Abstract
Setting: Sixty-eight primary health facilities, Koinadugu District, rural Sierra Leone.
Objectives: Sierra Leone, a country with one of the highest burdens of malaria, was severely affected by the 2014–2015 Ebola virus disease outbreak. In under-five children, we compared trends in the completeness of malaria reports sent to the district office during the pre-Ebola, Ebola and post-Ebola periods, including the number of children with reported fever, malaria diagnostic testing performed and treatment for malaria initiated with artemisinin-based combination therapy (ACT).
Design: A cross-sectional study.
Results: Of 1904 expected malaria reports, 1289 (68%) were received. Completeness of reporting was 61% pre-Ebola, increased to 88% during the outbreak and dropped to 44% post-Ebola (P = 0.003). Total malaria testing (n = 105 558) exceeded the number of fever cases (n = 105 320). Pre-Ebola, 75% (n = 43 245) of all reported fever cases received malaria treatment, dropping to 34% (n = 50 453) during the Ebola outbreak. Of 36 804 confirmed malaria cases during Ebola, 17 438 (47%) were treated, significantly fewer than in the pre-Ebola period (96%, P < 0.001). Of the fever cases, 95% in both the pre- and post-Ebola periods received ACT, a rate that increased to 99% during the Ebola outbreak.
Conclusion: Pre-existing gaps in malaria reporting worsened after the Ebola outbreak. Reassuringly, malaria testing matched fever cases, although only half of all confirmed cases received treatment during the outbreak, possibly explained by outbreak-related operational difficulties. These findings could be useful to guide health systems strengthening and recovery.
Keywords: health systems strengthening, operational research, SORT IT, rapid diagnostic test, artemisinin combination therapy
Abstract
Contexte : Soixante-huit structures de soins de santé primaires, dans le district de Koinadugu, dans la Sierra Leone rurale.
Objectifs : La Sierra Leone, un des pays les plus frappés par le paludisme, a été gravement affecté par la flambée épidémique d'Ebola en 2014–2015. Parmi les enfants âgés de <5 ans, nous avons comparé, dans les périodes avant, pendant et après Ebola, les tendances de l'exhaustivité des rapports relatifs au paludisme envoyés au bureau du district, le nombre d'enfants fébriles chez qui le test de diagnostic du paludisme a été réalisé et la prise en charge du paludisme par un traitement combiné à base d'artémisinine (TCA).
Schéma : Etude transversale.
Résultats : Sur 1904 rapports de paludisme attendus, 1289 (68%) ont été reçus. La complétude des rapports a été de 61% avant Ebola, a augmenté à 88% pendant Ebola et est tombée à 44% après Ebola (P = 0,003). Le nombre total de tests de paludisme (n = 105 558) a dépassé le nombre de cas de fièvre (n = 105 320). Avant Ebola, 75% de tous les cas de fièvre rapportés (n = 43 245) ont reçu un traitement du paludisme, nombre qui est tombé à 34% pendant Ebola (n = 50 453). Sur 36 804 cas de paludisme confirmés pendant Ebola, 17 438 (47%) ont été traités, significativement moins qu'avant Ebola (96% ; P < 0,001). Parmi les cas de fièvre, 95% ont reçu du TCA avant et après Ebola et jusqu'à 99% pendant Ebola.
Conclusion : Les lacunes préexistantes en matière de rapports relatifs au paludisme se sont aggravées après Ebola. Il est rassurant de voir que les tests du paludisme sont restés parallèles aux cas de fièvre mais seulement la moitié de tous les cas confirmés a reçu un traitement pendant Ebola, ce qui peut être expliqué par des problèmes opérationnels liés à la flambée épidémique. Ces résultats pourraient être utiles pour guider le renforcement et la récupération des systèmes de santé.
Abstract
Marco de referencia: Sesenta y ocho establecimientos de salud del distrito rural de Koinadugu en Sierra Leona.
