Abstract
Background
Dissociated strabismus complex (DSC) is an enigmatic form of strabismus that includes dissociated vertical deviation (DVD) and dissociated horizontal deviation (DHD). We employed magnetic resonance imaging (MRI) to evaluate the extraocular muscles in DSC.
Methods
We studied 5 patients with DSC and mean age of 25 years (range, 12–42 years), and 15 age-matched, orthotropic control subjects. All patients had DVD; 4 also had DHD. We employed high-resolution, surface coil MRI with thin, 2 mm slices and central target fixation. Volumes of the rectus and superior oblique muscles in the region 12 mm posterior to 4 mm anterior to the globe–optic nerve junction were measured in quasi-coronal planes in central gaze.
Results
Patients with DSC had no structural abnormalities of rectus muscles or rectus pulleys or the superior oblique muscle but exhibited modest, statistically significant increased volume of all rectus muscles ranging from 20% for medial rectus to 9% for lateral rectus (P < 0.05).
Conclusions
DSC includes various combinations of sursumduction, excycloduction, and abduction not conforming to Hering’s law. We have found modest generalized enlargement of all rectus muscles. DSC is associated with generalized rectus extraocular muscle hypertrophy in the absence of other orbital abnormalities.
Dissociated strabismus complex (DSC) has been recognized for more than a century.1 A slow elevation of the nonfixating eye is the most recognizable feature, but torsional or abducting components can predominate in one or both eyes. Latent or manifest-latent nystagmus and subnormal binocular vision are also associated. The most prominent early descriptions are from Bielschowsky.2 Dissociated vertical deviation (DVD), a term attributed to Raab,3 is the most commonly used clinical label for this type of strabismus, although it is not fully descriptive. There is no consensus on the etiology of DSC. Intricate mechanisms have been offered to explain DSC. Guyton and colleagues4 proposed that DVD is related to exaggerated vertical vergence, produced primarily by the oblique extraocular muscles damping cyclovertical nystagmus during visual attention. Brodsky5 proposed that DVD is a counter-rolling phenomenon related to the dorsal light reflex of fish.
Recently Demer and Dushyanth6 have demonstrated the utility of magnetic resonance imaging (MRI) as a noninvasive method to measure volume, contractility, and pulley position of extraocular muscles during normal and pathological eye movements.6 The present study aimed to determine by MRI the volume of each rectus and superior oblique muscle in DSC patients to clarify the pathophysiology of DSC as well as rectus pulley positions.
Subjects and Methods
A retrospective evaluation was performed of data prospectively collected by an ongoing study of strabismus conducted continuously since 1990 at the same institution. At the time of analysis in late 2015, a total of 647 patients recruited at the Stein Eye Institute, University of California, Los Angeles, had undergone high-resolution orbital MRI for strabismus. Subjects prospectively provided written informed consent according to protocol approved by the UCLA Institutional Review Board and compliant with the requirements of the US Health Insurance Portability and Accountability Act of 1996. Control subjects were recruited by advertising and underwent complete examinations to verify normal corrected vision, normal ocular versions, orthotropia in all gaze positions, and normal stereopsis of 40 arcsec by Titmus testing. Subjects with DSC underwent complete sensorimotor evaluation and MRI. Data were obtained from 15 orthotropic adult volunteers (mean age, 21 ± 2.2 [standard deviation]; range, 19–27 years; 10 females) and 5 patients with DSC (mean age, 25 ± 11; range, 12–42 years; 3 females). Clinically all patients had DVD, and 4 of these had DHD. Two cases had a torsional component, and nystagmus was evident in 1 case only. Patients with DSC had associated esotropia in 4 cases and exotropia in one. Prior to the MRI study, 2 cases had undergone both inferior oblique weakening surgery and superior rectus recession for correction of DVD in two separate sessions, 1 case underwent only surgery on the inferior oblique, and the remaining 3 cases had undergone no previous surgery for DVD.
It is assumed that DVD is always bilateral, even if not evident clinically. Two cases exhibited bilateral asymmetrical DVD. The remaining 3 cases were clinically unilateral. However, under the assumption that DVD is a bilateral condition, both orbits of all affected patients were analyzed.
