Abstract
Setting: All public health facilities in Bonthe District, rural Sierra Leone.
Objective: To compare, in the periods before and during the Ebola virus disease outbreak, 1) the submission and completeness of monthly human immunodeficiency virus (HIV) reports, and 2) the uptake of HIV testing and care for pregnant women and the general population.
Design: A cross-sectional study using routine programme data.
Results: Of the 627 HIV reports expected in each period, 406 (65%) were submitted in the pre-Ebola period and 376 (60%) during the Ebola outbreak (P = 0.08), of which respectively 318 (78%) and 335 (89%) had complete information (P < 0.001). In the pre-Ebola period, 5012 pregnant women underwent testing for HIV, of whom 25 were HIV-positive, compared to 4254 during the Ebola period, of whom 21 were HIV-positive (P < 0.001). Of those who were HIV-positive, respectively 14 (56%) and 21 (100%) received antiretroviral prophylaxis or antiretroviral therapy (ART) (P < 0.001). In the general population, 5770 persons underwent HIV testing pre-Ebola vs. 3095 in the Ebola period (P < 0.001); of those who tested positive for HIV, respectively 62% (33/53) and 81% (33/41) were started on ART (P = 0.06).
Conclusion: There was suboptimal reporting on HIV/acquired immune-deficiency disease syndrome activities before and during the Ebola virus disease outbreak. HIV testing decreased during the Ebola outbreak, while the uptake of prevention of mother-to-child transmission and ART increased. Pre-emptive actions are needed to maintain the levels of HIV testing in any future outbreak.
Keywords: antiretroviral therapy, PMTCT, pregnant women, SORT IT, operational research
Abstract
Contexte : Toutes les structures de santé publiques du district de Bonthe, Sierra Leone rurale.
Objectif : Au cours des périodes avant et pendant l'épidémie d'Ebola, comparer 1) la soumission et la complétude des rapports mensuels du virus de l'immunodéficience humaine (VIH), et 2) la couverture du test VIH et les soins des femmes enceintes et de la population générale.
Schéma : Étude transversale grâce à des données de routine du programme.
Résultats : Sur les 627 rapports relatifs au VIH attendus pour chaque période, 406 (65%) ont été soumis avant Ebola et 376 (60%) pendant l'épidémie d'Ebola (P = 0,08) ; 318 (78%) des rapports pré Ebola et 335 (89%) des rapports de la période Ebola comportaient des informations complètes (P < 0,001). Dans la période pré-Ebola, 5012 femmes enceintes ont eu un test VIH (dont 25 VIH positives) comparées aux 4254 (dont 21 VIH positives) pendant Ebola (P < 0,001), et parmi les femmes VIH positives, respectivement 14 (56%) et 21 (100%) ont reçu une prophylaxie antirétrovirale ou un traitement antirétroviral (TAR) (P < 0,001). Dans la population générale, 5770 personnes ont été testées pour le VIH avant Ebola et 3095 pendant Ebola (P < 0,001). Parmi les personnes VIH positives, respectivement 62% (33/53) et 81% (33/41) ont mis en route un TAR avant et pendant Ebola (P = 0,06).
Conclusion : Les rapports relatifs aux activités VIH/SIDA (syndrome de l'immunodéficience acquise) avant et après l'épidémie d'Ebola ont été sous-optimaux. La couverture du test VIH a diminué pendant l'épidémie d'Ebola, tandis que la couverture de la prévention de la transmission mère-enfant et TAR a augmenté. Des efforts de prévention sont requis pour maintenir l'utilisation du test VIH dans toute épidémie à venir.
Abstract
Marco de referencia: Todos los establecimientos públicos de atención de salud del distrito Bonthe en una zona rural de Sierra Leona.
Objetivo: Al analizar el período anterior a la epidemia de fiebre hemorrágica del Ébola y el período epidémico, se compararon los siguientes aspectos: 1) la rendición de informes mensuales sobre el virus de la inmunodeficiencia humana (VIH) y su integridad; y 2) la aceptación de las pruebas diagnósticas de la infección por el VIH y la adhesión al tratamiento por parte de las embarazadas y la población general.
