Abstract
Setting: Bombali District, rural Sierra Leone.
Objective: To compare the number of patients with presumptive tuberculosis (TB), the number of patients registered with TB (including testing for the human immunodeficiency virus [HIV] and initiation on antiretroviral therapy [ART]) and treatment outcomes during the pre-Ebola, Ebola and post-Ebola disease outbreak periods between 2013 and 2016.
Design: This was a cross-sectional study and retrospective cohort analysis of treatment outcomes.
Results: The mean monthly number of patients with presumptive TB before, during and post-Ebola was respectively 169, 145 and 210. The mean monthly number of registered TB cases was respectively 57, 57 and 96. Smear-positive TB was the most frequent type of TB, at 75%, 66% and 77%. The proportion of TB patients tested for HIV was 82% pre-Ebola, 74% Ebola and 99% post-Ebola. The proportion of HIV-positive patients with TB initiated on ART was respectively 46%, 85% and 100%. Treatment success among TB patients was 71% in the pre-Ebola period and 89% in the Ebola period (P < 0.001).
Conclusion: During the Ebola outbreak, there were decreases in the number of presumptive TB patients and in the proportions of patients diagnosed with smear-positive TB and tested for HIV. The initiation of ART in HIV-infected TB patients and treatment outcomes remained acceptable. Pre-emptive actions are needed to maintain adequate control activities in future outbreaks.
Keywords: HIV testing, HIV status, antiretroviral therapy, TB treatment outcomes, SORT IT, operational research
Abstract
Contexte : District de Bombali, Sierra Leone rurale.
Objectif : Comparer le nombre de patients présumés tuberculeux (TB), le nombre enregistrés comme TB (incluant le test du virus de l'immunodéficience humaine [VIH] et la mise en route du traitement antirétroviral [TAR]) et les résultats du traitement pendant les périodes avant, pendant et après Ebola, entre 2013 et 2016.
Schéma : Une étude transversale et analyse rétrospective de cohorte des résultats du traitement.
Résultats : Le nombre moyen mensuel de patients présumés TB a été 169 avant Ebola, 145 pendant Ebola et 210 après Ebola. Le nombre mensuel moyen de cas de TB enregistrés a été 57 avant Ebola, 57 pendant Ebola et 96 après Ebola. La TB à frottis positif a été le type le plus fréquent, avec 75% avant Ebola, 66% pendant Ebola et 77% après Ebola. Les proportions de patients tuberculeux testés pour le VIH ont été 82% avant Ebola, 74% pendant Ebola et 99% après Ebola et pour les patients tuberculeux VIH positifs mis sous TAR, ces proportions ont été 46% avant Ebola, 85% pendant Ebola et 100% après Ebola. Le taux de succès du traitement des patients tuberculeux a été de 71% pendant les périodes avant Ebola et de 89% pendant Ebola (P < 0,001).
Conclusion : Pendant la période de l'Ebola, il y a eu une diminution du nombre de patients présumés tuberculeux et dans les proportions de patients ayant eu un diagnostic de TB à frottis positif et un test VIH. La mise en route du TAR chez les patients infectés par le VIH et les résultats du traitement sont restés acceptables. Des actions préventives sont requises afin de maintenir des activités de lutte suffisantes lors de futures flambée épidémiques.
Abstract
Marco de referencia: El distrito rural de Bombali en Sierra Leona.
Objetivo: Comparar el número de pacientes con presunción clínica de tuberculosis (TB), el número de casos registrados de TB (con inclusión de las pruebas diagnósticas del virus de la inmunodeficiencia humana [VIH] y el inicio del tratamiento antirretrovírico [TAR]) y los desenlaces terapéuticos durante el período anterior a la epidemia del Ébola, durante la misma y después de ella del 2013 al 2016.
Método: Un estudio transversal con un análisis retrospectivo de cohortes de los desenlaces terapéuticos.
Resultados: El promedio mensual de pacientes con presunción clínica de TB fue como sigue: 169 antes de la epidemia del Ébola, 145 durante la epidemia y 210 después de la misma. El promedio mensual de casos registrados de TB fue 57 antes de la epidemia, 57 durante el brote y 96 después de la misma. La TB con baciloscopia positiva fue el tipo de enfermedad más frecuente y su proporción fue 75% antes de la epidemia del Ébola, 66% durante la misma y 77% después de ella. La proporción de pacientes TB que contaban con pruebas diagnósticas del VIH fue como sigue: 82% antes de la epidemia, 74% durante la misma y 99% después de ella y la proporción de pacientes con diagnóstico de TB y positivos frente al VIH que iniciaron el TAR fue 46% antes de la epidemia, 85% durante la misma y 100% después de ella. El éxito del tratamiento antituberculoso fue de 71% en el período anterior al brote de enfermedad del Ébola y de 89% en el período posterior al mismo (P < 0,001).
