Abstract
Setting: National Leprosy and Tuberculosis (TB) Control Programme, Liberia.
Objectives: To assess TB case finding, including human immunodeficiency virus (HIV) associated interventions and treatment outcomes, before (January 2013–March 2014), during (April 2014–June 2015) and after (July–December 2015) the Ebola virus disease outbreak.
Design: A cross-sectional study and retrospective cohort analysis of outcomes.
Results: The mean quarterly numbers of individuals with presumptive TB and the proportion diagnosed as smear-positive were: pre-Ebola (n = 7032, 12%), Ebola (n = 6147, 10%) and post-Ebola (n = 6795, 8%). For all forms of TB, stratified by category and age group, there was a non-significant decrease in the number of cases from the pre-Ebola to the Ebola and post-Ebola periods. There were significant decreases in numbers of cases with smear-positive pulmonary TB (PTB) from the pre-Ebola period (n = 855), to the Ebola (n = 640, P < 0.001) and post-Ebola (n = 568, P < 0.001) periods. The proportions of patients tested for HIV, found to be HIV-positive and started on antiretroviral therapy decreased as follows: pre-Ebola (respectively 72%, 15% and 34%), Ebola (69%, 14% and 30%) and post-Ebola (68%, 12% and 26%). Treatment success rates among TB patients were: 80% pre-Ebola, 69% Ebola (P < 0.001) and 73% post-Ebola (P < 0.001). Loss to follow-up was the main contributing adverse outcome.
Conclusion: The principal negative effects of Ebola were the significant decreases in diagnoses of smear-positive PTB, the declines in HIV testing and antiretroviral therapy uptake and poor treatment success. Ways to prevent these adverse effects from recurring in the event of another Ebola outbreak need to be found.
Keywords: smear-positive pulmonary tuberculosis, HIV testing, TB treatment outcomes, SORT IT, operational research
Abstract
Contexte : Programme national de la tuberculose (TB), Liberia.
Objectifs : Evaluer la détection des cas de TB, y compris les interventions associées au virus de l'immunodéficience humaine (VIH) et les résultats du traitement, avant (1 janvier 2013–31 mars 2014), pendant (1 avril 2014–30 juin 2015) et après la flambée d'Ebola (1 juillet–31 décembre 2015).
Schéma : Une etude transversale et analyse de cohorte rétrospective des résultats.
Résultats : Le nombre moyen mensuel par trimestre de présomptions de TB et de pourcentages de frottis positifs détectés était : pré-Ebola (n = 7032, 12%), pendant Ebola (n = 6147, 10%) et post-Ebola (n = 6795, 8%). Pour les TB toutes formes, stratifiées par catégorie et groupe d'âge, il y a eu une diminution non significative des nombres des périodes pré-Ebola à pendant Ebola et post-Ebola. Il y a eu une diminution significative des TB pulmonaire à frottis positif : pré-Ebola (855), pendant-Ebola (640, P < 0,001) et post-Ebola (568, P < 0,001). Les proportions de patients testés pour le VIH, trouvés VIH positifs et mis sous traitement antirétroviral (TAR) ont diminué : pré-Ebola (respectivement 72%, 15% et 34%), Ebola (69%, 14% et 30%) et post-Ebola (68%, 12% et 26%). Les taux de succès du traitement des patients TB ont été 80% pré-Ebola, 69% pendant Ebola (P < 0,001) et 73% post-Ebola (P < 0,001), avec les pertes de vue comme contributions principales aux résultats défavorables.
Conclusion : Les principaux effets négatifs d'Ebola ont été des diminutions significatives du diagnostic de TBP à frottis positif, des déclins du test VIH et de la prise du TAR et un résultat médiocre du traitement. Il faut trouver des moyens d'éviter que ces effets négatifs ne surviennent à nouveau en cas de nouvelle épidémie.
Abstract
Marco de referencia: El programa nacional contra la tuberculosis (TB) de Liberia.
Objetivos: Evaluar la búsqueda de casos de TB teniendo en cuenta las intervenciones asociadas con el virus de la inmunodeficiencia humana (VIH) y examinar los desenlaces terapéuticos antes del brote epidémico de fiebre hemorrágica del Ébola (de 1 enero del 2013 a 31 marzo del 2014), durante el brote (del 1 abril del 2014 a 30 junio del 2015) y después del mismo (de 1 julio a 31 diciembre del 2015).