Método: Un estudio transversal a partir de los datos corrientes del programa.
Resultados: De los 627 informes sobre el VIH previstos en cada período, antes de la epidemia del Ébola se enviaron 406 informes (65%) y durante la misma 376 (60%; P = 0,08); la información transmitida fue completa en 318 informes anteriores a la epidemia (78%) y en 335 de los informes presentados durante el brote (89%; P < 0,001). Antes de la epidemia del Ébola se practicó la prueba del VIH a 5012 embarazadas (25 resultados positivos), en comparación con 4254 durante la epidemia (21 resultados positivos; P < 0,001); antes del brote, de las pacientes con resultado positivo, 14 recibieron profilaxis o tratamiento con medicamentos antirretrovíricos (TAR) (56%) y 21 pacientes durante epidemia (100%; P < 0,001). En la población general, antes del brote del Ébola se practicó la prueba del VIH a 5770 personas y durante la epidemia a 3095 personas (P < 0,001). De las personas positivas frente al VIH antes del brote, el 62% inició el TAR (33 de 53) y el 81% durante la epidemia (33 de 41; P = 0,06).
Conclusión: Se constató una deficiencia en la notificación de las actividades de atención del VIH/sida antes del brote epidémico y durante el mismo. La realización de pruebas diagnósticas del VIH disminuyó durante la epidemia, pero aumentó la administración del TAR y la prevención de la transmisión madre al niño. Se precisan medidas anticipatorias que permitan conservar la tasa de utilización de la prueba del VIH durante todo episodio epidémico futuro.
Ebola virus disease is a zoonotic, filovirus infection that is part of a group of diseases known as viral haemorrhagic fevers.1 Transmission occurs through close contact with the body fluids of infected patients; the disease can therefore spread rapidly, especially where health systems are fragile and under-resourced.2,3
The 2014 Ebola outbreak in West Africa started in Guinea in December 2013 and spread rapidly to Liberia and Sierra Leone.4 By 3 January 2016, 28 637 cases had been reported in the region, of whom 11 315 were known to have died.5 The extent of the outbreak compelled the World Health Organization (WHO) to declare an international public health emergency in 2014.6 Sierra Leone was the most heavily burdened country, with almost half of all cases.5 The outbreak started in Sierra Leone in May 2014 and was declared over in November 2015, although occasional sporadic cases occurred thereafter. Prior to the Ebola outbreak, the country was struggling to recover from civil war and already had serious deficiencies in its health system and considerable health worker shortages.2,7 The Ebola outbreak adversely affected the quality of health service delivery, and had a major impact on health-seeking behaviour and access to care in the community.8,9
In all three countries in the region, including Sierra Leone, the outbreak led to disruption of the tuberculosis (TB), human immunodeficiency virus/acquired immune-deficiency syndrome (HIV/AIDS) and malaria programmes.10 In Sierra Leone, a large proportion of clinics offering services for pregnant women with HIV were reported to have closed, as had a large proportion of clinics providing HIV testing and antiretroviral therapy (ART), resulting in reported decreases throughout the country in the number of people with HIV being started and retained on therapy.10 Similar problems were reported in neighbouring Guinea.11
The extent and details of the disruption to HIV diagnostic and treatment services requires further assessment to prevent this from happening again should another Ebola outbreak threaten the health-care system and services. A rapid method of assessment is to observe whether HIV diagnostic and treatment services differed at the district level before and during the Ebola epidemic.
Bonthe District, one of 14 medical districts in Sierra Leone, is situated in a remote area in the south of the country. This was the district least affected by the Ebola outbreak, with only five recorded cases (the first on 18 May 2015 and the last on 20 December 2015). The measures put in place, i.e., quarantining, restricted travel, a ‘no touch’ policy and repurposing of health-care workers, were nevertheless imposed in all districts of the country, and we were interested to know whether this resulted in disruption of programmes in a remote district that was not directly or severely affected by the outbreak.