Conclusión: Durante el período de la epidemia de enfermedad del Ébola se observó una disminución del número de casos con presunción clínica de TB, de la proporción de diagnósticos de TB con baciloscopia positiva y de la proporción de pacientes con pruebas diagnósticas del VIH. La iniciación del TAR en los pacientes coinfectados por el VIH y TB y los desenlaces terapéuticos conservaron proporciones aceptables. Se precisan intervenciones anticipativas, con el fin de mantener actividades de control adecuadas durante los brotes epidémicos en el futuro.
The 2014 Ebola outbreak in West Africa started in Guinea in December 2013, and rapidly spread to Liberia and Sierra Leone,1 prompting the World Health Organization (WHO) in 2014 to declare an international public health emergency.2 By 3 January 2016, a total of 28 637 cases had been reported in the region, of whom 11 315 died.3 Sierra Leone was the country most affected, registering almost half of all Ebola cases in the region.3 Prior to the Ebola outbreak, the country was trying to recover from civil war, limitations in the health system and serious shortages of health-care workers.4,5 The Ebola outbreak adversely affected the quality of health service delivery and seriously affected health-seeking behaviour and access to care in the community.6,7
On 7 November 2015, Sierra Leone was declared free of Ebola,3 and although there have since been occasional sporadic cases, the challenging road to recovery has begun. In all three affected countries in the region, including Sierra Leone, the disease control programmes for tuberculosis (TB), human immunodeficiency virus/acquired-immune deficiency syndrome (HIV/AIDS) and malaria experienced disruptions.8 Clinical teams and facilities were often repurposed to the Ebola response, making it difficult for new and existing TB patients to access and receive treatment.8 It was predicted that the Ebola epidemic would hinder TB programme activities in terms of diagnosis and treatment as well as the provision of testing for HIV and antiretroviral therapy (ART) for those patients who were HIV-positive.9 In Macenta, Forested Guinea, where the Ebola outbreak started and which was severely affected by Ebola, there was an overall decline in reporting of new TB cases.10 By contrast, in Guinea's capital city, Conakry, where there were fewer Ebola cases and non-governmental organisations (NGOs) provided support in the form of a package of measures to guarantee a continuum of care, TB case finding and services were maintained at satisfactory levels.11
These reports, and the lack of published information to date from Sierra Leone, suggest that more information is needed about the extent to which the Ebola virus outbreak disrupted TB control programme services. A rapid method of undertaking this assessment is to observe and report on what happened to patients with presumptive and active TB at the district level before, during and after the Ebola epidemic. We therefore carried out a study in Bombali District, Sierra Leone, specifically to assess whether the numbers of patients recorded with presumptive TB, the numbers registered with TB (including receiving HIV testing and HIV care) and treatment outcomes were different during the pre-Ebola, Ebola and post-Ebola periods.
METHODS
Study design
This study used a cross-sectional design and a retrospective cohort approach for TB treatment outcomes among patients with presumptive and active TB.
Setting
General setting
Sierra Leone is a small country in West Africa, bordered by Guinea and Liberia. The country has a population of 7 075 641 based on the national census in 2015,12 and a gross national income per capita of US$1340.13 Administratively, the country is divided into four regions (North, South, East and West), which are further divided into 14 districts. Medical services are provided by a mix of public, private and industry-supported organisations and NGOs, with traditional medicine being an important component of primary health care.14 There is an established network of public health facilities in the country. The population has to pay for health-care services, with the exception of children aged <5 years, pregnant women and lactating mothers, who are covered by the free health-care policy; this also includes Ebola survivors and patients diagnosed and treated within disease-specific programmes such as those for TB, HIV/AIDS and malaria.
Study setting
Bombali District, the study site, located in the northern region of Sierra Leone, is the second largest district in the country, with 13 chiefdoms and an ethnically diverse population of 606 183.12 The district has 16 community health centres, 18 community health posts, 48 maternal child health posts, 1 government hospital, 1 military hospital, 1 community hospital, 3 mission clinics, 3 mission hospitals and 3 private clinics. Bombali District was one of the hardest hit areas during the Ebola epidemic, with, by October 2015, 1050 confirmed cases of a total 8704 confirmed cases nationwide.15 The district experienced its last Ebola case on 13 September 2015.