Método: Un estudio transversal con análisis de cohortes retrospectivo de los desenlaces terapéuticos.
Resultados: Antes de la epidemia, el promedio mensual por trimestres de los casos con presunción de TB fue 7032 (12% de baciloscopias positivas), durante el brote fue 6147 (10%) y después de la epidemia fue 6795 (8%). En todos los casos de TB, estratificados por categorías y grupos de edad, durante la epidemia y después de ella se observó una disminución no significativa de las cifras, en comparación con el período anterior a la epidemia. Ocurrió una neta disminución de los casos de TB pulmonar con baciloscopia positiva, a saber: antes de la epidemia del Ébola n = 855, durante el brote n = 640 (P < 0,001) y después del mismo n = 568 (P < 0,001). Se observó una disminución de la proporción de pacientes en quienes se practicó la prueba del VIH, que obtuvieron un resultado positivo y que iniciaron el tratamiento antirretrovírico (ART), respectivamente como sigue: antes del brote del Ébola 72%, 15% y 34%, durante la epidemia 69%, 14% y 30% y después del brote 68%, 12% y 26%. La tasa de éxito del tratamiento antituberculoso fue 80% antes del brote, 69% durante la epidemia (P < 0,001) y 7% después de la misma (P < 0,001); el desenlace desfavorable predominante fue la pérdida durante el seguimiento.
Conclusión: Las principales consecuencias negativas de la epidemia del Ébola fueron una disminución considerable en el diagnóstico de TB pulmonar con baciloscopia positiva, una reducción de la práctica de la prueba del VIH y de la iniciación del ART y tasas insuficientes de éxito terapéutico. Se recomienda definir las medidas que puedan evitar estas consecuencias adversas, en la eventualidad de una futura epidemia.
Despite the progress made globally in the last 20 years with the implementation of the World Health Organization's (WHO's) Stop TB strategy, tuberculosis (TB) remains a major public health threat. It is estimated that one third of the world's population is infected with Mycobacterium tuberculosis, and 5–10% of infected persons are at risk of developing active TB during their lifetime. In 2014, an estimated 9.6 million people worldwide developed new active TB and 1.5 million people died from the disease, of whom 390 000 had associated human immunodeficiency virus (HIV) infection.1
Liberia is among several countries in West Africa where TB is a significant public health problem. The National Leprosy and Tuberculosis Control Programme (NLTCP) has been implementing DOTS-based TB control activities for many years in Liberia in line with WHO recommendations.2 Between 2011 and 2013, the numbers of notified cases of new and relapse TB ranged from 7500 to 8000 per annum, with an estimated case detection rate of 55–60% and a treatment success rate in new TB cases of around 80%.3
In 2013, West Africa was hit by Ebola virus disease (EVD), a severe, often fatal, zoonotic filovirus infection;4 the outbreak started in Guinea in December 2013 and rapidly spread to Sierra Leone and Liberia.5,6 Liberia diagnosed its first cases of Ebola in late March 2014, with the peak of the outbreak occurring between August and October 2014. The WHO declared Liberia free of Ebola on 9 May 2015. However, the country experienced another cluster of six cases in June 2015, was declared free of transmission on 3 September, experienced three more cases in November and was again declared Ebola-free on 14 January 2016.6 By June 2016, Liberia had reported 10 678 cases, with 4810 associated deaths.6
In all three affected countries in the region, disruptions occurred in the national programmes for TB, HIV/AIDS (acquired immune-deficiency syndrome) and malaria.7 Programme officers became sick, of whom some died as a result of Ebola. Many clinical teams and facilities were repurposed as part of the Ebola response, which took staff away from their disease control activities. With the ‘no touch’ policy in place, problems were reported with the delivery of anti-tuberculosis treatment and reduced uptake of HIV testing, which is performed with fingerpricks using rapid dipstick technology.8 In Guinea, the Ebola outbreak was reported to have resulted in an overall decline in the reporting of new TB cases,9 although another study in the same country showed that in facilities supported by non-governmental organisations, and where contingency planning was implemented, TB case finding and TB services could be maintained at satisfactory levels.10
These conflicting reports make it important to further assess what happened to TB case finding and treatment outcomes as a result of the EVD outbreak, and whether there were any particular types or category of TB or TB-HIV interventions that were particularly affected and which subsequently recovered in the post-Ebola period. This information may be useful at programme level to maintain services if another Ebola outbreak occurs in the future. We therefore undertook an assessment of TB case finding and treatment outcomes to observe what happened to patients before, during and after the Ebola outbreak in Liberia at the national level.
