Abstract
Setting: The malaria-endemic country of Liberia, before, during and after the 2014 Ebola outbreak.
Objective: To describe the consequences of the Ebola outbreak on Liberia's National Malaria Programme and its post-Ebola recovery.
Design: A retrospective cross-sectional study using routine countrywide programme data.
Results: Malaria caseloads decreased by 47% during the Ebola outbreak and by 11% after, compared to the pre-Ebola period. In those counties most affected by Ebola, a caseload reduction of >20% was sustained for 12 consecutive months, while this lasted for only 4 consecutive months in the counties least affected by Ebola. Linear regression of monthly proportions of confirmed malaria cases—as a proxy indicator of programme performance—over the pre- and post-Ebola periods indicated that the malaria programme could require 26 months after the end of the acute phase of the Ebola outbreak to recover to pre-Ebola levels.
Conclusions: The differential persistence of reduced caseloads in the least- and most-affected counties, all of which experienced similar emergency measures, suggest that factors other than Ebola-related security measures played a key role in the programme's reduced performance. Clear guidance on when to abandon the emergency measures after an outbreak may be needed to ensure faster recovery of malaria programme performance.
Keywords: malaria diagnosis, malaria treatment, artemisinin-based combination therapy, rapid diagnostic test, operational research
Abstract
Contexte : Le Liberia, pays d'endémie palustre, avant, pendant et après l'épidémie d'Ebola de 2014.
Objectif : Décrire les conséquences de l'épidémie d'Ebola sur le programme national de lutte contre le paludisme et sa récupération après Ebola.
Schéma : Étude rétrospective transversale utilisant des données de routine du programme dans tout le pays.
Résultats : Le nombre de cas de paludisme déclarés a baissé de 47% pendant et de 11% après l'épidémie d'Ebola, comparé à la période pré-Ebola. Dans les comtés les plus affectés par Ebola, une réduction de plus de 20% a été maintenue pendant plus de 12 mois consécutifs, tandis que celle-ci n'a duré que pendant 4 mois consécutifs dans les comtés les moins affectés par Ebola. Une régression linéaire des proportions mensuelles de cas de paludisme confirmés—comme indicateur indirect de la performance du programme—sur les périodes pré- et post-Ebola a montré que le programme paludisme pourrait avoir besoin de 26 mois après la fin de la phase aiguë de l'épidémie d'Ebola pour revenir aux niveaux d'avant Ebola.
Conclusion: La persistance différentielle de réduction des cas déclarés dans les comtés les moins et les plus affectés, qui ont tous expérimenté des mesures d'urgence similaires, suggère que des facteurs autres que les mesures de sécurité liées à Ebola ont joué des rôles clés dans la réduction de la performance du programme. Des recommandations claires sur le moment auquel il faut abandonner les mesures d'urgence après une flambée pourraient être nécessaires pour assurer une récupération plus rapide de la performance du programme.
Abstract
Marco de referencia: El país de Liberia, con una situación endémica de paludismo, antes de la epidemia de fiebre hemorrágica del Ébola, durante el brote y después del mismo en el 2014.
Objetivos: Describir las consecuencias del brote epidémico del Ébola sobre el programa nacional contra el paludismo y su recuperación después de la epidemia.
Método: Fue este un estudio transversal retrospectivo a partir de los datos corrientes del programa en todo el país.
Resultados: La carga de morbilidad por paludismo disminuyó un 47% durante la epidemia y un 11% después de la misma, en comparación con el período anterior. En las provincias más afectadas por el brote se observó una disminución constante de más del 20% durante 12 meses consecutivos, comparada con 4 meses en las provincias menos afectadas. La regresión lineal de la proporción mensual de casos confirmados de paludismo, utilizada como indicador indirecto del desempeño del programa durante los períodos anterior y posterior a la epidemia del Ébola, puso de manifiesto que el programa precisó 26 meses después del final de la fase aguda de la epidemia hasta recuperar su nivel de desempeño anterior al brote.
Conclusiones: La recuperación diferencial de la notificación en las provincias menos afectadas y las más afectadas por la epidemia, pese a que en todas las regiones se ejecutaron intervenciones de emergencia equivalentes, indica que factores diferentes a las medidas de seguridad desencadenadas por la epidemia influyeron de manera importante en la disminución del desempeño del programa. Se precisan orientaciones claras con respecto al momento más oportuno para interrumpir las intervenciones de emergencia después de los brotes epidémicos, con el propósito de facilitar una recuperación más rápida del funcionamiento del programa contra el paludismo.