National and district TB programme
Sierra Leone launched the DOTS strategy for TB in 1994, and since then WHO guidance has been followed for diagnosis and treatment.16 Patients are identified as having presumptive TB if they have a cough of ⩾2 weeks, and undergo sputum smear microscopy and chest radiography. They are diagnosed as smear-positive pulmonary TB (PTB) or smear-negative PTB according to standard criteria; on occasion cases are registered as ‘sputum smear not performed’. Extra-pulmonary TB (EPTB) is diagnosed on the basis of clinical, radiographic and other supportive evidence. Diagnosed patients are registered according to the type of TB, and treatment is based on the category of TB (new or previously treated) using internationally recommended standardised treatment regimens.16 All TB patients are offered HIV testing; for those who are HIV positive, cotrimoxazole preventive treatment (CPT) and ART are given. All patients are monitored until the end of treatment. Final treatment outcomes are based on standardised criteria, with results recorded in the patient treatment cards and the TB patient registers. Monthly or quarterly reporting is conducted at the district level and is focused on case finding and treatment outcomes, with results collated centrally by the National TB Control Programme.
In Bombali District, the district TB officer coordinates the diagnostic, treatment and follow-up response, and is responsible for the supervision, collection and collation of data. There are 15 TB basic management units in the district, with laboratory registers for recording the numbers of presumptive TB patients, TB patient treatment cards and registers for recording the number of patients diagnosed, HIV testing parameters and final treatment outcomes.
Patient population
The study included all patients in Bombali District with presumptive and active TB registered in the pre-Ebola (1 June 2013–30 April 2014), Ebola (1 June 2014–30 April 2015) and post-Ebola (1 November 2015–30 April 2016) outbreak periods. It also included case finding and treatment outcome data of patients registered in Bombali District in the third quarter of 2013 (pre-Ebola) and the third quarter of 2014 (Ebola) only, so that final outcomes could be ascertained and compared.
Data variables, sources of data and data collection
Data variables included year, month, number of presumptive TB patients each month, TB patients registered (stratified by type and category of disease), number of TB patients tested for HIV, number diagnosed as HIV-positive, number started on ART and standardised final treatment outcomes of those registered in the third quarters of 2013 and 2014. The sources of data were the district laboratory registers and the TB patient registers, the monthly tally forms from the district TB officer and the monthly and quarterly reports from the district TB programme.
Analysis and statistics
The data were collected into an Excel spreadsheet (Microsoft Corp, Redmond, WA, USA) and exported to EpiData analysis software (v. 2.2.2.182, EpiData Association, Odense, Denmark), where further analysis was carried out. Numbers and proportions were presented and treatment outcomes were compared between the different periods using the χ2 test, relative risk (RR) ratios and 95% confidence intervals (CI). Levels of significance were set at 5%.
Ethics approval
Approval for this study was obtained from the National Ethics Committee and the Sierra Leone Ethics and Scientific Review Committee, Ministry of Health and Sanitation (Freetown). Ethics approval was also obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France). As aggregate data were used without identifiers, informed patient consent was not required.
RESULTS
Presumptive TB
Monthly trends in numbers of patients reported with presumptive TB in the pre-Ebola, Ebola and post-Ebola periods are shown in Figure 1. The most striking finding was a sharp decrease in presumptive TB cases 2 months into the Ebola period, which remained low for the next 6 months before increasing. The mean monthly numbers of presumptive TB cases for the three periods were 169 for the pre-Ebola, 145 in the Ebola period and 210 for the post-Ebola period.
FIGURE 1.