METHODS
Study design
This was a cross-sectional study combined with a retrospective cohort study of new smear-positive pulmonary TB (PTB) patients and their treatment outcomes using NLTCP data.
Setting
General setting
Liberia, situated in West Africa, is bordered by Guinea, Sierra Leone, Ivory Coast and the Atlantic Ocean. The estimated population is approximately 4 million, of whom approximately half live in urban areas. The per capita gross national income is US$2012.11 The country is divided administratively into 15 counties, which include 91 health districts. Medical services are provided by a mixture of public, private and non-governmental organisations (NGOs). In the public sector, health services are free of charge, including those for TB and HIV/AIDS.
TB control services in Liberia
Liberia started using the DOTS strategy for TB in 1999, and coverage at the county level is 100%.12 The NLTCP is responsible for all TB control activities, including the country's two specialised TB hospitals and 161 TB diagnostic/treatment centres distributed among the 727 health facilities in Liberia. Most TB diagnosis and treatment is conducted in the public sector, but some private, faith- and industry-based facilities undertake these activities free of charge based on a memorandum of understanding between the government and the private sector.
Clinicians and nurses provide routine diagnostics, management and care for patients with presumptive and diagnosed TB in the health facilities in line with WHO recommendations.2 Patients are identified as having presumptive TB if they have cough of ⩾2 weeks, and are investigated at TB diagnostic/treatment centres by sputum smear microscopy and chest radiography with set criteria used to diagnose smear-positive PTB, smear-negative PTB and PTB with smear not performed. Extra-pulmonary TB (EPTB) is diagnosed on the basis of clinical, radiographic and other supportive evidence. Diagnosed patients are registered according to the type of TB, and treatment is based on the category of TB (new or previously treated) using internationally recommended standardised treatment regimens of 6 months for new patients or 8 months for previously treated patients (provided there is no evidence of multidrug resistance), in line with WHO recommendations.2,13 All TB patients are offered HIV testing; those found to be HIV-positive are started on cotrimoxazole preventive therapy (CPT) and antiretroviral therapy (ART).
All patients are monitored until the end of treatment and are then assessed in relation to their treatment outcomes based on standardised WHO criteria, with the results recorded on the patient treatment cards and in the TB patient registers.2 Data at clinic level are collected daily and quarterly by clinicians and/or nurses, with supervision to check data accuracy undertaken monthly at the county level and quarterly by the central unit. The checked data are then sent to the district health office, transferred up the system to the county level, and from there to the Ministry of Health (MoH) through the Health Management Information System (HMIS). The data are collated as quarterly aggregate numbers in the HMIS.
Study population
The study included all patients in Liberia with presumptive and active TB who were investigated, diagnosed, registered or treated between 2013 and 2015 in the pre-Ebola (1 January 2013–31 March 2014), Ebola (1 April 2014–30 June 2015) and post-Ebola (1 July–31 December 2015) periods. Treatment outcomes were reported for new smear-positive PTB patients during the time periods studied for those enrolled in treatment up to 1 year previously.
Data variables, sources of data and data collection
The key data variables included, on a quarterly basis: year; quarter; number of presumptive TB patients investigated; patients with a positive sputum smear result; types and categories of TB stratified by age (0–4, 5–14 and ≥15 years); HIV testing and, of those found to be HIV-positive, the numbers started on CPT and ART; and the treatment outcomes of patients with new and relapse smear-positive TB. The primary source of data was the HMIS at the MoH headquarters.
Analysis and statistics
Data were extracted from the District Health Information System version 2 (Oslo, Norway) into an Excel database (Microsoft Corp, Redmond, WA, USA). A descriptive analysis was performed for the case finding, HIV testing and treatment outcomes in the pre-Ebola (1 January 2013–31 March 2014), Ebola (1 April 2014–30 June 2015) and post-Ebola (1 July–31 December 2015) periods. Quarterly means and standard errors were calculated for absolute numbers, and average proportions were derived during the pre-Ebola, Ebola and post-Ebola periods. Comparisons were made between the pre-Ebola and Ebola periods and between the pre-Ebola and post-Ebola periods. The quarterly means and standard errors were compared using Student's t-test, and proportions were compared using the χ2 test. Levels of significance were set at 5% (P < 0.05).