Objetivos: Sierra Leona es uno de los países con más alta carga de morbilidad por paludismo y sufrió una grave epidemia de enfermedad por el virus del Ébola en el 2014 y el 2015. Se compararon las tendencias en el carácter integral de los informes sobre el paludismo de los niños <5 años de edad enviados a la oficina distrital, antes, durante y después del brote; se analizaron las cifras de los informes con el número de casos de fiebre en los cuales se practicaron las pruebas diagnósticas de paludismo y de tratamientos combinados a base de artemisinina (TCA).
Método: Une estudio transversal.
Resultados: De las 1904 notificaciones de paludismo previstas, se recibieron 1289 (68%). La exhaustividad de los informes durante el período antes del brote del Ébola fue de 61%, aumentó a 88% durante el brote y disminuyó a 44% después del mismo (P = 0,003). El total de las pruebas diagnósticas de paludismo practicadas (n = 105 558) excedió el número disminuido de casos (n = 105 320). Durante el período anterior al brote, el 75% de todos los casos notificados de fiebre (n = 43 245) recibió tratamiento antipalúdico y esta cifra disminuyó al 34% durante el brote (n = 50 453). De los 36 804 casos confirmados de paludismo durante el brote, 17 438 (47%) recibieron tratamiento, lo cual representa una proporción significativamente inferior a la del período antes del brote (96%; P < 0,001). Del reducido número de casos, el 95% recibió TCA antes y después del brote y esta proporción alcanzó el 99% durante el brote epidémico.
Conclusión: Las deficiencias prexistentes en la notificación del paludismo se agravaron después del brote epidémico del Ébola. Un dato alentador es que el número de pruebas diagnósticas realizadas fue equiparable al número reducido de casos notificados de paludismo, pero solo la mitad de todos los casos confirmados recibieron tratamiento durante el brote del Ébola, probablemente por causa de dificultades operativas relacionadas la epidemia. Estos resultados pueden ser útiles a fin de orientar el fortalecimiento y la reactivación de los sistemas de salud.
In 2015, there were an estimated 214 million new cases of malaria worldwide, with 438 000 malaria-related deaths, 90% of which occurred in Africa.1 Children in Africa are particularly susceptible to malaria infection and mortality, with 95% of global deaths among children aged <5 years (292 000/306 000) occurring in this region.1 Sierra Leone is one of the countries with the highest malaria burdens in the world, with 1.3 million malaria cases reported in 2015.1
In the 2014–2015 Ebola outbreak in West Africa, Sierra Leone was one of the worst affected countries, with almost half (n = 14 122) of all reported cases and 3955 deaths.2–5 Prior to the outbreak, the country was emerging from over a decade of civil war that had depleted the health system and caused serious health worker shortages. With 50 000 patients per physician (compared to approximately 300 patients/physician in France), Sierra Leone has one of the highest patient-to-physician ratios in the world.6 These underlying health system challenges were aggravated by the sustained Ebola outbreak,7 as several health facilities shut down due to fear of Ebola virus disease (EVD) among health care workers (HCWs): over 300 HCWs were infected and there were 220 Ebola-related health worker deaths.5,8 Community fears of contracting Ebola at health facilities and strict community quarantines may also have hindered access to care.4
Few studies have assessed the effects of the Ebola outbreak on the health system in West Africa. One study from Sierra Leone showed a drop in surgical interventions during the outbreak,9 while others revealed gaps in the utilisation of maternal health services,10 a decline in malaria consultations11 and deterioration in the quality of human immunodeficiency virus/acquired immune-deficiency syndrome (HIV/AIDS) care.12
The effect of the Ebola outbreak is particularly relevant to malaria control, as both malaria and EVD present with fever, and malaria-related case management is largely dependent on functional and accessible health facilities.11 World Health Organization (WHO) and national malaria management guidelines stipulate that all children with fever should be offered malaria diagnostic testing (a rapid diagnostic test [RDT] or laboratory-based microscopy) prior to being placed on artemisinin-based combination therapies (ACTs).13,14 We hypothesised that the Ebola outbreak may have influenced the completeness of malaria reporting, health facility attendance for fever, the use of malaria diagnostic tests and administration of ACTs. A Medline search revealed no publications that had assessed these parameters in relation to the pre-Ebola, Ebola and post-Ebola disease outbreak periods in West Africa. Such information may guide future malaria control strategies and assist in post-Ebola recovery efforts.