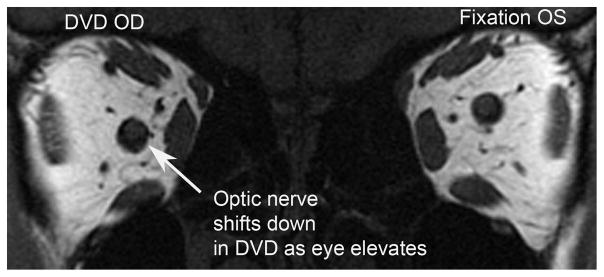
A 1.5-T MRI scanner (Signa; General Electric, Milwaukee, WI) was used with T1- or T2-weighted fast spin-echo pulse sequences.6 Technical aspects, described elsewhere,7 include dual-phased surface coil array (Medical Advances, Milwaukee, WI) and a fixation target consisting of a fine, afocal, fiber optic light that does not elicit vergence. We have verified the absence of vergence in scores of nonstrabismic subjects tested with these fiber optic targets. We have also conducted multiple series of experiments in separate, different groups of 8–12 normal subjects each employing various accommodative binocular stimuli to induce and measure convergence,7 divergence (unpublished data), and vertical vergence.21 Resolution of MRI images was the same for all subjects. High-resolution (312 μm) quasi-coronal images (Figure 1) of 2 mm thickness and matrix of 256 × 256 perpendicular to the long axis of the orbit were obtained in central gaze for each eye fixing monocularly on a centered target so that eye position was unaffected by strabismus angle. Interocular differences of individual patients were not analyzed. Imaging of the nonfixating orbit of each subject verified the presence of strabismus during the MRI procedure (Figure 1), although the nonfixating orbit was not otherwise analyzed quantitatively. Only the fixating orbit in central gaze was analyzed quantitatively to avoid confounding by eye position, which influences maximum muscle cross section and volume. Image analysis was similar to published methods.8,9
FIG 1.
Coronal magnetic resonance imaging (MRI) of both eyes during left eye (OS) fixation with dissociated vertical deviation (DVD) manifesting in the right eye (OD).
Investigators did not have a strong prior hypothesis regarding expected effect of DSC. MRIs were quantified using ImageJ (Rasband WS. ImageJ, US National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/, 1997–2009, accessed February 2009). Each rectus muscle and the superior oblique muscle cross-sectional area was determined automatically after manually outlining it (Figure 2). Horizontal rectus muscle cross sections were determined in 9 contiguous, 2 mm thick midorbital image planes ranging 12 mm posterior to 4 mm anterior to the globe–optic nerve junction (Figure 3). The plane of the globe–optic nerve junction served as the reference plane zero, with image planes more posterior designated negative and anteriorly positive. Volumes of extraocular muscles in the region were determined by multiplying the summed cross sections by 2 mm image plane thickness. The inferior oblique muscle cannot be measured reliably in quasi-coronal image planes. The location of the pulley of each of the four rectus muscles of the patients was measured in oculocentric coordinates using the technique, and compared with normative data, of Clark, Miller, and Demer.10 Because images were available only in central gaze where inflections in rectus muscle paths are present to indicate anteroposterior position of the pulleys, horizontal and vertical pulley coordinates of pulleys in patients with DSC were measured at the normal anteroposterior locations of normal pulleys.10 The measurement was done by the same author (GZR) for the cases and control, repeating all measurements twice over a period of 6 months to ensure accuracy.
FIG 2.