Ethics
Approval for the study was obtained from the Pacific Institute for Research and Evaluation Ethics Committee, University of Liberia Pacific (Monrovia, Liberia) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France). As aggregate data were used, informed patient consent was not required.
RESULTS
Presumptive tuberculosis
The numbers of persons with presumptive TB registered at laboratories and the proportions smear-positive are shown in Table 1 and Figure 1. Presumptive TB declined in the Ebola period, particularly at the peak of the outbreak in the third quarter of 2014, and then increased in the post-Ebola period, although these changes were not significant. The proportion of persons found to be smear-positive declined significantly in the Ebola period and decreased again in the post-Ebola period.
TABLE 1.
National cases of presumptive and registered TB along with HIV testing and care in the pre-Ebola, Ebola and post-Ebola disease outbreak periods, * Liberia, 2013–2015

FIGURE 1.
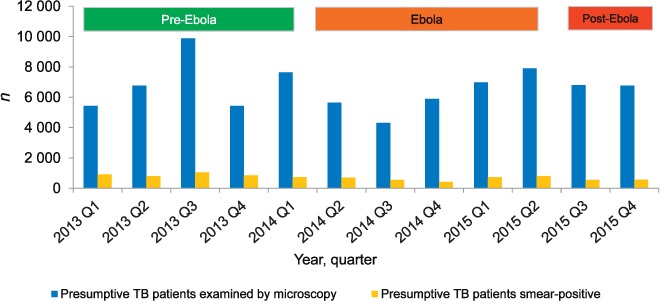
Numbers of persons with presumptive TB investigated nationally per quarter along with those identified as sputum smear-positive before, during and after the Ebola outbreak in Liberia, 2013–2015. Pre-Ebola period = 1 January 2013–31 March 2014; Ebola period = 1 April 2014–30 June 2015; post-Ebola period = 1 July–31 December 2015. TB = tuberculosis.
Tuberculosis cases
The numbers of patients registered with TB, stratified by type, category and age group, are shown in Table 1, Figure 2 and Figure 3. For TB all forms, stratified by category and age group, there was a non-significant decrease in numbers from the pre-Ebola period to the Ebola and post-Ebola periods. The most marked decreases in TB cases were in the last two quarters of 2014 during the peak of the outbreak. The key finding was a significant decrease in smear-positive PTB in the Ebola and post-Ebola periods, and a significant decrease in EPTB in the Ebola period.
FIGURE 2.
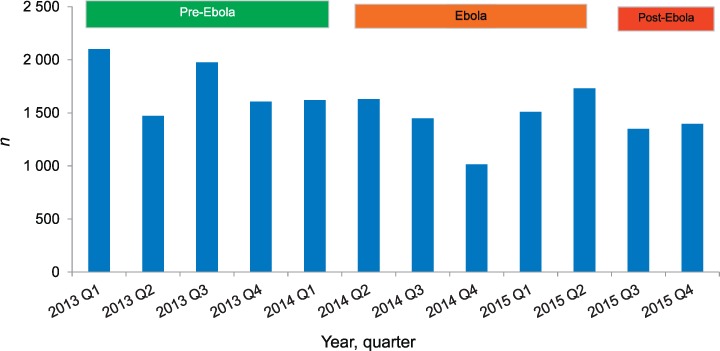
Numbers of TB cases (all forms) registered nationally per quarter before, during and after the Ebola outbreak in Liberia, 2013–2015. Pre-Ebola period = 1 January 2013–31 March 2014; Ebola period = 1 April 2014–30 June 2015; post-Ebola period = 1 July–31 December 2015. TB = tuberculosis.
FIGURE 3.

Numbers of TB cases registered nationally each quarter, stratified by type of TB, retreatment and age, before, during and after the Ebola outbreak in Liberia, 2013–2015. A) New TB cases, stratified by type of disease. B) Retreatment TB cases. C) All TB cases, stratified by children (aged 0–14 years) and adults (aged ≥ 15 years). Pre-Ebola period = 1 January 2013–31 March 2014; Ebola period = 1 April 2014–30 June 2015; post-Ebola period = 1 July–31 December 2015. TB = tuberculosis.