At the primary health-care level in the rural district of Koinadugu in Sierra Leone, among children aged <5 years, we compared trends in 1) the completeness of malaria reports sent to the district information office, 2) the number of reported fever cases and malaria tests performed and 3) the number of patients treated for malaria with ACT within 24 h of fever onset before, during and after the Ebola outbreak.
METHODS
Design
The study team utilised a cross-sectional design using routine programme data.
Setting
General setting
Sierra Leone, situated in West Africa, is bordered by Guinea in the north-east and Liberia in the south-east. The capital, Freetown, is a port city close to the Atlantic Ocean. The country has an estimated population of 7 million,15 and is among the poorest in the world,16 with approximately 40% of the population aged <14 years. There are 14 districts nationwide. The health infrastructure is tiered into five levels and includes tertiary referral hospitals, district hospitals and peripheral health units (PHUs). PHUs include community health centres (CHCs), community health posts (CHPs) and maternal and child health posts (MCHPs). Details of the geographic locations and services offered is shown in Table 1.
TABLE 1.
The tiered structure and function of health facilities in Sierra Leone, 2015

Specific setting
Koinadugu, the study site, is the largest district in the country. It shares an international border with Guinea and is mountainous, with rugged roads that become inaccessible during the rainy season. The population is estimated at 408 097, of whom approximately 20% are children aged <5 years. Koinadugu has 11 chiefdoms, one district hospital and 72 functioning PHUs (10 CHCs, 28 CHPs and 34 MCHPs), of which 68 continued to function during the entire study period; these were the study sites.
Although the Ebola outbreak started in May 2014 in Sierra Leone, Koinadugu was the last district to register Ebola cases, with the first case confirmed in October 2014. The outbreak was largely confined to one chiefdom, with a total of 109 confirmed cases and 57 deaths, including one HCW.
Malaria management in Sierra Leone
Malaria management in Sierra Leone is conducted in line with national guidelines.14 All individuals presenting with fever to any given health facility are subjected to malaria diagnostic testing, which may involve an RDT or microscopy. Only confirmed malaria cases should receive ACTs. Oral first-line drug regimens include artesunate-amodiaquine (AS+AQ) and artesunate-lumifantrine. Severe malaria cases may receive parenteral AS, artemeter or quinine. Pregnant women receive sulfadoxine-pyrimethamine (Fansidar) as intermittent preventive therapy during their pregnancy. To ensure community access to malaria treatment, trained community health workers provide RDT and oral first-line drug treatment in the communities.
Study population and period
All children aged <5 years with fever who presented to 68 primary health-care units were included in the analysis, as accurate records were only available for this age group. This age group is the most vulnerable to malaria morbidity and mortality. The study time-frame across all the PHUs spanned the pre-Ebola period (1 June 2013–30 April 2014), the Ebola outbreak period (1 June 2014–30 April 2015) and the post-Ebola period (1 November 2015–30 April 2016). These periods were selected to minimise the effects of possible seasonal variations in malaria transmission on the study.
Data collection, sources and statistical analysis
Data related to the study objectives were sourced directly from routine morbidity registers available at each of the 68 health facilities. Paper-based malaria reporting forms are expected to be sent each month by each of the health facilities from the PHU level to the district health information office. For the pre-Ebola and Ebola periods, which each spanned 11 months, the total number of expected reports for each period from the 68 PHUs was (68 × 11) = 748; and for the post-Ebola period, spanning 6 months, the total expected reports from the 68 PHUs was (68 × 6) = 408. Differences between the number of expected reports and the number actually received by the district information office represent the gaps in reporting completeness.
A data manager was responsible for data quality and a dedicated data entry clerk completed data entry. The principal investigator supervised aspects related to data quality. EpiData software was used for data entry and analysis (v. 3.1 for entry and v. 2.2.2.182 for analysis; EpiData Association, Odense, Denmark).