The 2014 Ebola virus disease (EVD) outbreak in West Africa was the largest in history, with over 28 000 confirmed cases and over 11 000 deaths.1 The outbreak started in Guinea and spread to Sierra Leone and Liberia, leading to a major breakdown of an already weak health system in Liberia.2 By 8 April 2015, EVD had infected a total of 9862 people and led to 4408 deaths in Liberia, of which 372 infections and 184 deaths occurred among health care workers.2 Several health facilities closed down or suspended various health care activities.2 There was a generalised loss of trust and a resulting decrease in health-seeking behaviour among the population,2,3 likely affecting the delivery of life-saving care to patients.4
The extent to which the health care system was compromised and the time that was required to recover to pre-Ebola levels have been assessed to some detail in various surveys,3,5 but not on the basis of countrywide data. The issue of health care system breakdown and recovery during and/or after a disaster such as the EVD outbreak is particularly relevant to malaria control efforts, as they have considerable impact on morbidity and mortality.6
EVD posed a particular challenge for malaria management due to the overlap of symptoms. Before the outbreak, malaria was already a major public health problem in Liberia, exacting a high toll among young children and pregnant women.6 Malaria is among the leading causes of mortality in the country, and it was an important source of morbidity during the outbreak.3,7 Adaptations of various malaria control strategies to the EVD context were attempted during the outbreak.8,9 There are anecdotal observations that many malaria cases went untreated due to fear of being sent to Ebola isolation units, and that health care providers abandoned their posts due to fear of contracting the disease.10,11
To better understand where the weaknesses and resilience of the malaria programme lay in the Liberian health care system, we set out to document the numbers of malaria cases diagnosed and treated, disaggregated by the extent of the outbreak in the different counties. We also attempted to model the anticipated time needed for the malaria programme to recover to pre-Ebola levels.
METHODS
Study design
This was a retrospective cross-sectional study using routine programme data. A county was considered ‘most affected’ if at the time of writing it reported >70 confirmed cases in the outbreak, while ‘least affected’ counties reported <10 confirmed cases. The most affected counties included Montserrado, Lofa, Margibi, Bong and Grand Cape Mount, while the least affected were Grand Gedeh, Grand Kru, Mary Land, River Cess and River Gee.
The period from January 2013 to March 2014 was considered the pre-Ebola period, from April 2014 to May 2015 the Ebola period and from June to December 2015 the post-Ebola period.
Setting
General setting
Liberia, a West African country bordered by Sierra Leone, Guinea and Côte d'Ivoire,12 has 15 counties subdivided into 91 health districts,13 with an estimated population of 4 million, 60% of whom live in urban areas, and a growth rate of 2.1%; 67% of the population are literate, and more than half of the population live below the poverty line.14 Liberia has a rainy and a dry season: malaria cases exhibit a seasonal pattern associated with the rainy season (mid-April to mid-October).14
National Malaria Control Programme
The National Malaria Strategic Plan has four main activities: case management of malaria, management of malaria in pregnancy, integrated vector management and advocacy and behaviour change interventions.15 The plan also aims to strengthen the National Malaria Control Programme (NMCP) by improving programme management, operational research, monitoring and evaluation, and overall health systems strengthening. Case management activities are designed to increase the availability and promote the use of artemisinin-based combination therapy (ACT) as first-line treatment for malaria among children aged <5 years according to the Liberia Ministry of Health and World Health Organization (WHO) guidelines.15
Every suspected case of malaria is required to be confirmed by either a rapid diagnostic test (RDT) or microscopy, and treated with ACT for uncomplicated malaria or artemether or quinine for severe cases. In several instances antimalarials are also offered to clinically diagnosed cases; while this is not in line with the national guidelines, it was recommended during the EVD outbreak as an emergency measure when the need to make a confirmatory diagnosis was challenged by the ‘no touch’ rule instituted in health facilities in August 2014. RDTs are supplied to all the health facilities.15 RDTs and ACTs can also be accessed through a system of community health workers, who are also supplied and managed through the health centres. Malaria diagnosis and treatment are offered free of charge to children aged <5 years and pregnant women in all public facilities; in some private facilities, partners subsidise malaria-related services for children aged <5 years and pregnant women at an affordable cost.15 However, the adoption of WHO guidelines recommending clinical diagnosis and treatment of malaria in the early stages of the Ebola outbreak, and a temporary moratorium imposed by malaria partners on malaria commodities due to leakages and stockouts at facilities in Liberia, might have resulted in low numbers of malaria cases being detected or treated at some point.16
Preventive measures to reduce the number of fever cases presenting at health facilities included the distribution of anti-malaria commodities, ACT in private pharmacies and medicine stores, mass drug distribution and nationwide distribution of insecticide-treated nets.17 Malaria prevalence in under-fives was 46%, and the number of diagnosed malaria cases was 1 583 754 in 2013.17 A two-round (Round 1: 18 October–21 November 2014; Round 2: 25 November–19 December 2014) campaign of mass drug administration of malaria chemoprophylaxis with artesunate/amodiaquine in the administrative zones of Monrovia showed an increase in treatment adherence from 91% to 99% from Round 1 to Round 2.8,17
Study participants
Aggregate data from all cases diagnosed and treated for malaria and reported through the Liberia Health Management Information System (HMIS) from January 2013 to December 2015 were included in the study.
