-Gomez M, rellano-Orden V, Marin-Caballos A, maya-Villar R, Marin A, Puppo-Moreno A, Ferrandiz-Millon C, Flores-Cordero JM, Murillo-Cabezas F. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Critical Care Medicine. 2008;36:1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- Lelubre C, Vincent JL. Relationship between red cell storage duration and outcomes in adults receiving red cell transfusions: a systematic review. Crit Care. 2013;17:R66. doi: 10.1186/cc12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban N, Rais-Bahrami K, Short B. I want to say one word to you - just one word - “plastics”. Transfusion. 2006;46:503–506. doi: 10.1111/j.1537-2995.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- McCullough J. Red cell storage: does duration matter? Blood. 2013;121:1491–1492. doi: 10.1182/blood-2013-01-475988. [DOI] [PubMed] [Google Scholar]
- McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, Tapson VF, Channick RN, Rubin LJ. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest. 2013;143:324–332. doi: 10.1378/chest.11-3060. [DOI] [PubMed] [Google Scholar]
- Middelburg RA, van de Watering LM, Briet E, van der Bom JG. Storage time of red blood cells and mortality of transfusion recipients. Transfusion Medicine Reviews. 2013;27:36–43. doi: 10.1016/j.tmrv.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. Journal of Clinical Investigation. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroff G, Holme S, Keegan T, Heaton A. Storage of ADSOL-preserved red cells at 2.5 and 5.5 degrees C: comparable retention of in vitro properties. Vox Sanguinis. 1990;59:136–139. doi: 10.1111/j.1423-0410.1990.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–785. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–964. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- Nester T, AuBuchon JP. Hemotherapy decisions and their outcomes. In: Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical Manual. AABB; Bethesda: 2011. pp. 571–615. [Google Scholar]
- Nicholson SE, Johnson RA, Craig T, Myers JG, Durante W, Stewart RM, Johnson FK. Transfusion-related acute lung injury in a rat model of trauma-hemorrhage. J Trauma. 2011;70:466–471. doi: 10.1097/TA.0b013e3182032584. [DOI] [PubMed] [Google Scholar]
- Orlina AR, Josephson AM. Comparative viability of blood stored in ACD and CPD. Transfusion. 1969;9:62–69. doi: 10.1111/j.1537-2995.1969.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Otani T, Oki K, Akino M, Tamura S, Naito Y, Homma C, Ikeda H, Sumita S. Effects of helicopter transport on red blood cell components. Blood Transfus. 2012;10:78–86. doi: 10.2450/2011.0029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenski K, Saidenberg E, Lavoie M, Tokessy M, Branch DR. Red blood cell storage lesions and related transfusion issues: a Canadian Blood Services research and development symposium. Transfusion Medicine Reviews. 2012;26:68–84. doi: 10.1016/j.tmrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Pereira A. Will clinical studies elucidate the connection between the length of storage of transfused red blood cells and clinical outcomes? An analysis based on the simulation of randomized controlled trials. Transfusion. 2013;53:34–40. doi: 10.1111/j.1537-2995.2012.03656.x. [DOI] [PubMed] [Google Scholar]
- Pettila V, Westbrook AJ, Nichol AD, Bailey MJ, Wood EM, Syres G, Phillips LE, Street A, French C, Murray L, Orford N, Santamaria JD, Bellomo R, Cooper DJ. Age of red blood cells and mortality in the critically ill. Crit Care. 2011;15:R116. doi: 10.1186/cc10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Janssen C, Fretz EB, Berry B, Chase AJ, Siega AD, Carere RG, Fung A, Simkus G, Klinke WP, Hilton JD. Red blood cell storage duration and mortality in patients undergoing percutaneous coronary intervention. American Heart Journal. 2010;159:876–881. doi: 10.1016/j.ahj.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Rock G, Tocchi M, Ganz PR, Tackaberry ES. Incorporation of plasticizer into red cells during storage. Transfusion. 1984;24:493–498. doi: 10.1046/j.1537-2995.1984.24685066808.x. [DOI] [PubMed] [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. Journal of the American Medical Association. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- Saager L, Turan A, Dalton JE, Figueroa PI, Sessler DI, Kurz A. Erythrocyte storage duration is not associated with increased mortality in noncardiac surgical patients: a retrospective analysis of 6,994 patients. Anesthesiology. 2013;118:51–58. doi: 10.1097/ALN.0b013e3182746ba4. [DOI] [PubMed] [Google Scholar]