Results are presented descriptively. Differences between groups were assessed using the Pearson's χ2 test and the χ2 for linear trends. The level of significance was set at P < 0.05.
Ethics approval
Permission for the study was obtained from the Sierra Leone Scientific and Ethics Review Board, Ministry of Health and Sanitation, Freetown and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As the study used aggregated data, informed consent was not required.
RESULTS
Completeness of malaria reports sent to the district information office
The Figure shows the completeness of the malaria reports transmitted to the district health information office. Of 1904 expected reports, 1289 (68%) actually arrived. Completeness of reporting was 61% in the pre-Ebola period, increasing to 88% during the outbreak and dropping to 44% in post-Ebola (χ2 for trend, P = 0.003).
FIGURE.
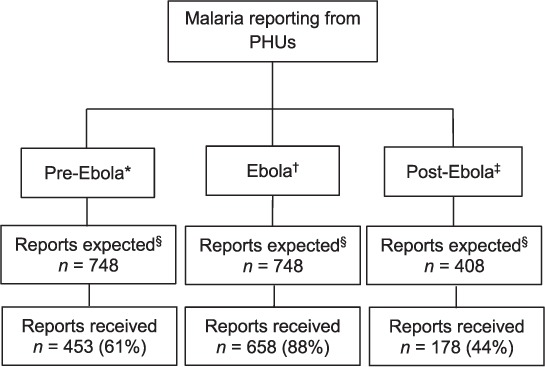
Flow chart showing completeness of malaria reporting for children aged <5 years from 68 PHUs in the pre-Ebola, Ebola and post-Ebola disease outbreak periods, Koinadugu District, Sierra Leone. * 1 June 2013–30 April 2014; † 1 June 2014–30 April 2015; ‡1 November 2015–30 April 2016. § For the pre-Ebola and Ebola periods, each spanning 11 months, the total expected reports for each period from the 68 PHUs was (68 × 11) = 748; for the post-Ebola period, spanning 6 months, the total expected reports from the 68 PHUs was (68 × 6) = 408. PHU = peripheral health unit.
Numbers of reported fever cases and malaria tests performed
The monthly average of reported fever cases was similar in the pre-Ebola (n = 4324) and Ebola outbreak (n = 4586) periods, but dropped significantly in the post-Ebola period by 47% (n = 2324) (Table 2).
TABLE 2.
Fever cases reported (suspected malaria) and malaria tests performed during the pre-Ebola, Ebola and post-Ebola outbreak periods at the primary health-care level, Koinadugu District, Sierra Leone
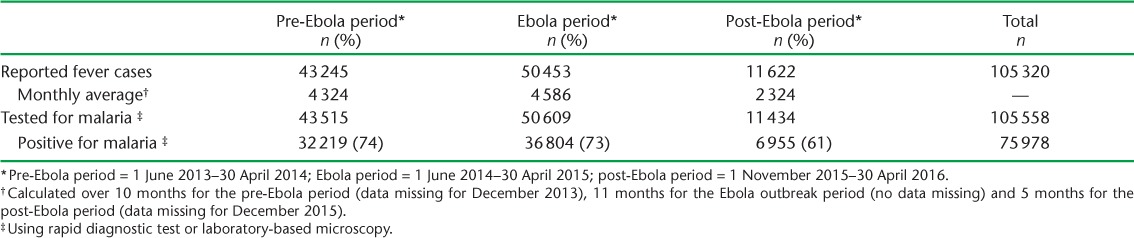
The total number (n = 105 558) of malaria diagnostic tests performed exceeded the total number of reported fever cases (n = 105 320). The trend in the monthly utilisation of malaria diagnostic tests was similar for the pre-Ebola, Ebola and post-Ebola periods.
Numbers treated for malaria in relation to artemisinin-based combination therapy and fever onset
In the pre-Ebola period, 75% of reported fever cases were treated for malaria, decreasing to 34% during the Ebola period. Of 36 804 malaria cases confirmed during the Ebola period, only 17 438 (47%) were actually treated, which is significantly lower than in the pre-Ebola period (96%, P < 0.001) (Table 3).