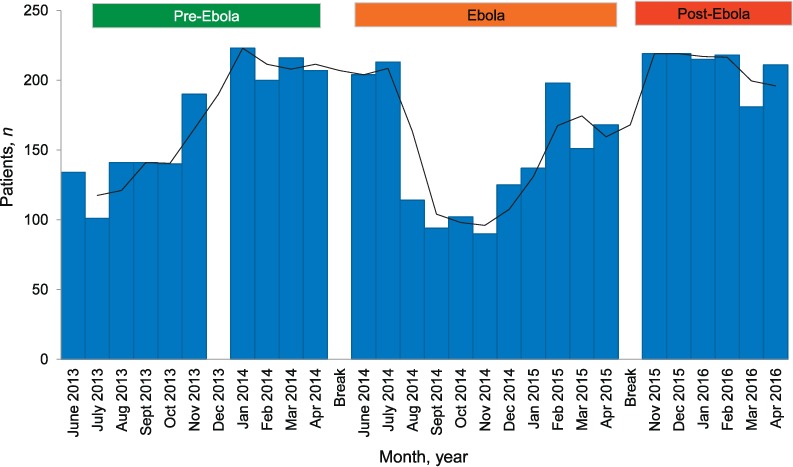
Numbers of patients with presumptive TB who were screened and investigated for TB in the laboratory each month before, during and after the Ebola disease outbreak in Bombali District, Sierra Leone, 2013–2016. Pre-Ebola period = 1 June 2013–30 April 2014; Ebola period = 1 June 2014–30 April 2015; post-Ebola period = 1 November 2015–30 April 2016. The gap in data in the pre-Ebola period in December 2013 was due to missing data. TB = tuberculosis.
Registered TB patients stratified by type and category of disease
The monthly trends in the numbers of patients registered with TB, stratified by type and category of TB in the pre-Ebola, Ebola and post-Ebola periods, are shown in Figure 2. For all TB cases, the monthly numbers were initially low, at 35–60, in the pre-Ebola period before increasing to 65–90 per month in early 2014 (Figure 2A). In the Ebola period, there was a sharp decrease in the third and fourth months to respectively 43 and 27 TB cases per month, with numbers gradually increasing thereafter. In the post-Ebola period, the monthly numbers of TB cases increased to 80–100. The mean monthly numbers of TB cases for the three periods were 57 pre-Ebola, 57 Ebola and 96 post-Ebola. Trends in types of TB and category of TB are shown in Figure 2B and C. Smear-positive PTB predominated, accounting for 72% of all TB cases over the study period: this proportion was 75% pre-Ebola, 66% in the Ebola period and 77% post-Ebola. New cases of TB accounted for 92% of all TB categories over the study period: 95% pre-Ebola, 91% during Ebola and 92% post-Ebola.
FIGURE 2.
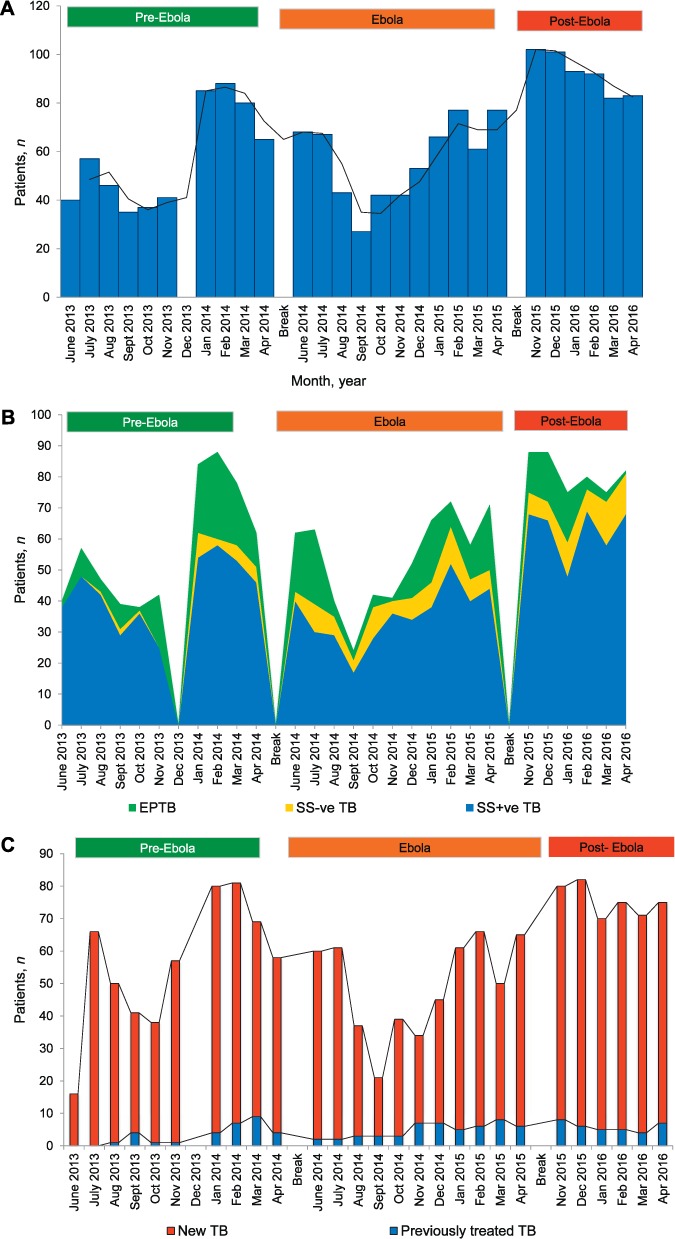
Numbers of patients diagnosed and reported with TB each month, stratified by type and category of disease, before, during and after the Ebola outbreak in Bombali District, Sierra Leone, 2013–2016. A) All registered TB cases. B) Types of TB: smear-positive PTB; smear-negative PTB; EPTB. Pulmonary TB with sputum smears not performed are not shown in the Figure. Trend lines are shown for different types of TB. C) Category of TB – new and previously treated tuberculosis. Pre-Ebola period = 1 June 2013–30 April 2014; Ebola