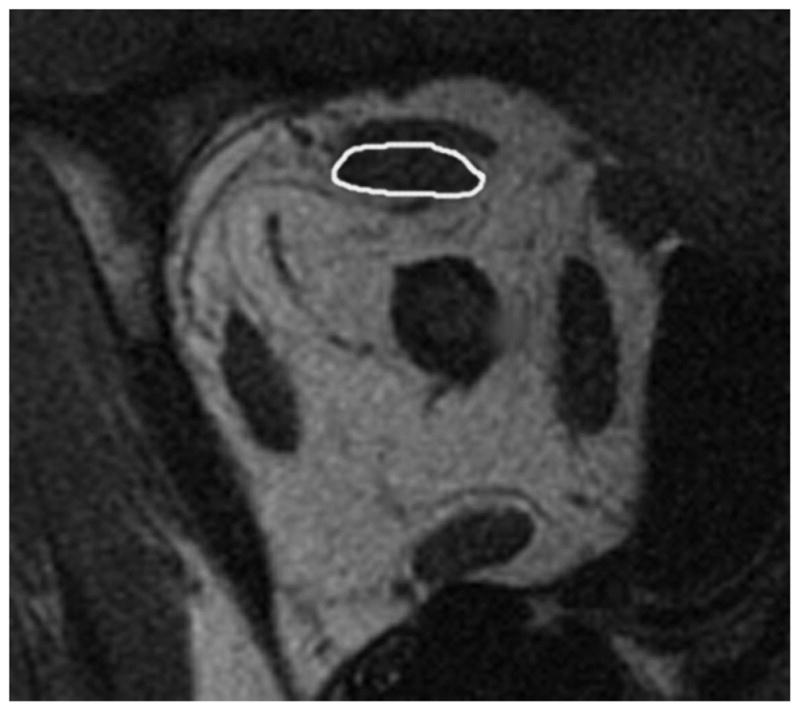
Quasi-coronal plane MRI of right orbit showing superior rectus outlined in image plane zero.
FIG 3.
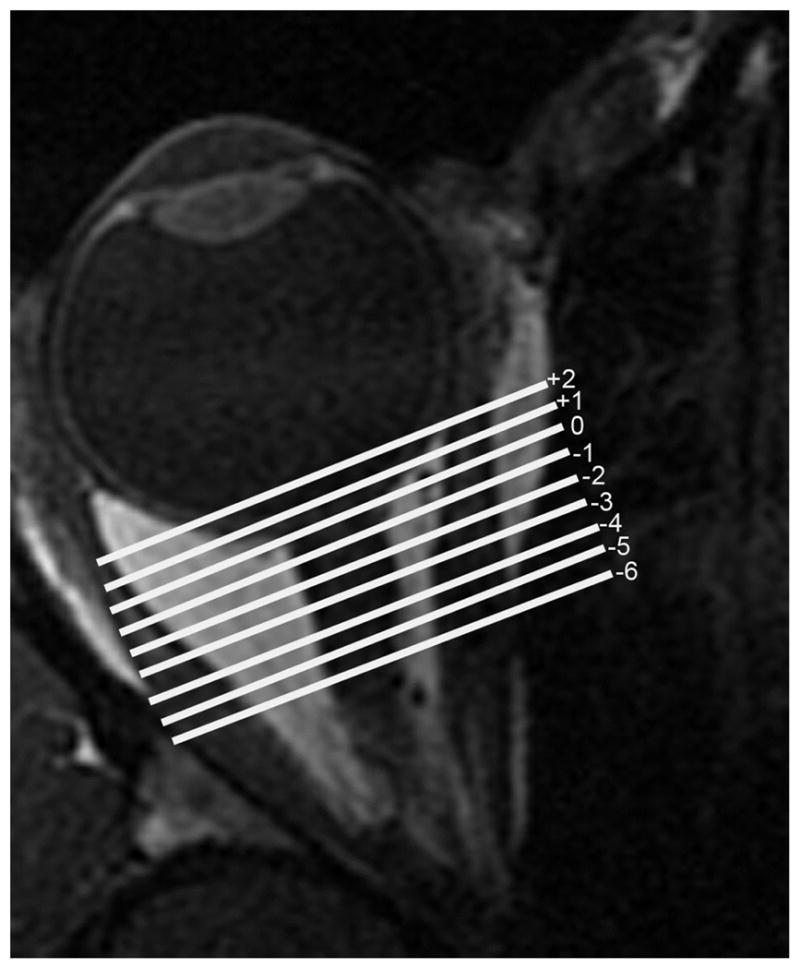
Axial MRI of a right orbit showing the numbered quasi-coronal planes employed in analysis of extraocular muscle cross sections.
Data were analyzed statistically (Prism software; Graph Pad, San Diego GA) using the independent, two-tailed t test with P values <0.05 considered significant.