HIV testing and care
Information on HIV testing results, positive diagnoses and care are shown in Table 1 and Figure 4. From the pre-Ebola period to the Ebola and post-Ebola periods, there were progressive decreases in the proportion of TB patients tested for HIV, found to be HIV-positive and started on CPT and ART, and these were especially marked in the last two quarters of 2014. Significant decreases were seen in HIV testing, the number of HIV-positive results and the uptake of ART in the post-Ebola period compared with the pre-Ebola period.
FIGURE 4.

HIV testing and HIV care for TB cases registered nationally per quarter before, during and after the Ebola outbreak in Liberia, 2013–2015. A) Numbers of TB cases tested for HIV. B) HIV-positive TB cases of those tested for HIV. C) HIV-positive TB patients placed on CPT and ART. Pre-Ebola period = 1 January 2013–31 March 2014; Ebola period = 1 April 2014–30 June 2015; post-Ebola period = 1 July–31 December 2015. TB = tuberculosis; HIV = human immunodeficiency virus; CPT = cotrimoxazole preventive therapy; ART = antiretroviral therapy.
Treatment outcomes
The treatment success rates in new smear-positive PTB patients enrolled 1 year previously are shown in Table 2 and Figure 5. Treatment success was significantly lower among the patients reported in the Ebola and post-Ebola periods, although it improved slightly post-Ebola. The key contributing adverse outcomes that significantly increased in these two periods were loss to follow-up, transfer-out and failure.
TABLE 2.
Treatment outcomes in new smear-positive TB patients placed on treatment between 2012 and 2014, with outcomes assessed in the pre-Ebola, Ebola and post-Ebola disease outbreak periods, * Liberia, 2013–2015
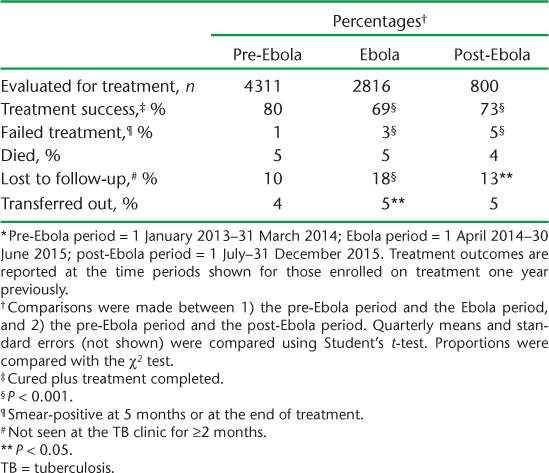
FIGURE 5.

Treatment success* rates in new smear-positive PTB patients registered nationally per quarter, before, during and after the Ebola outbreak in Liberia, 2012–2014. *Cured and treatment completed. Pre-Ebola period = 1 January 2013–31 March 2014; Ebola period = 1 April 2014–30 June 2015; post-Ebola period = 1 July–31 December 2015. PTB = pulmonary tuberculosis.
DISCUSSION
This is the first study to report on the effects of the EVD outbreak on TB case finding and treatment outcomes in Liberia. There were some important findings. First, there was a striking decline in the detection of presumptive smear-positive TB and in patients registered with smear-positive PTB in the Ebola period, and this continued into the post-Ebola period. Second, there were progressive decreases in HIV testing, HIV-positive test results and uptake of ART from the pre-Ebola to the Ebola and post-Ebola periods, reaching statistical significance in the post-Ebola period. Third, treatment success rates decreased, although this did improve somewhat in the post-Ebola period. The main contributory adverse outcomes were loss to follow-up, transfer-out and failure. Finally, the most marked declines in case finding, HIV testing and treatment outcomes occurred in the last two quarters of 2014, when the Ebola outbreak was at its peak.