The aim of this study was therefore to determine whether the diagnosis and treatment of people living with HIV, including pregnant women, differed before Ebola and during the Ebola outbreak period in the Bonthe District of Sierra Leone. Specific objectives during the two periods were to document the submission and completeness of monthly reporting for HIV data, the uptake of HIV testing and care for pregnant women attending antenatal clinics and HIV testing and treatment in the general population.
METHODS
Study design
This was a comparative cross-sectional study assessing monthly reporting and HIV diagnosis and treatment at the district level by the use of routine record systems.
Setting
General setting: country and district
Sierra Leone, a small country in West Africa, with a population of approximately 7 million (2015 national census),12 is bordered by Guinea and Liberia. Its gross national income per capita is US$1340.13 There is an established network of public health facilities delivering health-care services for which the population has to pay, except for children aged <5 years, pregnant women and lactating mothers, who are covered by the free health-care policy, as are patients who are diagnosed and treated within disease-specific programmes such as those for TB and HIV/AIDS. The public health care services are supplemented by the private sector, industry-supported clinics and non-governmental organisations (NGOs), including faith-based facilities.
Bonthe District, situated in a remote area in southern Sierra Leone, is divided into the mainland and the islands, with the latter constituting the larger part of the district. There are many isolated islands, and these are the most remote, hardest-to-reach areas of the country. Bonthe District, divided administratively into 11 chiefdoms and a municipality, has a total population of approximately 200 000, of whom 70 000 live on Bonthe Island. Health-care delivery is provided by the government hospital on the island, a mission hospital on the mainland and 55 peripheral health units (PHUs) distributed between the island and the mainland. During the 2014 Ebola epidemic, these health-care facilities recorded a sharp fall in patient load.
HIV testing and treatment services: national and district
The HIV epidemic in Sierra Leone is described as mixed, generalised and heterogeneous. The latest data (2014) estimated an HIV prevalence of 1.4% in the adult population aged 15–49 years, and 55 000 people living with HIV/AIDS (PLHIV), of whom 4700 were children.14 New HIV infections and AIDS-related deaths were estimated at 2700 for the year. ART coverage in 2014 was 38%, i.e., 10 289/26 495 PLHIV receiving ART. Prevention of mother-to-child transmission (PMTCT) coverage with antiretroviral (ARV) prophylaxis, with either single-dose nevirapine at labour or with zidovudine during pregnancy, was much better, at 85%, i.e., 2585/3055 women living with HIV receiving ARV.14
Details of the national response to HIV/AIDS are shown in the Table.14–17 In Bonthe District the two hospitals and 55 PHUs provide HIV testing and counselling, including for pregnant women, and ARV prophylaxis for pregnant women as part of PMTCT coverage. Cotrimoxazole preventive therapy is initiated in all persons who are HIV-positive, and recommended ART regimens16,17 are offered at the PHUs to pregnant women, children and adults in WHO clinical stages 3 and 4. Asymptomatic adults and those in WHO clinical stages 1 and 2 are referred to the hospitals for CD4 cell testing to determine their eligibility for ART. Data on HIV testing and ART registration are recorded in standardised registers (both antenatal care [ANC] and general population registers) based at the hospitals or PHUs. Monthly reports are sent to the district health management team (DHMT), where they are collated into monthly summary forms by the Monitoring and Evaluation Officers.
TABLE.
The national response to HIV/AIDS in Sierra Leone

Study population
The study population included aggregate numbers of persons and pregnant women in Bonthe District who were tested for HIV, found to be HIV-positive and started on PMTCT and/or ART in the pre-Ebola (1 June 2013–30 April 2014) and Ebola (1 June 2014–30 April 2015) periods.
Data variables, sources of data and data collection
Data variables included the period, year, month, site (PHU or hospital), number of monthly reports submitted and completed, number of pregnant women undergoing HIV testing each month and number testing positive for HIV and administered PMTCT prophylaxis or who started ART. In the general population, the data variables were the monthly number who underwent HIV testing, tested positive for HIV and started ART. The sources were the antenatal registers, HIV testing registers and the monthly summary forms at the PHUs and the hospitals.