Results
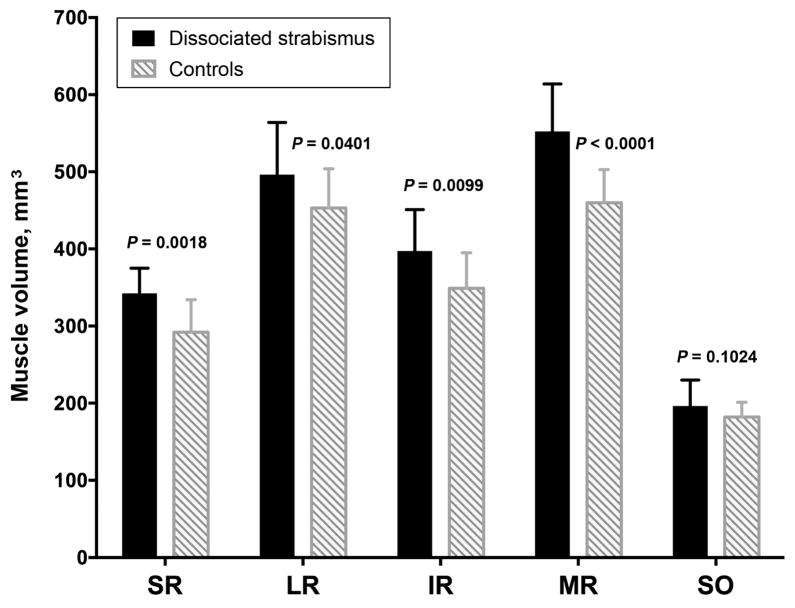
High-quality MRI was obtained in each subject, clearly demonstrating the rectus and superior oblique muscles in quasi-coronal planes. The configurations and sizes of all extraocular muscles in patients with DSC appeared similar to normal. However, midorbital partial volumes of all of the rectus muscles were modestly but significantly larger in patients with DSC than in normal subjects, with differences ranging from 20% for medial rectus muscle to 9% for lateral rectus muscle (P < 0.05). These data are plotted in Figure 4. Volume of the superior oblique muscle, however, did not significantly differ from normal in patients with DSC (P= 0.102). Table 1 illustrates the absence of any obvious relationship between laterality and variations in individual muscle volumes.
FIG 4.
Midorbital volumes of extraocular muscles in patients with dissociated strabismus complex (DSC), and controls. IR, inferior rectus muscle; LR, lateral rectus muscle; MR, medial rectus muscle; SO, superior oblique muscle; SR, superior rectus muscle.
Table 1.
Extraocular muscle volume in clinically dissociated vertical deviationa
| Muscle volume, mm3
|
|||
|---|---|---|---|
| Case 2 (DVD left eye) | Case 4 (DVD right eye) | Case 5 (DVD right eye) | |
| Right eye | |||
| Superior rectus | 413 | 392 | 318 |
| Lateral rectus | 575 | 423 | 480 |
| Inferior rectus | 445 | 400 | 389 |
| Medial rectus | 627 | 495 | 527.932 |
| Superior oblique | 299 | 260 | 213 |
| Left eye | |||
| Superior rectus | 489 | 361 | 360 |
| Lateral rectus | 562 | 496 | 480 |
| Inferior rectus | 464 | 410 | 367 |
| Medial rectus | 645 | 531 | 454 |
| Superior oblique | 212 | 187 | 213 |
Predominant laterality of the dissociated vertical deviation (DVD) was not systematically related to unilateral differences in extraocular muscle volume.
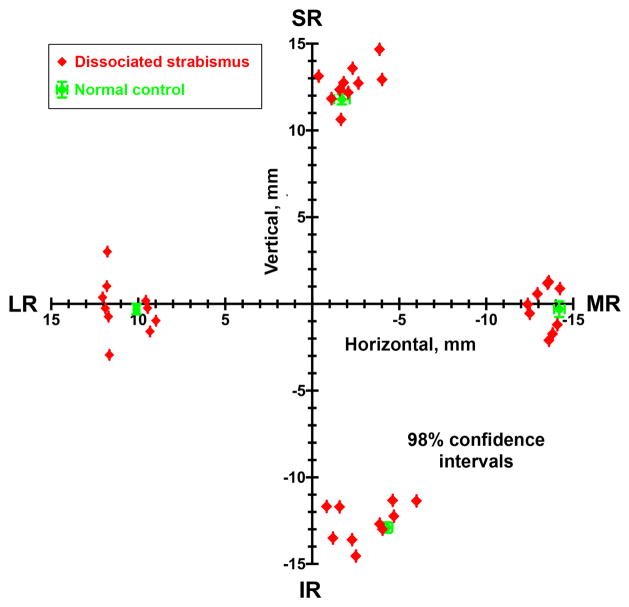
The horizontal and vertical coronal plane coordinates of the four rectus pulleys of each orbit of each patient with DSC are plotted individually as red symbols in Figure 5, along with the 95% confidence intervals for normal pulley coordinates from the normative data of Clark and Demer.10 Note that although the rectus pulley coordinates for the rectus pulleys in DSC are scattered, the means of their distributions do not differ statistically from normal pulley coordinates. Confidence intervals for pulley coordinates for patients with DSC (not shown) overlapped completely with normal coordinates for all four rectus pulleys, indicating absence of statistical differences in pulley positions in DSC.