Data variables, data sources and data collection
Data variables included malaria cases diagnosed using RDT and/or microscopy, or on the basis of clinical diagnosis alone, and treated with ACT and/or injectables, classified by county. Data were extracted from the Liberia HMIS. The principal investigator (NKD) supervised the overall data collection process and validated the data.
Analysis and statistics
The aggregated data were exported to an Excel v. 14 database (Microsoft, Redmond, WA, USA) for analysis. Data were described in monthly trends, with proportions being presented for test-confirmed cases and intravenous quinine treatment and by level of impact of the outbreak in the counties. Changes in numbers of cases diagnosed were assessed by comparing the numbers of cases before, during and after the outbreak for the corresponding months. The anticipated time to recovery of the NMCP was modelled through a linear fit of the data in the pre- and post-Ebola periods, and by calculating the intercept of the two linear models in accordance with a previously documented methodology.18
Ethics approval
Ethics approval was obtained from the University of Liberia-Pacific Institute for Research and Evaluation Institutional Review Board, Monrovia, Liberia, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France. As aggregate data were used for the study, informed patient consent was not needed.
RESULTS
Malaria caseloads
Figure 1 shows the monthly trends in reported malaria cases and proportions confirmed using RDT or microscopy across all counties and for the most and least affected EVD counties in the country. Countrywide, the number of cases dropped considerably as of August 2014, when emergency measures to contain the EVD outbreak were implemented; an overall reduction of 47% during the outbreak and of 11% post-outbreak was observed compared to the pre-Ebola period. When comparing monthly caseloads after the start of the outbreak with the corresponding months in 2013 (pre-Ebola), a reduction of more than 20% was observed for 7 consecutive months. In the counties most affected by EVD, this reduction was sustained for 12 consecutive months, while for the least affected counties the reduction was limited to 4 consecutive months.
FIGURE 1.
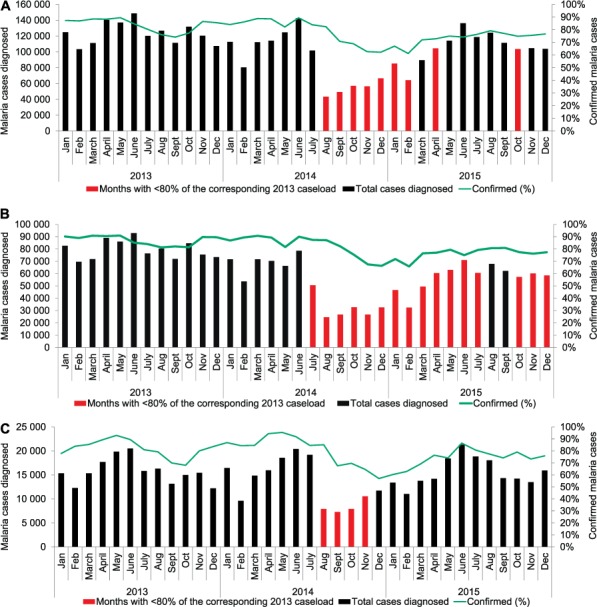
Diagnosed cases of malaria and proportions of confirmed malaria cases, Liberia, 2013–2015: A) nationwide, B) in counties most affected by the 2014 Ebola outbreak, and C) in counties least affected by the outbreak.
Treated cases
A similar pattern was observed for all treated cases: while numbers decreased countrywide during and post-outbreak (reductions of 46% and 12%, respectively), the decrease was sustained for longer in the most affected counties, but remained limited to 4 months in the least affected counties (Figure 2).
FIGURE 2.