period = 1 June 2014–30 April 2015; post-Ebola period = 1 November 2015–30 April 2016. The gap in data in the pre-Ebola period in December 2013 was due to missing data. TB = tuberculosis; EPTB = extra-pulmonary TB; SS+ve TB = sputum smear-positive pulmonary TB; SS−ve TB = sputum smear-negative pulmonary TB; PTB = pulmonary tuberculosis.
HIV testing and ART uptake
The monthly uptake of HIV testing and ART initiation in HIV-positive TB patients is shown in Figure 3A and B. Data are missing for the whole of 2013. For the 4 months with data in the pre-Ebola period, the proportion of TB patients who were tested for HIV was 82% (262/318). During the Ebola period, HIV testing decreased to its lowest level, at 26%, by the fourth month; it then increased, along with the proportion of all TB patients tested in the Ebola period, to 74% (460/623). The proportion of TB patients tested for HIV was high post-Ebola, at 99% (546/553). Of the 1268 TB patients who underwent testing for HIV throughout the whole study period, 110 (9%) were HIV-positive: 10% pre-Ebola, 7% during Ebola and 9% post-Ebola. Of 95 (86%) HIV-positive TB patients reported to have started ART, 46% started in the pre-Ebola period, 85% in the Ebola period and 100% in the post-Ebola period.
FIGURE 3.
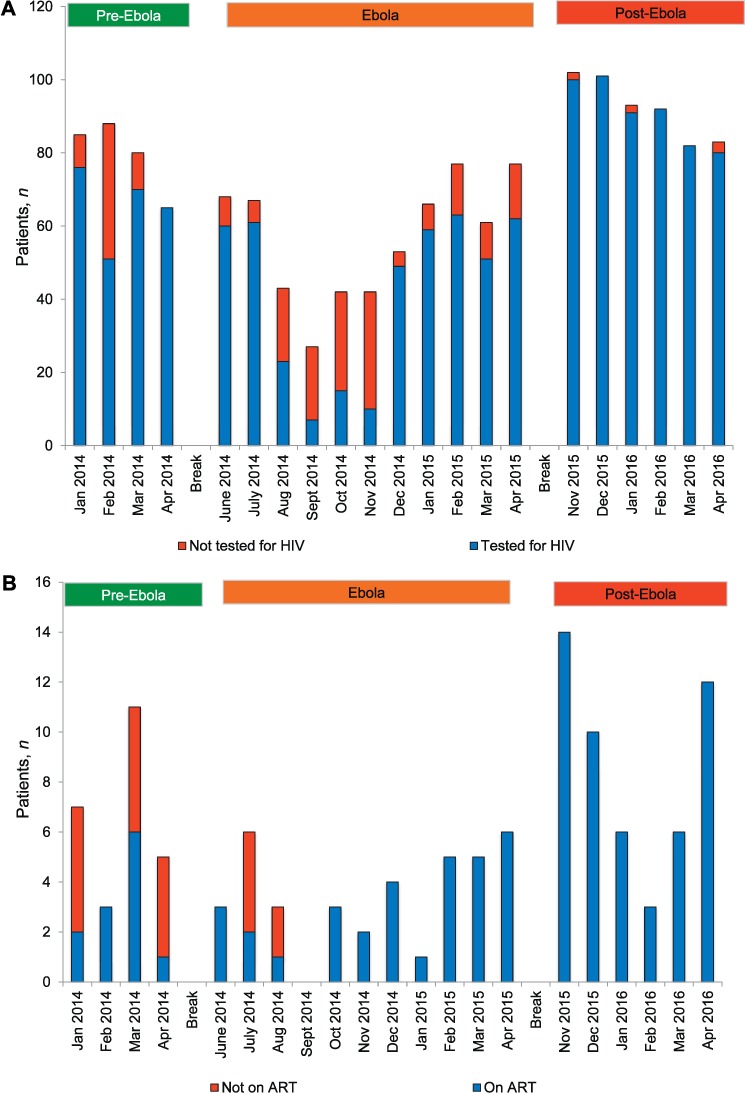
HIV collaborative services in diagnosed TB patients each month before, during and after the Ebola outbreak in Bombali District, Sierra Leone, 2014–2016. A) HIV testing in TB patients. B) HIV-positive TB patients started on ART. Pre-Ebola period = 1 January–30 April 2014; Ebola period = 1 June 2014–30 April 2015; post-Ebola period = 1 November 2015–30 April 2016. The gap in data in the Ebola period in September 2014 was due to missing data. HIV = human immunodeficiency virus; ART = antiretroviral therapy; TB = tuberculosis.
Treatment outcomes
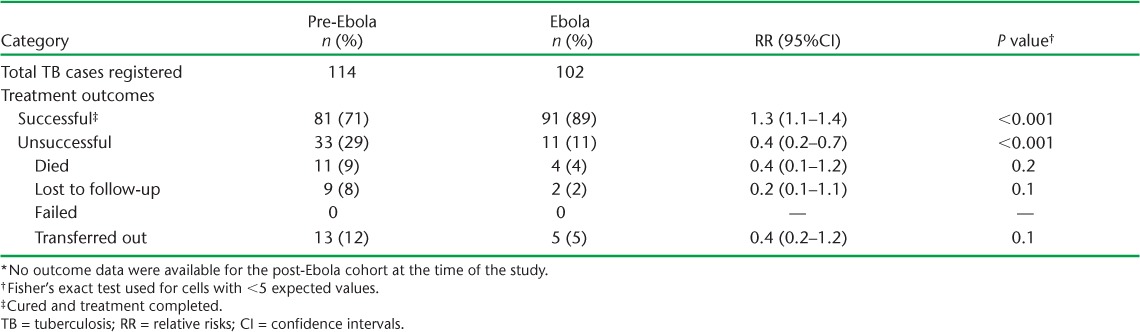
Treatment outcomes for all TB patients registered in the pre-Ebola period (July–September 2013) and the Ebola period (July–September 2014) are shown in the Table. The treatment success rate was significantly higher in the Ebola period, with death, loss to follow-up and transfer-out rates all lower than in the pre-Ebola period. No outcome data were available for the post-Ebola cohort at the time of the study.