Analysis and statistics
The data were entered into an Excel file (Microsoft Corp, Redmond, WA, USA), and exported and analysed using EpiData software (v. 2.2.2.182, EpiData Association, Odense, Denmark). A submitted monthly report was judged to be complete if it contained the number of patients tested for HIV, the number who tested positive for HIV and the number initiated on PMTCT and/or ART. The means and proportions were analysed and compared between the pre-Ebola and the Ebola periods using respectively the t-test and the χ2 test, with levels of significance set at 5%.
Ethics approval
Permission to carry out the study was granted by the Ministry of Health and Sanitation (MoHS, Freetown, Sierra Leone). Local ethics approval was obtained from the Sierra Leone National Ethics Committee (MoHS, Freetown), and international ethics approval was provided by the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France). As this was a record review of aggregate data, the need for informed patient consent was waived.
RESULTS
Submission and completeness of monthly HIV data
Figure 1 shows the monthly submissions of HIV reports from the 57 health facilities and whether the reports had complete information in the pre-Ebola and the Ebola periods. Of the expected 627 reports for each 11-month period, 406 (65%) were submitted in the pre-Ebola period; this was not very different from the 376 (60%) submitted in the Ebola period (P = 0.08). Of the submitted reports, in the pre-Ebola period 318 (78%) had complete information, significantly fewer than the 335 (89%) in the Ebola period (P < 0.001).
FIGURE 1.
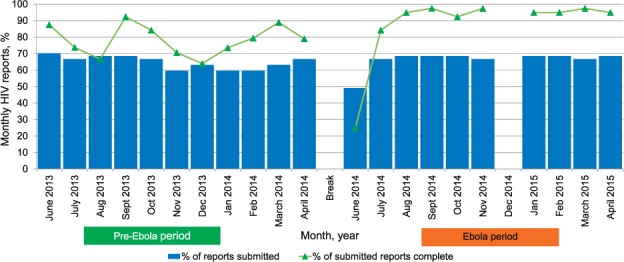
Submission of monthly HIV reports and their completeness in Bonthe District, Sierra Leone, before and during the Ebola disease outbreak, 2013–2015. Pre-Ebola period = 1 June 2013–30 April 2014; Ebola period = 1 June 2014–30 April 2015. There were no data for any of the health facilities in December 2014. HIV = human immunodeficiency virus.
HIV testing and care for pregnant women
Figure 2A shows the monthly HIV testing rates in pregnant women attending antenatal clinics in the pre-Ebola and Ebola periods. In the pre-Ebola period, 5012 pregnant women were tested for HIV (monthly mean 456, standard deviation [SD] 69), compared to 4254 in the Ebola period (monthly mean 425, SD 84); these differences were statistically significant (P < 0.001). Figure 2B shows the monthly uptake of PMTCT/ART in HIV-infected pregnant women. Respectively 25 (<1%) and 21 (<1%) pregnant women tested positive for HIV in the pre-Ebola and Ebola periods. In the pre-Ebola period, 56% (14/25) were given PMTCT/ART compared with 100% of the women in the Ebola period (P < 0.001).
FIGURE 2.
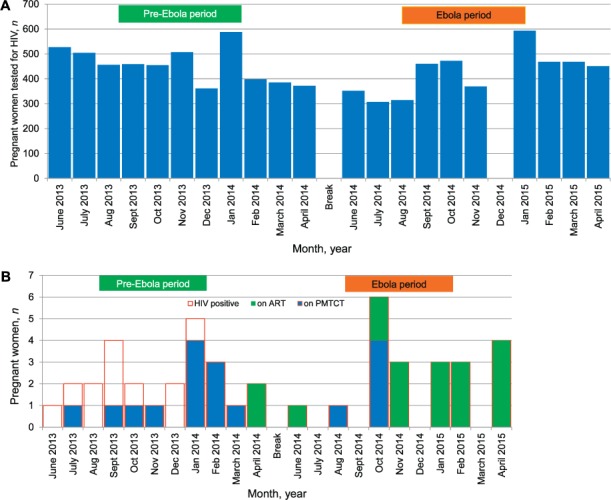
HIV testing and PMTCT/ART interventions in pregnant women attending antenatal clinics in Bonthe District, Sierra Leone, before and during the Ebola outbreak period, 2013–2015. A) Numbers of pregnant women tested for HIV. B) HIV-positive pregnant women started on ART or given PMTCT. No pregnant woman tested positive for HIV in the months of July and September 2014 and March 2015. There were no data for any of the health facilities in December 2014. Pre-Ebola period = 1 June 2013–30 April 2014; Ebola period = 1 June 2014–30 April 2015. HIV = human immunodeficiency virus; PMTCT = Prevention of Mother-to-Child Transmission; ART = antiretroviral therapy.