FIG 5.
Rectus pulley locations in coronal plane oculocentric coordinates in patients with DSC, compared with published norms and 98% confidence intervals from Clark, Miller, and Demer10 that are nearly the same size as plot symbols. On average, pulley coordinates in DSC (red) did not differ significantly from normal, although was appreciable interindividual variability. Abbreviations as in Figure 4.
Discussion
DSC comprises various combinations of sursumduction, excycloduction, and abduction that violate Hering’s law. In this study MRI demonstrated modest generalized enlargement of all rectus extraocular muscles without any particular pattern that would account for the characteristic eye movements. This muscle enlargement cannot be attributed to position of the scanned eye or by strabismus angle because each orbit was imaged during central monocular fixation. Although the outlining of muscles was not masked, all analysis subsequent to that step was digital. Also, these results are not predicted by any prior hypothesis concerning extraocular muscle volume in DSC, so they could not result from biased expectation.
It was surprising that volumes of all rectus muscles are significantly increased in DSC despite absence of thyroid ophthalmopathy or any other hypertrophic pathology. Although 4 patients in the current study had undergone previous strabismus surgery for esotropia and DVD, prior strabismus surgery cannot explain this finding: it has been elsewhere demonstrated that strabismus surgery does not alter extraocular muscle volume or maximum cross section.11 Recession of a muscle does not remove tissues and has a negligible effect on volume.11 Whatever effect previous strabismus surgery might have had on the muscles studied here, it could not have directly produced the observed size increases.
Schoeff and colleagues11 noted supernormal volume of the medial rectus muscle and a trend to enlargement of the lateral rectus muscle in patients with esotropia. Generalized hypertrophy may be related to abnormal discharge patterns of motor neurons innervating the extraocular muscles previously demonstrated in monkeys with DSC and is consistent with similar changes in both agonist and antagonist muscles in experimentally induced strabismus in monkeys.12,13 The cause of this enlargement is unknown. However, Altick and colleagues16 showed that 25% muscle-specific genes in humans are significantly down-regulated in surgically excised extraocular muscles.14 An analogous change in gene expression might underlie the increase in muscle volume.
The eye movements of DVD have previously been described as a form of vertical vergence,15–17 and technically this interpretation is correct. Enright18 has argued that the bulk of vertical fusional vergence is implemented by the superior oblique muscle, without important contribution by the inferior oblique. Van Rijn and Collewijn19 refuted Enright’s hypothesis that only the superior oblique muscle is the primary mediator of vertical vergence. Guyton and colleagues4 argued that the cyclovertical component of DVD may help stabilize the fixing eye by damping vertical nystagmus, while the accompanying hypertropia is a consequent but undesirable side effect. On the other hand, recent MRI studies of compartmental contractility in extraocular muscles during prism-induced vertical vergence have demonstrated differential compartmental function of multiple oblique and rectus muscles.20 For instance, the lateral compartment of inferior rectus muscle contracted vigorously during infraduction in response to monocular viewing through ipsilateral base-up prism.20
Das has demonstrated vergence-related neuronal activity within the supraoculomotor area of monkeys reared with alternative monocular occlusion who developed horizontal strabismus associated with DSC.21 This supports the concept that DSC could be due to altered supranuclear innervation, as long ago hypothesized by Bielschowsky.2
Limitations of the current study include the relatively small sample size of patients. Also, extraocular muscle contractility was not measured. Although MRI investigations have demonstrated that congenital horizontal or vertical rectus heterotopy can mimic both overelevation in adduction and V pattern strabismus,22 the present study provides evidence that neither selective size abnormalities of the rectus or superior oblique muscles, nor rectus pulley heterotopy, are causes or results of DSC. Our knowledge of the secondary effects of DSC on the size of the extraocular muscles remains incomplete, and further studies of DSC remain warranted.