TABLE 3.
Number of patients treated for malaria with ACT, stratified by type of medication and timing (within 24 h of fever onset) in the pre-Ebola, Ebola and post-Ebola outbreak periods at the primary health-care level, Koinadugu District, Sierra Leone
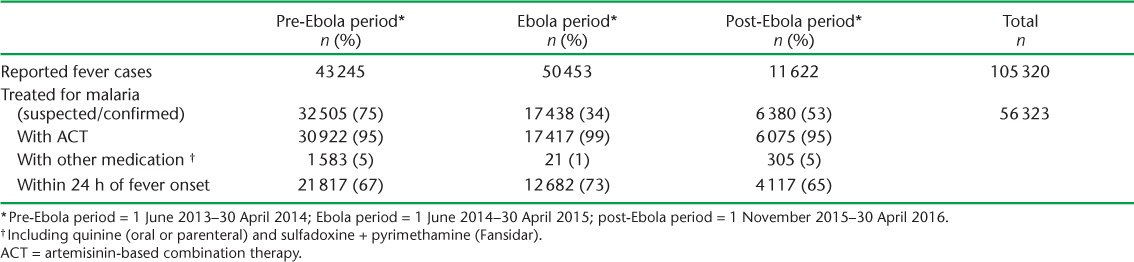
Of all the fever cases (suspected and confirmed malaria), 95% in both the pre- and post-Ebola periods received ACT; this increased to 99% during the Ebola outbreak. Delays in administering ACT within the recommended time following self-reported onset of fever (<24 h) were apparent during the three study periods.
DISCUSSION
This is one of the first studies to assess the management of children with fever for malaria in the context of Ebola in a rural district setting. The findings show pre-existing gaps in the completeness of malaria reporting that worsened in the post-Ebola period, with approximately four of 10 expected reports reaching the district level. Of additional concern is that less than half of all confirmed malaria cases were actually treated for malaria during the outbreak. This may be attributed to the fact that as fever is a symptom common to both malaria and EVD, HCWs might have been inclined to be prudent by referring affected children to the Ebola management sites.
This study shows that a previously weak malaria reporting system improved during the Ebola outbreak and deteriorated thereafter. It is important to correct this situation, as district management teams rely on malaria reports for projections of drug and consumable requirements. This study highlights the urgent need for a rapid point-of-care test for EVD, which is a prerequisite for the differentiation of Ebola-related fever from other fevers.
The strengths of this study include the selection of the largest district in the country as the study site and the inclusion of all primary health-care units that remained open throughout the study period. As the data were also directly sourced from the health facility morbidity forms, double-entered and validated, we believe the findings reflect the operational reality on the ground. The study also addresses an identified operational research priority of the Ministry of Health and Sanitation at national level, and it may thus contribute to policy and practice.
One of the main limitations is the missing data on morbidity; the real malaria situation may be much worse than indicated in the results. The aggregate, non-individualised data did not allow us to directly link malaria diagnosis and ACT treatment. Although national and WHO malaria guidelines13,14 recommend the rational use of ACTs, it was impossible to assess this parameter. The problem lies at the level of the morbidity forms, which will need to be modified in such a way as to be able to decipher this information accurately. Clarity on indicators that need to be reported at national programme level is also needed.
These limitations notwithstanding, the study has a number of implications for policy and practice. First, although malaria reporting was incomplete prior to the outbreak, it actually improved during the outbreak and subsequently deteriorated. We do not know the exact reasons for this finding, but it may be attributed to the fact that during the Ebola outbreak there were increased numbers of reporting staff at district level, with closer monitoring and supervision and enhanced transport logistics. This may have had a favourable effect on staff motivation in reporting. During the Ebola outbreak, more opportunities were also available for transfer of paper-based forms from the PHU level to the district health information office, which may have improved the completeness of reporting. This viewpoint seems logical, as there was significant deterioration following the withdrawal of the Ebola-related emergency resources (post-Ebola). Possible ways of improving reporting include improving supervision, monitoring and feedback to enhance the motivation of PHU staff. Periodic data quality control audits may also be warranted.