HIV testing and treatment in the general population
Figure 3A shows the monthly HIV testing rates in the general population (excluding pregnant women) in the pre-Ebola and Ebola periods. In the pre-Ebola period, a total of 5770 persons were tested for HIV (monthly mean 577, SD 103) compared to 3095 in the Ebola period (monthly mean 310, SD 78); these differences were statistically significant (P < 0.001). Figure 3B shows the monthly initiation rates of ART in HIV-positive patients. In the pre-Ebola and the Ebola periods, respectively 53 and 41 persons tested positive for HIV. Of these, 62% (33/53) were started on ART in the pre-Ebola period, which was not significantly different from the 81% (33/41) started on ART in the Ebola period (P = 0.06).
FIGURE 3.
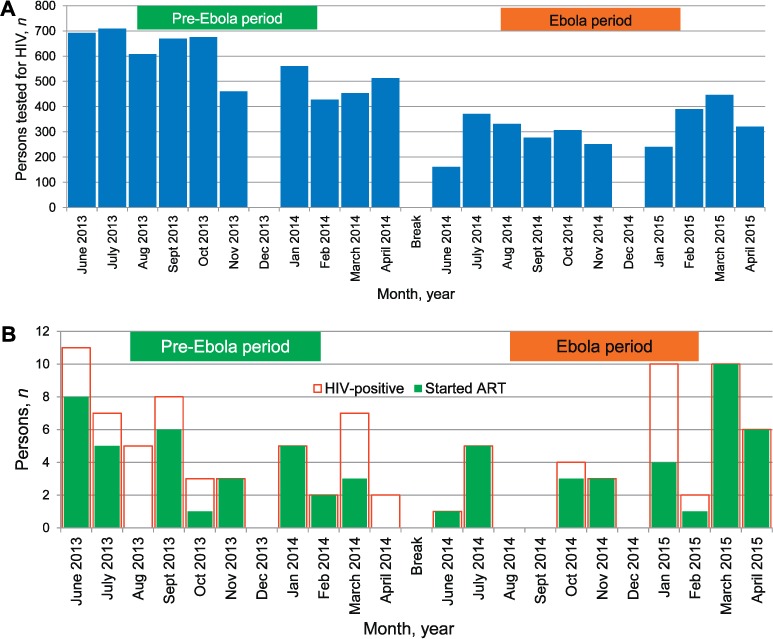
HIV testing and uptake of ART in the general population in Bonthe District, Sierra Leone, before and during the Ebola outbreak. A) HIV testing in the general population. B) Uptake of ART in people testing positive for HIV. There were no data for any of the health facilities in December 2013 and December 2014. Pre-Ebola period = 1 June 2013–30 April 2014; Ebola period = 1 June 2014–30 April 2015. HIV = human immunodeficiency virus; ART = antiretroviral therapy.
DISCUSSION
This is the first study to report on the effects of the Ebola outbreak on HIV testing and care services in the general population, including pregnant women, in a remote, hard-to-reach district of Sierra Leone. It is also among the first studies to report on the submission and completeness of HIV data in this region.
There were some interesting findings. First, the monthly reporting rates for HIV were generally low in this district during the two periods, with over one third of reports not submitted. The completeness of the submitted reports improved during the Ebola outbreak, however. Second, HIV testing rates dropped in the Ebola outbreak period, in both the general population and pregnant women. Conversely, uptake of ART and/or PMTCT services for PLHIV, including pregnant women, increased in the Ebola period.