The strengths of this study were the use of the national database, for which the data are checked at facility and county level, and the conduct and reporting of the study in line with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines and sound ethics principles.14,15 The study's limitations are related to the usual problems of accuracy and completeness of routinely collected data, particularly as data quality during the Ebola and post-Ebola periods may have declined due to a lack of supportive supervision. There is also no information about the timing of CPT or ART initiation in TB patients infected with HIV, both of which are important for treatment success and survival.16,17
The overall decreases in the numbers of patients presenting with presumptive TB, which in turn led to decreases in the numbers diagnosed and registered with TB, may have been due to symptomatic patients with cough and fever avoiding health facilities due to fears of contracting or being diagnosed with Ebola or because of imposed quarantine measures.18 Decreases in the numbers of cases with TB may also have been due to EVD increasing mortality in this patient group at the time that patients developed symptoms. The significant declines in diagnoses of smear-positive TB may have been due to health-care workers, including laboratory technicians, becoming ill or dying from Ebola, being redeployed in the community to help manage presumed and confirmed Ebola cases, and difficulties in performing laboratory procedures due to the ‘no touch’ policy. This policy may also have been one of the reasons for the significant decline in EPTB, for which clinical assessment and investigative procedures are crucial for diagnosis. As testing for HIV with rapid diagnostic test kits requires fingerprick testing, which is generally discouraged during an Ebola outbreak,8 it is not surprising to see a decline in HIV testing rates. In the post-Ebola period, however, the proportions of patients diagnosed with smear-positive TB and undergoing testing for HIV continued to decrease. The reasons for this are incompletely understood, but include delays in the procurement and distribution of consumables, delays in the training and replacing of essential staff and a lack of supervision, which is essential for maintaining standards.19
Treatment success rates deteriorated during the Ebola outbreak and did not return to the pre-Ebola levels. This was due mainly to high losses to follow-up, which increased from 10% to nearly 20% during the Ebola outbreak. Loss to follow-up can include unreported deaths,20 which may have been a factor among registered TB patients in Liberia. Interestingly, treatment failure rates increased. This may be due to patients interrupting treatment due to the various reasons discussed earlier, and is of concern, as failure may lead to drug-resistant TB.21
It is clear that the Ebola outbreak in Liberia had serious negative effects on many aspects of the NLTCP, which had still not fully recovered in the 6-month post-Ebola period. What can be done to hasten the recovery and prepare for future outbreaks? Laboratory capacity needs to be strengthened and consideration given to increased coverage of rapid point-of-care molecular diagnostic tests for TB, such as Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), which can eliminate some of the burdensome laboratory work involved with the preparation and reading of sputum smears.22 The NLTP needs to strengthen collaboration with the HIV/AIDS programme and identify alternative ways of performing HIV testing other than using fingerpricks, such as oral salivary testing,23 and ensuring uninterrupted supplies of HIV test kits and antiretroviral drugs. Despite the quarantine measures and travel restrictions, ways need to be found to maintain key NLTCP activities such as providing personal protective measures against Ebola for key front-line staff, ensuring regular and timely distribution of anti-tuberculosis drugs and consumables to facilities and maintaining facility-based supervision.19 Finally, guidance is needed from the WHO about when to start and when to stop emergency procedures, which would help programmes to be more resilient in the event of another Ebola outbreak.