TABLE.
Treatment outcomes of registered TB cases in the pre-Ebola (July–September 2013) and Ebola (July–September 2014) periods in Bombali District, Sierra Leone *
DISCUSSION
This is the first study from a district in Sierra Leone reporting on TB case finding, control activities and treatment outcomes before, during and after the Ebola virus disease outbreak. There were some interesting findings. First, in the pre-Ebola period, case finding, TB-HIV collaborative activities and overall treatment outcomes were poor compared with the post-Ebola period, suggesting that factors other than Ebola, such as a lack of human resources, poor quality control, inadequate supervision and interrupted supplies of TB-related commodities, were adversely affecting TB control efforts at this time. Second, the negative effects on TB control activities observed during the Ebola outbreak compared with the pre- and post-Ebola periods were the decreases in the number of patients presenting with presumptive TB, the proportion of patients diagnosed with smear-positive PTB and the proportion of TB patients tested for HIV. Third, there were some surprising observations, such as the fact that the initiation of ART in HIV-positive TB patients and the overall TB treatment outcomes were better in the Ebola period compared with the pre-Ebola period, similar to recent reports from Guinea.11 We do not know the reasons for this, but they might be related to the extra resources, both human and material, that were deployed by international organisations and the government during the Ebola outbreak, which may have led to better attention and support for disease-specific control activities such as TB. Finally, the best TB control activity indicators were observed in the post-Ebola period, with high proportions of registered TB patients having smear-positive PTB, an almost 100% uptake of HIV testing and 100% initiation of ART for those found to be HIV-positive. Again, this may have been due to the additional resources provided during the Ebola outbreak.