A key strength of this study is that as it included all the public health facilities in the district, it is a good representation of the whole district. Furthermore, the conduct and reporting of the study adhered to the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines and sound ethics principles.18,19
There were several limitations to the study. No data were available for any of the health facilities for December 2014, while no data were available for HIV care services for the general population for December 2013. The monthly reports indicated the number of persons undergoing HIV testing, but did not provide reliable information either on the number of pregnant women or on the number in the general population who were eligible for HIV testing, and thus the proportions being HIV tested could not be ascertained. The study was based on routine programme records, which often suffer from issues of data quality. There was also no information in the various registers examined about the eligibility criteria for ART uptake in assessing treatment uptake in PLHIV in the general population.
Previous studies have highlighted the disruption of HIV, TB and malaria programmes as a result of the Ebola outbreak in the region.10 In particular, the closure of clinics may have led to declines in the number of persons tested for HIV and/or initiated and maintained on treatment and care. This was likely due to the repurposing of resources, including health-care workers, to the hard hit regions. Bonthe District reported only five of the 14 122 Ebola cases in the country, and no facilities were closed during the Ebola outbreak. While there was no official redeployment of the health workforce from Bonthe to other districts, more than half of the workforce consisted of volunteers, who were not as restricted in their movements as others. These volunteers also offered HIV testing services. In addition to the lack of trust between community and health workforce, there was a ‘no touch’ policy implemented in the district, in compliance with recommendations for the country as a whole, and there was also a general discouragement of HIV testing using the fingerprick test.20 These factors are likely to have contributed to the reduced testing rates for HIV in the population during the Ebola outbreak.20
The increased uptake of ART in the Ebola outbreak was reassuring. This may have been due to active uptake of ART by patients due to the fear that any exacerbation or worsening of their condition could be misinterpreted as Ebola. The national policy that restricted the movement of people between districts may also have assisted in the initiation and retention of HIV patients on care within the district.
Two main implications arise from this study. First, measures are needed to ensure regular, comprehensive reporting and completeness of health data, including for HIV. This includes improving the quantity and quality of the health workforce responsible for data monitoring and encouraging retention strategies, training, regular supervision, feedback and strategic use of the data. Constant availability of data collection and reporting tools is also key, and consideration should be given to setting up and deploying electronic medical record systems at district level. One model could be the robust, point-of-care touch screen electronic medical record system that has been successfully scaled up in Malawi.21
Second, pre-emptive measures are needed to mitigate the neglect of disease control programmes, including HIV, in the event of future outbreaks. These include planning for and ensuring safety measures and an adequate supply of logistics and resources, including those that can be provided by the donor community.20 Programmes need to be innovative. HIV testing methods other than the fingerprick test need to be considered, such as oral salivary testing and using established community-based testing approaches.22,23 A study in neighbouring Guinea showed that TB control services could be maintained during an Ebola outbreak provided there is contingency planning that includes support for the health systems, services and health-care workers.24
Sierra Leone was declared free of Ebola on 7 November 2015, and although there have been occasional sporadic cases since that date, the road to recovery has begun. Recovery measures have to address those problems that existed even before the outbreak, including the data and reporting systems. This study has shown that reporting rates for HIV data were low even in the pre-Ebola period, while during the Ebola outbreak testing for HIV decreased both in the general population and in pregnant women. Pre-emptive actions are needed to maintain HIV testing rates in future outbreaks.