- Sakakibara T, Juji T. Post-transfusion graft-versus-host disease after open heart surgery [Letter] Lancet. 1986;2:1099. doi: 10.1016/s0140-6736(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman CI, Nathe K, Brown M, Cohn SM. Impact of age of transfused blood in the trauma patient. J Trauma. 2002;52:1224–1225. doi: 10.1097/00005373-200206000-00036. [DOI] [PubMed] [Google Scholar]
- Simonova G, Tung JP, Fraser JF, Do HL, Staib A, Chew MS, Dunster KR, Glenister KM, Jackson DE, Fung YL. A comprehensive ovine model of blood transfusion. Vox Sanguinis. 2013:10. doi: 10.1111/vox.12076. in press. [DOI] [PubMed] [Google Scholar]
- Solheim BG, Flesland O, Seghatchian J, Brosstad F. Clinical implications of red blood cell and platelet storage lesions: an overview. Transfus Apher Sci. 2004;31:185–189. doi: 10.1016/j.transci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, Solomon MA, Alimchandani M, Quezado M, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10:s7–s11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinella PC, Carroll CL, Staff I, Gross R, Mc QJ, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, Triulzi D, Stowell CP. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43:107–116. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfusion Medicine Reviews. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- Tung JP, Fung YL, Nataatmadja M, Colebourne KI, Esmaeel HM, Wilson K, Barnett AG, Wood P, Silliman CC, Fraser JF. A novel in vivo ovine model of transfusion-related acute lung injury (TRALI) Vox Sanguinis. 2011;100:219–230. doi: 10.1111/j.1423-0410.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- Tung JP, Fraser JF, Nataatmadja M, Colebourne KI, Barnett AG, Glenister KM, Zhou AY, Wood P, Silliman CC, Fung YL. Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI) Crit Care. 2012;16:R19. doi: 10.1186/cc11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Blood Transfusion & Tissue Transplantation Services. Guidelines for the Blood Transfusion Services in the United Kingdom. 8. The Stationary Office; London: 2013. [Google Scholar]
- UK Legislation. Statutory Instruments 2005. 50. 2005. The Blood Safety and Quality Regulations 2005. HEALTH AND SAFETY. © Crown copyright 2005. [Google Scholar]
- Vamvakas EC. Why have meta-analyses of randomized controlled trials of the association between non-white-blood-cell-reduced allogeneic blood transfusion and postoperative infection produced discordant results? Vox Sanguinis. 2007;93:196–207. doi: 10.1111/j.1423-0410.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- van Buskirk CM, Kashyap R, Thakur SJ, Murray DL, Bryant SC, Winters JL, Stubbs JR, Gajic O. Red blood cell storage has no impact on clinical outcome in critically ill patients. (Abstract) Transfusion. 2009;49:10A. [Google Scholar]
- van de Watering LM. Pitfalls in the current published observational literature on the effects of red blood cell storage. Transfusion. 2011;51:1847–1854. doi: 10.1111/j.1537-2995.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- van de Watering LM. Effects of red blood cell storage in heavily transfused patients. Current Opinion in Anaesthesiology. 2013;26:204–207. doi: 10.1097/ACO.0b013e32835e7408. [DOI] [PubMed] [Google Scholar]
- van de Watering LM, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–1718. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- van Straten AH, Soliman Hamad MA, van Zundert AA, Martens EJ, ter Woorst JF, de Wolf AM, Scharnhorst V. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. Journal of Thoracic and Cardiovascular Surgery. 2011;141:231–237. doi: 10.1016/j.jtcvs.2010.02.059. [DOI] [PubMed] [Google Scholar]
- Wagner SJ, Glynn SA, Welniak L. Research opportunities in optimizing storage of red blood cell products. Transfusion. 2014 doi: 10.1111/trf.12244. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JP, Wells AW, Babb RG, Stainsby D, Hamilton PJ. Effect of storage age of transfused blood on 48 hour Hb increment and recovery of 2,3 DPG in haematology patients. (Abstract) British Journal of Haematology. 2005;129:1. [Google Scholar]
- Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, McClelland DB. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Critical Care Medicine. 2004;32:364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- Wang D, Cortes-Puch I, Sun J, Solomon SB, Kanias T, Remy KE, Feng J, Alimchandani M, Quezado M, Helms CC, Perlegas A, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion. 2013 doi: 10.1111/trf.12607. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrop KJ. Selection of anticoagulant-preservatives for canine and feline blood storage. Vet Clin North Am Small Anim Pract. 1995;25:1263–1276. doi: 10.1016/s0195-5616(95)50153-6. [DOI] [PubMed] [Google Scholar]
- Wardrop KJ, Tucker RL, Mugnai K. Evaluation of canine red blood cells stored in a saline, adenine, and glucose solution for 35 days. JVet Intern Med. 1997;11:5–8. doi: 10.1111/j.1939-1676.1997.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Wasser MN, Houbiers JG, D’Amaro J, Hermans J, Huysmans HA, van Konijnenburg GC, Brand A. The effect of fresh versus stored blood on post-operative bleeding after coronary bypass surgery: a prospective randomized study. British Journal of Haematology. 1989;72:81–84. doi: 10.1111/j.1365-2141.1989.tb07656.x. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G, Jr, Griffin RL, Huynh VQ, Cherry SA, III, Marques MB, Reiff DA, Kerby JD, Rue LWI., II Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008a;65:279–282. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G, Jr, Marques MB, Cherry SA, III, Reiff DA, Kerby JD, Rue LWI., II Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008b;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., III Duration of red cell storage influences mortality after trauma. J Trauma. 2010;69:1427–1431. doi: 10.1097/TA.0b013e3181fa0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–920. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Feiner J, Toy P, Twiford J, Shimabukuro D, Lieberman J, Looney MR, Lowell CA, Gropper MA. Fresh and stored red blood cell transfusion equivalently induce subclinical pulmonary gas exchange deficit in normal humans. Anesth Analg. 2012;114:511–519. doi: 10.1213/ANE.0b013e318241fcd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KL, Brunskill SJ, Doree C, Hopewell S, Stanworth S, Murphy MF, Hyde C. The clinical effects of red blood cell transfusions: an overview of the randomized controlled trials evidence base. Transfusion Medicine Reviews. 2011;25:145–155. doi: 10.1016/j.tmrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Willy C, Reithmeier W, Kuhlmann WD, Gerngross H, Flegel WA. Leukocyte depletion of red cell components prevents exposure of transfusion recipients to neutrophil elastase. Vox Sanguinis. 2000;78:19–27. doi: 10.1159/000031144. [DOI] [PubMed] [Google Scholar]
- Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Annals of Thoracical Surgery. 2008;86:554–559. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–236. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yürük K, Milstein DM, Bezemer R, Bartels SA, Biemond BJ, Ince C. Transfusion of banked red blood cells and the effects on hemorrheology and microvascular hemodynamics in anemic hematology outpatients. Transfusion. 2013;53:1346–1352. doi: 10.1111/j.1537-2995.2012.03905.x. [DOI] [PubMed] [Google Scholar]
- Zehnder L, Schulzki T, Goede JS, Hayes J, Reinhart WH. Erythrocyte storage in hypertonic (SAGM) or isotonic (PAGGSM) conservation medium: influence on cell properties. Vox Sanguinis. 2008;95:280–287. doi: 10.1111/j.1423-0410.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- Zimring JC, Welniak L, Semple JW, Ness PM, Slichter sJ, Spitalnik SL. Current problems and future directions of transfusion-induced alloimmunization: summary of an NHLBI working group. Transfusion. 2011;51:435–441. doi: 10.1111/j.1537-2995.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair AC. Clinical impact of blood storage lesions. American Journal of Hematology. 2010;85:117–122. doi: 10.1002/ajh.21599. [DOI] [PubMed] [Google Scholar]