The strengths of this study are the inclusion of the whole district sample with data collected over the three time periods, and the conduct and reporting of this study according to the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines and sound ethics principles.17,18 The limitations of the study are related to the use of routine records with missing data (there were data missing for the HIV parameters, for example, for all of 2013, due to the register being lost) or possibly incorrect data. There was no information in the registers about the timing of ART for HIV-infected TB patients, which is an important factor for treatment success and survival.19–21 Furthermore, as Bombali District was severely affected by the Ebola outbreak, we are not sure how generalisable our findings are to less severely affected districts.
How do the findings from Bombali District compare with national data from Sierra Leone reported in the WHO global TB reports during the study period? During 2013 and 2014, with 2014 including both the pre-Ebola and Ebola periods, the country registered respectively 12 072 and 12 721 TB cases.22,23 The national rates of bacteriologically confirmed PTB were approximately 60% in both years; the rates of HIV testing were respectively 93% and 87%, and of those found to be HIV-positive, 65–70% were started on ART.22,23 For the 2013 and 2014 patient cohorts, respectively 87% and 85% were reported to have successfully completed treatment.23,24 In Bombali District during the Ebola outbreak there were thus differences compared with the national data, with decreases in the number of patients notified with smear-positive PTB and in the proportions of patients tested for HIV. These differences were probably due to the 2014 national data overlapping the pre-Ebola and Ebola periods and including districts that were not severely affected by the Ebola outbreak.
Anecdotal reports that the community avoided visiting health facilities due to fears of contracting or being diagnosed with Ebola, or due to imposed quarantine measures, could explain some of the negative effects of the Ebola outbreak on TB control activities in Bombali District, which may have contributed to the decrease in the reported numbers of presumptive TB patients presenting for investigation. Similarly, health-care workers, including laboratory technicians and assistants, were redeployed in the community to help manage presumed and confirmed Ebola cases or to work in Ebola treatment units, and this may have led to a decline in skilled human resources to perform smear microscopy and perform HIV testing. Furthermore, HIV testing using rapid diagnostic testing kits usually requires fingerprick testing, which was discouraged during the Ebola outbreak.9
There are some important policy implications from this study. First, a study in neighbouring Guinea showed that TB control services can be maintained during the Ebola outbreak provided there is contingency planning and support for health systems, health services and health-care workers.11 These lessons need to be shared, discussed and taken up where appropriate.
Second, it is important to set up systems to ensure that patients with presumptive TB can be investigated if there is another Ebola outbreak. Depending on available resources, this could involve sputum specimens being dropped off at specified community locations for onward transport to laboratories. In the longer term, more attention could also be paid to point-of-care diagnostic tests based on rapid molecular technology.25
Third, the programmes need to identify other ways of performing HIV testing apart from the fingerprick test, such as oral salivary testing, and adopting established community-based approaches during emergency outbreaks such as Ebola.26,27
Finally, a well-functioning TB control programme must have timely and accurate data on case finding and treatment outcomes, not only for monitoring programme performance, but also for forecasting and procurement, to ensure a robust supply chain for drugs and consumables; some thought should go toward the establishment of real-time electronic monitoring systems at the district level. This has been successfully undertaken for the HIV/AIDS response in another resource-poor country, Malawi,28 and a similar model could be considered for Sierra Leone.
In conclusion, this is the first study from a district in Sierra Leone to look at the effect of the Ebola outbreak on TB control activities. The main negative effects were the decreases in the number of presumptive TB patients presenting with symptoms, the proportions diagnosed with smear-positive PTB and the proportions tested for HIV, while the initiation of ART in HIV-infected TB patients and overall treatment outcomes remained at acceptable levels.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the WHO/TDR, the Sierra Leone Ministry of Health (Freetown), the WHO Sierra Leone Country Office (Freetown) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of the SORT IT workshops were provided through the Centre for Operational Research, The Union; The Union South-East Asia Office (New Delhi, India); the Ministry of Health, Government of Karnataka (Karnataka, India); the Operational Research Unit (LUXOR), MSF Brussels Operational Centre (Luxembourg); Academic Model Providing Access to Health Care (AMPATH, Eldoret, Kenya); Alliance for Public Health (Kiev, Ukraine); Institute of Tropical Medicine (Antwerp, Belgium); University of Toronto (Toronto, ON, Canada); Dignitas International (Zomba, Malawi); Partners in Health-Sierra Leone (Boston, MA, USA); and the Baroda Medical College (Vadodara, India).