Improving the information flow from the PHU to district level requires the creative deployment of a number of possible strategies. The recent introduction of the Integrated Disease Surveillance and Response (IDSR) system, along with monthly meetings of the PHU managers at district level, could be an opportunity to combine the transmission of reports. Furthermore, as chiefdom health supervisors routinely visit all PHUs and have access to motorbikes, they may be able to support this activity. The current reality is that PHU staff have neither Internet access nor other electronic means for transmitting morbidity forms to the district level. There is thus clear reliance on transport logistics, but this is limited or unavailable at primary level. Further operational research on these issues, including use of independent electronic data systems,17,18 is necessary, as is greater investment.
Second, a reassuring finding was that the monthly utilisation of malaria diagnostics closely matched the number of reported fever cases, implying that fever cases are being routinely subjected to malaria testing. Surprisingly, there was no decline in this trend during the Ebola outbreak despite a ‘no touch’ policy and a general recommendation to avoid invasive procedures as a means of minimising Ebola-related occupational risks. This observation may be linked to the fact that Koinadugu was the last district to register Ebola cases in Sierra Leone, and that these were confined to one of 11 chiefdoms. This may have given time for health workers to receive infection prevention and control (IPC) training as well as IPC supplies, which might have resulted in boosted confidence and motivation among health workers to continue routine malaria management activities. IPC training may also explain why parenteral medication was avoided in almost all (99%) fever cases (significantly more than in the pre-Ebola period). This finding is in stark contrast with a study from Guinea, which reported substantial decreases in malaria care during the Ebola outbreak,11 and suggests that IPC training and support for HCW safety can yield dividends in upholding essential services.
Finally, less than half of all confirmed malaria cases received treatment during Ebola, significantly fewer than in the pre- and post-Ebola periods. This could be attributed to the fact that all cases of fever were considered as possible Ebola patients, resulting in their referral to Ebola holding or treatment centres. Although understandable, the drawback to this approach is that children (and their parents) who may not have had Ebola may have been put at risk of being exposed and contracting the disease at such facilities. Furthermore, as Ebola holding and treatment centres were already overwhelmed with confirmed cases, such referrals may have contributed to increased workloads and unnecessary congestion.
We strongly recommend acceleration of the development of a rapid point-of-care Ebola test that would allow Ebola testing to be executed simultaneously with malaria tests,19,20 and to be made readily available at an affordable cost. This has the potential to enhance rational screening for fever, referral of confirmed Ebola cases and uptake of malaria treatment. In sustained Ebola outbreaks such as in 2014–2015, which lasted for over a year, this will be vital to sustain malaria management and avoid malaria-related mortality.