In conclusion, this is the first national study from Liberia to assess the effect of the Ebola outbreak on TB control activities both during and after the outbreak. The principal negative effects were a significant decrease in the diagnosis of smear-positive PTB, a decline in HIV testing and ART uptake, and poor treatment success, largely due to high losses to follow-up. Ways to hasten the recovery of and to mitigate these adverse effects on TB control in the case of a future outbreak are needed.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the WHO/TDR, the Liberia Ministry of Health and Social Welfare (Monrovia), the WHO Country Office for Liberia (Monrovia) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of the SORT IT workshops were provided through the Centre for Operational Research, The Union; The Union South-East Asia Office (New Delhi, India); the Ministry of Health, Government of Karnataka, Bangalore, India; the Operational Research Unit (LUXOR), MSF (Brussels Operational Centre, Luxembourg); Academic Model Providing Access to Healthcare (AMPATH, Eldoret, Kenya); Baroda Medical College (Vadodara, India); the Institute of Medicine, University of Chester (Chester, UK); the Lighthouse Trust (Lilongwe, Malawi); and Aklilu Lemma Institute of Pathobiology (Addis Ababa, Ethiopia). The programme was funded by the Department for International Development (London, UK) and the WHO/TDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. World Health Organization. . Global tuberculosis report, 2015. WHO/HTM/TB/2015.22 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. World Health Organization. . Treatment of tuberculosis. Guidelines. 4th ed WHO/HTM/TB/2009.420 Geneva, Switzerland: WHO, 2010. [PubMed] [Google Scholar]
- 3. World Health Organization. . Global tuberculosis report, 2014. WHO/HTM/TB/2014.08 Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 4. Beeching N J, Fenech M, Houlihan C F.. Ebola virus disease. BMJ 2014; 349: 26– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baize S, Pannetier D, Oestreich L.. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 15: 1418– 1425. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. . Situation report. Ebola virus disease. 10 June 2016. Geneva, Switzerland: WHO, 2016. http://apps.who.int/iris/bit-stream/10665/208883/1/ebolasitrep_10Jun2016_eng.pdf Accessed March 2017. [Google Scholar]
- 7. Edelstein M, Angelides P, Heyman D L.. Ebola: the challenging road to recovery. Lancet 2015; 385: 2234– 2235. [DOI] [PubMed] [Google Scholar]
- 8. Zachariah R, Ortuno N, Hermans V, . et al. Ebola, fragile health systems and tuberculosis care: a call for pre-emptive action and operational research. Int J Tuberc Lung Dis 2015; 19: 1271– 1275. [DOI] [PubMed] [Google Scholar]
- 9. Leuenberger D, Hebelamou J, Strahm S, . et al. Impact of the Ebola outbreak on general and HIV care in Macenta, Forest Guinea. 2014. AIDS 2015; 29: 1883– 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortuno-Gitierrez N, Zachariah R, Woldeyohannes D, . et al. Upholding tuberculosis services during the 2014 Ebola storm: an encouraging experience from Conakry, Guinea. PLOS ONE 2016; 11: e0157296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. . World health statistics, 2014. Geneva, Switzerland: WHO, 2014. [Google Scholar]
- 12. National Leprosy and TB Control Programme. . National TB Strategic Plan 2007–2012. Monrovia, Liberia: Ministry of Health and Social Welfare, 2007. [Google Scholar]
- 13. Ministry of Health and Social Welfare. . National therapeutic guidelines for Liberia. Essential medicines list 2011. Monrovia, Liberia: Ministry of Health and Social Welfare, 2011. [Google Scholar]
- 14. von Elm E, Altman D G, Egger M, . et al. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 15. Edginton M, Enarson D, Zachariah R, . et al. Why ethics is indispensable for good-quality operational research. Public Health Action 2012; 2: 21– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blanc F X, Sok T, Laureillard D.. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365: 1471– 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suthar A B, Vitoria M A, Nagata J M, . et al. Cotrimoxazole prophylaxis in adults, including pregnant women with HIV: a systematic review and meta-analysis. Lancet HIV 2015; 4: e137– e150. [DOI] [PubMed] [Google Scholar]
- 18. Dynes M M, Miller L, Sam T, Vandi M A, Tomczyk B.. Perceptions of the risk for Ebola and health facility use among health workers and pregnant and lactating women—Kenema District, Sierra Leone. MMWR 2015; 6: 1226– 1227. [PubMed] [Google Scholar]
- 19. Frieden T R, Brudney K F, Harries A D.. Global tuberculosis: perspectives, prospects, and priorities. JAMA 2014; 312: 1393– 1394. [DOI] [PubMed] [Google Scholar]
- 20. Kruyt M L, Kruyt N D, Boeree M J, Harries A D, Salaniponi F M, van Noord P A.. True status of smear-positive pulmonary tuberculosis defaulters in Malawi. Bull World Health Organ 1999; 77: 386– 391. [PMC free article] [PubMed] [Google Scholar]
- 21. Caminero J A. Guidelines for clinical and operational management of drug-resistant tuberculosis. Paris, France: International Union Against Tuberculosis and Lung Disease, 2013. [Google Scholar]
- 22. Lawn S D, Mwaba P, Bates M, . et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 2013; 13: 349– 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choko A T, Desmond N, Webb E L, . et al. The uptake and accuracy of oral kits for HIV-self-testing in high HIV prevalence settings: a cross-sectional feasibility study in Blantyre, Malawi. PLOS Med 2011; 8: e100112. [DOI] [PMC free article] [PubMed] [Google Scholar]