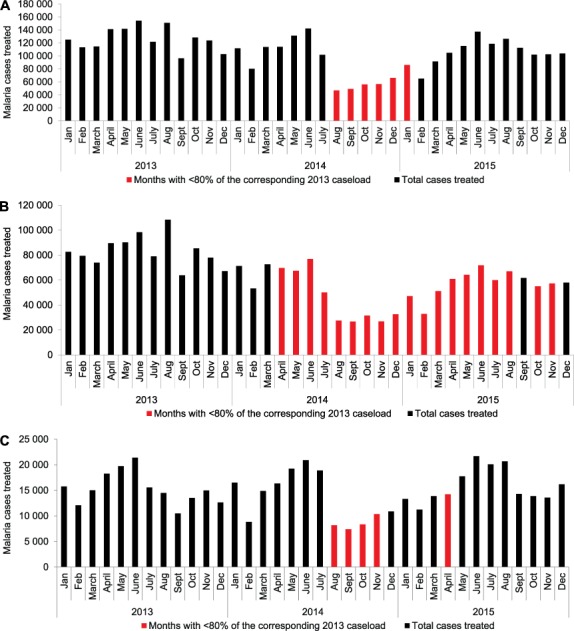
Treated cases of malaria (all treatment regimens) in Liberia, 2013–2015: A) nationwide, B) in counties most affected by the 2014 Ebola outbreak, and C) in counties least affected by the outbreak.
Programme performance
The proportion of confirmed malaria cases out of all notified cases was used as a proxy indicator for programme performance in the pre- and post-Ebola periods. During the Ebola period, the imposed no touch rule largely prohibited diagnosis from taking place. In the pre-Ebola period, the proportion of confirmed cases was 85%, falling to 67% in the Ebola period and rebounding to 76% post-Ebola. For the post-Ebola period, we attempted to model the time required for the proportion of confirmed cases to be restored to pre-Ebola levels. A linear regression was applied to the monthly confirmed proportions in the pre- and post-Ebola periods (Figure 3). We then extrapolated the linear trends for both periods: the intersect between the two linear trends was considered the timepoint at which the NMCP could be considered to have recovered to pre-Ebola levels. This point was reached 26 months after the end of the acute phase of the Ebola outbreak.
FIGURE 3.
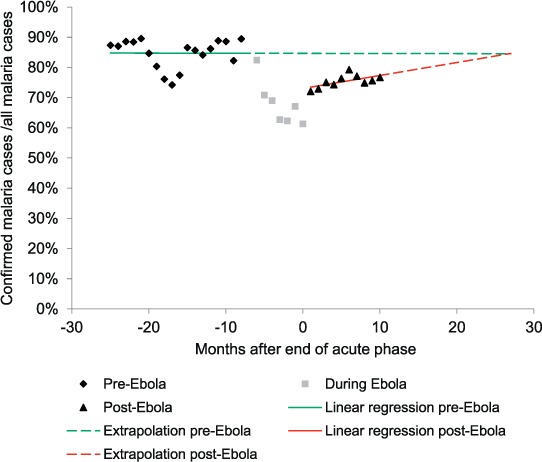
Linear regression analysis of the proportion of confirmed malaria cases, before and after the 2014 Ebola outbreak, Liberia, 2013–2015.
DISCUSSION
This is the first study to use national countrywide data to assess the consequences of the 2014 West Africa Ebola outbreak on the functioning of a national malaria programme. Starting in August 2014, the number of malaria cases diagnosed and treated decreased during the peak of the EVD outbreak, and subsequently rebounded. The rebound occurred much earlier in counties least affected by the EVD outbreak, and was considerably delayed in the most affected counties, despite the fact that similar emergency measures were in place.
Although the numbers of malaria cases diagnosed and treated returned to pre-EVD levels in the first year following the outbreak, the performance of the NMCP did not necessarily follow suit: while the proportion of confirmed malaria cases out of all cases—considered a proxy indicator for programme performance—recovered to some extent, modelling suggested that a period of more than 2 years is required for performance to recover to pre-EVD levels.