In conclusion, we have highlighted a number of shortcomings and opportunities in the management of malaria in children in the context of an Ebola outbreak. These findings could be useful to guide current efforts towards health systems strengthening and recovery in Sierra Leone and other West African countries.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the WHO/TDR, the Sierra Leone Ministry of Health (Freetown), WHO Country Office for Sierra Leone (Freetown) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of the SORT IT workshops were provided through the Centre for Operational Research, The Union; The Union South-East Asia Office (New Delhi, India); the Health and Family Welfare Services, Government of Karnataka (Karnataka, India); the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre (Luxembourg); AMPATH (Eldoret, Kenya); Alliance for Public Health (Kiev, Ukraine); Institute of Tropical Medicine (Antwerp, Belgium); University of Toronto (Toronto, ON, Canada); Dignitas International (Zomba, Malawi); Partners in Health (Boston, MA, USA); and the Baroda Medical College (Vadodara, India). The programme was funded by the Department for International Development (London, UK) and the WHO/TDR. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. World Health Organization. . World malaria report, 2015. Geneva, Switzerland: WHO, 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ Accessed January 2017. [Google Scholar]
- 2. Baize S, Pannetier D, Oestereich L, . et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371: 1418– 1425. [DOI] [PubMed] [Google Scholar]
- 3. Gostin L O, Lucey D, Phelan A.. The Ebola epidemic: a global health emergency. JAMA 2014; 312: 1095– 1096. [DOI] [PubMed] [Google Scholar]
- 4. Piot P, Muyembe J-J, Edmunds W J.. Ebola in west Africa: from disease outbreak to humanitarian crisis. Lancet Infect Dis 2014; 14: 1034– 1035. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. . Ebola situation report, 6 January 2016. Geneva, Switzerland: WHO, 2016. http://apps.who.int/ebola/sites/default/files/atoms/files/who_ebola_situation_report_06-01-2016.pdf?ua=1 Accessed January 2017. [Google Scholar]
- 6. Zachariah R, Ortuno N, Hermans V, . et al. Ebola, fragile health systems and tuberculosis care: a call for pre-emptive action and operational research. Int J Tuberc Lung Dis 2015; 19: 1271– 1275. [DOI] [PubMed] [Google Scholar]
- 7. Dallatomasina S, Crestani R, Sylvester Squire J, . et al. Ebola outbreak in rural West Africa: epidemiology, clinical features and outcomes. Trop Med Int Health 2015; 20: 448– 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. . Global Health Observatory data. Density of nursing and midwifery personnel, per 1000 population. Geneva, Switzerland: WHO, 2015. http://www.who.int/gho/health_workforce/nursing_mid-wifery_density/en/ Accessed January 2017. [Google Scholar]
- 9. Bolkan H A, Bash-Taqi D A, Samai M, Gerdin M, von Schreeb J.. Ebola and indirect effects on health service function in Sierra Leone. PLOS Curr 2014; 19: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delamou A, Hammonds R M, Caluwaerts S, Utz B, Delvaux T.. Ebola in Africa: beyond epidemics, reproductive health in crisis. Lancet 2014; 384: 2105. [DOI] [PubMed] [Google Scholar]
- 11. Plucinski M M, Guilavogui T, Sidikiba S, . et al. Effect of the Ebola virus disease epidemic on malaria case management in Guinea, 2014: a cross-sectional survey of health facilities. Lancet Infect Dis 2015; 15: 1017– 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leuenberger D, Hebelamou J, Strahm S, . et al. Impact of the Ebola epidemic on general and HIV care in Macenta, Forest Guinea, 2014. AIDS 2015; 29: 1883– 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. . Guidelines for the treatment of malaria. 3rd ed Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 14. Ministry of Health and Sanitation National Malaria Control Programme. . Revised guidelines for the case management of malaria in Sierra Leone. Freetown, Sierra Leone: MoHS, 2016. [Google Scholar]
- 15. Statistics Sierra Leone. . Sierra Leone 2015 population and housing census provisional results. Freetown, Sierra Leone: Statistics Sierra Leone, 2015. [Google Scholar]
- 16. World Health Organization. . World Health Statistics, 2014–2015. Geneva, Switzerland: WHO, 2015. http://www.who.int/gho/publications/world_health_statistics/2014/en/ Accessed January 2017. [Google Scholar]
- 17. Gadabu O, Munthali C, Zachariah R, . et al. Is transcription of data on antiretroviral treatment from electronic to paper-based registers reliable in Malawi? Public Health Action 2011; 1: 10– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oluoch T, Katana A, Ssempijja V, . et al. Electronic medical record systems are associated with appropriate placement of HIV patients on antiretroviral therapy in rural health facilities in Kenya: a retrospective pre-post study. J Am Med Inform Assoc 2014; 21: 1009– 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zachariah R, Harries A D.. The WHO clinical case definition for suspected cases of Ebola virus disease arriving at Ebola holding units: reason to worry? Lancet Infect Dis 2015; 15: 989– 990. [DOI] [PubMed] [Google Scholar]
- 20. Broadhurst M J, Kelly J D, Miller A, . et al. ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study. Lancet 2015; 386: 867– 874. [DOI] [PubMed] [Google Scholar]