Acknowledgments
Grant support: U.S. Public Health Service grant EY008313 and an Unrestricted Grant from Research to Prevent Blindness. J. Demer holds the Arthur Rosenbaum Chair of Pediatric Ophthalmology.
Footnotes
Presented at the 42nd Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Vancouver, British Columbia, April 6–10, 2016.
FDA disclosure: Investigational surface coils were used in MRI imaging.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stevens GT. On double vertical strabismus. Ann Ocularist. 1895;113:225. [Google Scholar]
- 2.Bielschowsky A. Die einseitigen und gegensinnigen (“dissoziierten”) Vertikalbewegungen der Augen. Graefes Arch Ophthalmol. 1930;125:493. [Google Scholar]
- 3.Raab EL. Dissociative vertical deviation. J Pediatr Ophthalmol Strabismus. 1970;7:146. doi: 10.3928/0191-3913-19990701-10. [DOI] [PubMed] [Google Scholar]
- 4.Guyton DL, Cheeseman EW, Jr, Ellis FJ, Straumann D, Zee DS. Dissociated vertical deviation: an exaggerated normal eye movement used to damp cyclovertical latent nystagmus. Trans Am Ophthalmol Soc. 1998;96:389–429. [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky MC. Dissociated vertical divergence: a righting reflex gone wrong. Arch Ophthalmol. 1999;117:1216–22. doi: 10.1001/archopht.117.9.1216. [DOI] [PubMed] [Google Scholar]
- 6.Demer JL, Dushyanth A. T2-weighted fast spin-echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. doi: 10.1016/j.jaapos.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–85. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA, Demer JL. Functional morphometry of horizontal rectus extraocular muscles during horizontal ocular duction. Invest Ophthalmol Vis Sci. 2012;53:7375–9. doi: 10.1167/iovs.12-9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129:904–8. doi: 10.1001/archophthalmol.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 11.Schoeff K, Chaudhuri Z, Demer JL. Functional magnetic resonance imaging of horizontal rectus muscles in esotropia. JAAPOS. 2013;17:16–21. doi: 10.1016/j.jaapos.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011;52:6697–705. doi: 10.1167/iovs.11-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narasimhan A, Tychsen L, Poukens V, Demer JL. Horizontal rectus muscle anatomy in naturally and artificially strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:2576–88. doi: 10.1167/iovs.06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altick AL, Feng CY, Schlauch K, Johnson LA, von Bartheld CS. Differences in gene expression between strabismic and normal human extraocular muscles. Invest Ophthalmol Vis Sci. 2012;53:5168–77. doi: 10.1167/iovs.12-9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheeseman EW, Jr, Guyton DL. Vertical fusional vergence. The key to dissociated vertical deviation. Arch Ophthalmol. 1999;117:1188–91. doi: 10.1001/archopht.117.9.1188. [DOI] [PubMed] [Google Scholar]
- 16.Helveston EM. Dissociated vertical deviation: a clinical and laboratory study. Trans Am Ophthalmol Soc. 1980;78:734–79. [PMC free article] [PubMed] [Google Scholar]
- 17.Zubcov AA, Goldstein HP, Reinecke RD. Dissociated vertical deviation (DVD): The saccadic and slow eye movements. Strabismus. 1994;2:1–11. doi: 10.3109/09273979409105048. [DOI] [PubMed] [Google Scholar]
- 18.Enright JT. Unexpected role of the oblique muscles in the human vertical fusional reflex. J Physiol. 1992;451:279–93. doi: 10.1113/jphysiol.1992.sp019164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rijn LJ, Collewijn H. Eye torsion associated with disparity-induced vertical vergence in humans. Vision Res. 1994;34:2307–16. doi: 10.1016/0042-6989(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 20.Demer JL, Clark RA. Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence. J Neurophysiol. 2015;113:2150–63. doi: 10.1152/jn.00871.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das VE. Cells in the supraoculomotor area in monkeys with strabismus show activity related to the strabismus angle. Ann N Y Acad Sci. 2011;1233:85–90. doi: 10.1111/j.1749-6632.2011.06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic rectus muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]