The emergency measures implemented by the Ministry of Health and its partners were aimed at preventing the spread of Ebola to areas that were untouched by the outbreak. These included the use of a sensitive case definition for Ebola in all health care settings: patients, and in particular febrile individuals, were screened mainly for Ebola instead of other conditions such as malaria. These unusual measures, including the fear among health-care workers of contracting EVD and the fear in the community of contracting the virus in the facilities or being sent to Ebola treatment units for their fever,11 might have contributed to the reduced malaria caseload during the peak of the Ebola period. In addition to these conditions, reporting and notification of malaria cases decreased, further contributing to the observed decrease in overall malaria caseloads. Reporting coverage during the pre-Ebola period for the 715 health facilities in Liberia was 83% in 2013, which decreased to 72% (723 facilities) in 2014 and rebounded to 83% (724 facilities) in 2015.17,19,20 A mass antimalarial drug administration campaign conducted in major communities in Montserrado County might have negatively contributed to malaria testing and diagnosis, both by reducing the malaria caseload at facility level and by reducing trust in malaria treatment due to side effects experienced during the campaign.8
The more rapid recovery of malaria caseloads in the least affected counties suggests that emergency measures per se, implemented nationwide, were possibly not the only drivers of the reduction in malaria diagnosis and treatment. In the least-affected counties, there was likely less fear and perhaps more ‘normality’ in daily life than in the most affected counties. Health facilities were kept open more regularly, as opposed to the most affected counties, where even the main referral hospital in Liberia was abandoned by its staff. A study in Guinea also documented differences between areas that were highly and less affected by Ebola.4
Strengths of the study include the use of national-level 2013–2015 data from the Liberia HMIS across all 15 counties of the country. Study data are thus representative of the countrywide malaria situation. Furthermore, the study adhered to STROBE (STrengthening the Reporting of OBservational Studies in Epidemiology) guidelines for the reporting of observational data and sound ethics principles.21,22 The main weakness of the study was likely the data quality: only data from the Liberian HMIS could be included in the study, and the quality of reporting before, during and after the outbreak is unknown. Statistical analyses were kept to the minimum to avoid over-interpreting the data; however, this may have introduced some biases. Other weaknesses included the lack of further disaggregation of national data for the full study period, precluding subanalyses of different populations, such as children aged <5 years and pregnant women.
The findings of our study carry two main implications. First, as our results suggest that the fear and stigma of Ebola may have been a more important driver of reduced malaria care during and immediately after the EVD outbreak than the emergency measures per se, there may be a need for stronger emphasis on community engagement and community-based health education during outbreaks of diseases such as EVD. Maintaining and/or restoring faith in the health system could have prevented or mitigated the huge fall in malaria diagnosis and treatment, even in the presence on the unusual security measures. Second, the continued reliance of presumptive treatment for malaria, modelled to last up to 2 years after the end of the acute phase of the EVD outbreak, suggests that insufficient attention may have been given to reinstating the NMCP after the emergency. While presumptive treatment of malaria was permissible during the outbreak—particularly in the absence of adequate personal protective equipment23—pro-active measures could have aided frontline staff to transition smoothly from the emergency to the post-emergency phase. Such measures could include clear directives as to when to resume malaria confirmation as standard, adequate stocking of kits and possible psychological support of staff (due to the trauma experienced), in addition to implementing primary health care approaches in general.24 The temporary moratorium imposed in July 2013 and the irregular availability of diagnostic and treatment agents during the Ebola outbreak resulted in fewer malaria cases being confirmed or treated.17 Liberia should nevertheless aim to treat only confirmed cases of malaria, in line with WHO guidelines.25