The programme was funded by the Department for International Development (London, UK) and the WHO/TDR. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. Baize S, Pannetier D, Oestereich L.. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 15: 1418– 1425. [DOI] [PubMed] [Google Scholar]
- 2. Gostin L O, Lucey D, Phelan A.. The Ebola epidemic: a global health emergency. JAMA 2014; 11: 1095– 1096. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. . Ebola situation report—6 January 2016. Geneva, Switzerland: WHO, 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-6-january-2016 Accessed March 2017. [Google Scholar]
- 4. Boozary A S, Farmer P E, Jha A K.. The Ebola outbreak, fragile health systems, and quality as a cure. JAMA 2014; 18: 1859– 1860. [DOI] [PubMed] [Google Scholar]
- 5. Ulrich C M. Ebola is causing moral distress among African healthcare workers. BMJ 2014; 349: g6672. [DOI] [PubMed] [Google Scholar]
- 6. National Ebola Response Centre. . Bo District, 2015. Freetown, Sierra Leone: NERC, 2015. http://www.nerc.sl/?q=sldistricts/bo Accessed March 2017. [Google Scholar]
- 7. Piot P, Muyembe J J, Edmunds W J.. Ebola in West Africa: from disease outbreak to humanitarian crisis. Lancet Infect Dis 2014; 14: 1034– 1035. [DOI] [PubMed] [Google Scholar]
- 8. Edelstein M, Angelides P, Heyman D L.. Ebola: the challenging road to recovery. Lancet 2015; 385: 2234– 2235. [DOI] [PubMed] [Google Scholar]
- 9. Zachariah R, Ortuno N, Hermans V, . et al. Ebola, fragile health systems and tuberculosis care: a call for pre-emptive action and operational research. Int J Tuberc Lung Dis 2015; 19: 1271– 1275. [DOI] [PubMed] [Google Scholar]
- 10. Leuenberger D, Hebelamou J, Strahm S, . et al. Impact of the Ebola epidemic on general and HIV care in Macenta, Forest Guinea, 2014. AIDS 2015; 29: 1883– 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortuno-Gutierrez N, Zachariah R, Woldeyohannes D, . et al. Upholding tuberculosis services during the 2014 Ebola storm: an encouraging experience from Conakry, Guinea. PLOS ONE 2016; 11: e0157296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Statistics Sierra Leone. . Sierra Leone 2015 population and housing census. Provisional results. March 2016. Freetown, Sierra Leone: Statistics Sierra Leone, 2015. [Google Scholar]
- 13. World Health Organization. . World Health Statistics, 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 14. Ministry of Health and Sanitation. . National health sector strategic plan 2010–2015. Freetown, Sierra Leone: MoHS, 2009. [Google Scholar]
- 15. Ministry of Health and Sanitation. . Ebola virus disease situation report 504. 14 October 2015. Freetown, Sierra Leone: MoHS, 2015. [Google Scholar]
- 16. World Health Organization. . Treatment of tuberculosis: guidelines. 4th ed WHO/HTM/TB.2009.420 Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 17. von Elm E, Altman D G, Egger M, . et al. The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 18. Edginton M, Enarson D, Zachariah R, . et al. Why ethics is indispensable for good-quality operational research. Public Health Action 2012; 2: 21– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blanc F X, Sok T, Laureillard D, . et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365: 1471– 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havlir D V, Kendall M A, Ive P, . et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365: 1482– 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karim S S A, Naidoo K, Grobler A, . et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365: 1492– 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. . Global tuberculosis report, 2014. WHO/HTM/TB/2014.08 Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 23. World Health Organization. . Global tuberculosis report, 2015. WHO/HTM/TB/2015.22 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 24. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 25. Lawn S D, Mwaba P, Bates M, . et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013; 13: 349– 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choko A T, Desmond N, Webb E L, . et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence settings: a cross-sectional feasibility study in Blantyre, Malawi. PLOS Med 2011; 8: e100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suthar A B, Ford N, Bachanas P J, . et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLOS Med 2013; 10: e1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Douglas G P, Gadabu O J, Joukes S, . et al. Using touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLOS Med 2010; 7: e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]