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the WHO/TDR, the Sierra Leone Ministry of Health and Sanitation (Freetown), the WHO Country Office for Sierra Leone (Freetown) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of the SORT IT workshops were provided through the Centre for Operational Research, The Union; The Union South-East Asia Office (New Delhi, India); the Ministry of Health, Government of Karnataka (Bangalore, India); the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre (Luxembourg); Academic Model Providing Access to Health Care (AMPATH, Eldoret, Kenya); Alliance for Public Health (Kiev, Ukraine); Institute of Tropical Medicine (Antwerp, Belgium); University of Toronto (Tornoto, ON, Canada); Dignitas International (Zomba, Malawi); Partners in Health, Sierra Leone (Boston, MA, USA); and Baroda Medical College (Vadodara, India). The authors would also like to thank K Y Gamanga for the data entry and J E Nyuma at the National AIDS Control Programme (Freetown) for oversight efforts on the data. The SORT IT programme was funded by the Department for International Development (London, UK) and the WHO/TDR. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. Beeching N J, Fenech M, Houlihan C F.. Ebola virus disease. BMJ 2014; 349: 26– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boozary A S, Farmer P E, Jha A K.. The Ebola outbreak, fragile health systems, and quality as a cure. JAMA 2014; 18: 1859– 1860. [DOI] [PubMed] [Google Scholar]
- 3. Philips M, Markham A.. Ebola: a failure of international collective action. Lancet 2014; 384: 1181. [DOI] [PubMed] [Google Scholar]
- 4. Baize S, Pannetier D, Oestereich L.. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 15: 1418– 1425. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. . Ebola situation reports: 6 January 2016. Geneva, Switzerland: WHO, 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-6-january-2016. Accessed March 2017. [Google Scholar]
- 6. Gostin L O, Lucey D, Phelan A.. The Ebola epidemic: a global health emergency. JAMA 2014; 11: 1095– 1096. [DOI] [PubMed] [Google Scholar]
- 7. Ulrich C M. Ebola is causing moral distress among African healthcare workers. BMJ 2014; 349: g6672. [DOI] [PubMed] [Google Scholar]
- 8. National Ebola Response Centre. . Bo District, 2015. Freetown, Sierra Leone: NERC, 2017. http://www.nerc.sl/?q=sldistricts/bo Accessed March 2017. [Google Scholar]
- 9. Piot P, Muyembe J J, Edmunds W J.. Ebola in West Africa: from disease outbreak to humanitarian crisis. Lancet Infect Dis 2014; 14: 1034– 1035. [DOI] [PubMed] [Google Scholar]
- 10. Edelstein M, Angelides P, Heyman D L.. Ebola: the challenging road to recovery. Lancet 2015; 385: 2234– 2235. [DOI] [PubMed] [Google Scholar]
- 11. Leuenberger D, Hebelamou J, Strahm S, . et al. Impact of the Ebola epidemic on general and HIV care in Macenta, Forest Guinea, 2014. AIDS 2015; 29: 1883– 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Statistics Sierra Leone. . Sierra Leone 2015 population and housing census. Provisional results. March 2016. Freetown, Sierra Leone: Statistics Sierra Leone, 2016. https://www.statistics.sl/wp-content/uploads/2016/06/2015-Census-Provisional-Result.pdf Accessed March 2017. [Google Scholar]
- 13. World Health Organization. . World Health Statistics, 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 14. Joint United Nations Programme on HIV/AIDS. . Sierra Leone National AIDS response progress report 2015. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 15. Joint United Nations Programme on HIV/AIDS. . 90–90–90. An ambitious treatment target to help end the AIDS epidemic. 2014. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 16. World Health Organization. . Antiretroviral drugs for treating pregnant women and preventing HIV infections in infants: recommendations for a public health approach. 2010 version. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 17. World Health Organization. . Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010 Revision. Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 18. von Elm E, Altman D G, Egger M, . et al. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 19. Edginton M, Enarson D, Zachariah R, . et al. Why ethics is indispensable for good-quality operational research. Public Health Action 2012; 2: 21– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zachariah R, Ortuno N, Hermans V, . et al. Ebola, fragile health systems and tuberculosis care: a call for pre-emptive action and operational research. Int J Tuberc Lung Dis 2015; 19: 1271– 1275. [DOI] [PubMed] [Google Scholar]
- 21. Douglas G P, Gadabu O J, Joukes S, . et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLOS Med 2010; 7: e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choko A T, Desmond N, Webb E L, . et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence settings: a cross-sectional feasibility study in Blantyre, Malawi. PLOS Med 2011; 8: e100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suthar A B, Ford N, Bachanas P J, . et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLOS Med 2013; 10: e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortuno-Gitierrez N, Zachariah R, Woldeyohannes D, . et al. Upholding tuberculosis services during the 2014 Ebola storm: an encouraging experience from Conakry, Guinea. PLOS ONE 2016; 11: e0157296. [DOI] [PMC free article] [PubMed] [Google Scholar]