In conclusion, we observed reductions in the number of malaria cases and treatments reported during the acute phase of the Ebola outbreak in Liberia. The difference in time to recovery in the least and most affected counties, despite similar nationwide security measures, highlights the role of other drivers in this process. The longer time required to restore the quality of the programme, as assessed by the time to restore confirmation of malaria, suggests a need for recovery measures to be instated, including clear directives as to when to resume malaria confirmation as standard.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union; Paris, France) and Médecins Sans Frontières (MSF; Paris, France). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by the WHO/TDR, the Liberia Ministry of Health (Monrovia), WHO Liberia (Monrovia, Liberia) and the Centre for Operational Research, The Union. Mentorship and the coordination/facilitation of the SORT IT workshops were provided through the Centre for Operational Research, The Union; The Union SouthEast Asia Office, New Delhi, India; the Ministry of Health, Government of Karnataka, Bangalore, India; the Operational Research Unit (LUXOR), MSF, Brussels Operational Centre, Luxembourg; Academic Model Providing Access to Healthcare (AMPATH), Eldoret, Kenya; Baroda Medical College, Vadodora, India; the Institute of Medicine, University of Chester, Chester, UK; The Lighthouse Trust, Lilongwe, Malawi; and Aklilu Lemma Institute of Pathobiology, Addis Ababa, Ethiopia. The programme was funded by the Department for International Development (London, UK) and WHO/TDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution Intergovernmental organizations license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
References
- 1. World Health Organization. . Ebola situation report. Geneva, Switzerland: WHO, 2016: 12. [Google Scholar]
- 2. Ministry of Health, Government of Liberia. . Investment plan for building a resilient health system in Liberia: 2015 to 2021. Monrovia, Liberia: MoH, 2015: 71. [Google Scholar]
- 3. Kuehne A, Lynch E, Marshall E, . et al. Mortality, morbidity and health-seeking behaviour during the Ebola epidemic 2014–2015 in Monrovia: results from a mobile phone survey. PLOS Negl Trop Dis 2016; 10: e0004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plucinski M M, Guilavogui T, Sidikiba S, Diakité N.. Effect of the Ebola virus disease epidemic on malaria case management in Guinea, 2014: a cross-sectional survey of health facilities. Lancet Infect Dis 2015; 15: 1017– 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McLean K E, Abramowitz S A, Ball J D, . et al. Community-based reports of morbidity, mortality, and health-seeking behaviours in four Monrovia communities during the West African Ebola epidemic. Glob Public Health 2016; July: 1– 17. [DOI] [PubMed] [Google Scholar]
- 6. Government of Liberia. . Liberia Demographic and Health Survey 2013. Monrovia, Liberia: Government of Liberia, 2014. [Google Scholar]
- 7. World Health Organization. . World health statistics, 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 8. Kuehne A, Tiffany A, Lasry E, . et al. Impact and lessons learned from mass drug administrations of malaria chemoprevention during the Ebola outbreak in Monrovia, Liberia, 2014. PLOS ONE 2016; 11: e0161311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aregawi M, Smith S J, Sillah-Kanu M, . et al. Impact of the mass drug administration for malaria in response to the Ebola outbreak in Sierra Leone. Malar J 2016; 15: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ukpai O M, Ubiaru P C.. Impact of the 2014/2015 Ebola virus disease epidemic on malaria cases and control in some West African countries: a mini review. Am J Med Case Rep 2015; 3: 325– 328. [Google Scholar]
- 11. Edelstein M, Angelides P, Heymann D L.. Ebola: the challenging road to recovery. Lancet 2015; 385: 2234– 2235. [DOI] [PubMed] [Google Scholar]
- 12. Liberia Institute of Statistics & Geo-Information Services. . Population and housing census. Monrovia, Liberia: LISGIS, 2008. [Google Scholar]
- 13. Ministry of Health, Government of Liberia. . Liberia National Health Policies and Plan (2011–2021). Monrovia, Liberia: MoH, 2011. [Google Scholar]
- 14. Liberia Institute of Statistics & Geo-Information Services. . Household income and expenditure survey, 2014. Monrovia, Liberia: LISGIS, 2016. [Google Scholar]
- 15. Ministry of Health, Government of Liberia. . National Malaria Strategic Plan: 2010–2015. Monrovia, Liberia: MoH, 2016. [Google Scholar]
- 16. Government of Liberia. . President's Malaria Initiative; Malaria Operational Plan, Liberia. Monrovia, Liberia: Government of Liberia, 2016. [Google Scholar]
- 17. Ministry of Health, Government of Liberia. . Annual health report. Monrovia, Liberia: MoH, 2013. [Google Scholar]
- 18. Benedetti G, Mossoko M, Nyakio Kakusu J P, . et al. Sparks creating light? Strengthening peripheral disease surveillance in the Democratic Republic of Congo. Public Health Action 2016; 6: 54– 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liberia Ministry of Health. . Annual health report. Monrovia, Liberia: MoH, 2014. [Google Scholar]
- 20. Liberia Ministry of Health. . Annual health report. Monrovia, Liberia: MoH, 2015. [Google Scholar]
- 21. Elm von E, Altman D G, Egger M, . et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 22. Edginton M, Enarson D, Zachariah R, . et al. Why ethics is indispensable for good-quality operational research. Public Health Action 2012; 2: 21– 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. . Guidance on temporary malaria control measures in Ebola-affected countries. Geneva, Switzerland: WHO, 2015: 5. [Google Scholar]
- 24. Scott V, Crawford-Browne S, Sanders D.. Critiquing the response to the Ebola epidemic through a primary health care approach. BMC Public Health 2016; 16: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization. . Guidelines for the treatment of malaria. 3rd ed Geneva, Switzerland: WHO, 2015. [Google Scholar]


