Abstract
Background and study aims
Endoscopic mucosal resection (EMR) offers a minimally invasive therapy for advanced esophageal dysplasia and early cancers but stricture formation limits its applicability. We aimed at assessing the efficacy of placement of a commercially available biological mesh for preventing stricture formation following esophageal EMR.
Methods
25 swine were submitted to circumferential esophageal EMR with 10-cm extent and divided in five groups: one group with EMR only (control); one receiving an uncovered stent (stent-only group); and three groups receiving a stent covered with one of three extracellular matrices, namely small intestine submucosa (SIS group), acellular dermal matrix (ADMgroup), or urinary bladder matrix (UBM group). Stricture formation was evaluated with weekly esophago-grams.
Results
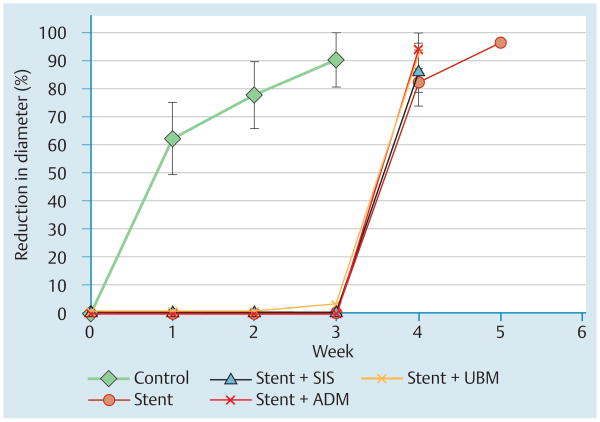
The stent-only group had significantly less stricture formation and survival was extended compared with controls (4.8 vs. 2.4 weeks). Compared with stenting only, the addition of a biological mesh did not reduce stricture formation: percent reductions in esophageal diameter for the groups were SIS 86%, ADM94%, and UBM 94%, compared with 82% in the stent-only group.
Conclusions
Placement of commercially available biological meshes did not alter remodeling sufficiently to prevent stricture formation after esoph-ageal EMR.
Introduction
Early esophageal cancer and high grade dysplasia (HGD) associated with Barrett's esophagus have traditionally been treated with esophagectomy. Ablative techniques such as photodynamic therapy, radiofrequency ablation, and cryotherapy have demonstrated some success but entail the risk of undertreatment, bleeding, perforation, and stricture development [1, 2] and require multiple sessions.
Endoscopic mucosal resection (EMR) maintains a low rate of morbidity and mortality while providing an intact tissue specimen that supplies valuable staging information [3], but contraction of the wound edges results in debilitating stricture. Biological mesh or extracellular matrix (ECM) has been used to treat esophageal defects and the effects ofcircumferential EMR in canine models [4– 8]. ECM has been reported to induce reparative effects by which the injured tissue is remodeled to resemble native tissue [9]. If ECM can prevent stricture formation, EMR could be rapidly implemented for treating HGD, Barrett's esophagus, or intramucosal esophageal cancer. Using a porcine model we investigated the efficacy of three commercially available ECM products, mounted on self-expanding stents, for reducing the extent of esophageal stricture following circumferential EMR.
Materials and methods
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University, Cleveland, Ohio, USA. A total of 25 swine were divided into five groups: Control (no stent,); stent-only (self-expanding silicone stent,); SIS (stent covered with small intestine submucosal ECM; Biodesign, Cook Medical, Bloomington, Indiana, USA), ADM(acellular dermal matrix-covered stent; Strattice, Lifecell Corporation, Bridgewater, New Jersey, USA), UBM (stent with urinary bladder membrane ECM; Matristem, Acell Corporation, Columbia, Maryland). All the stents used were self-expanding silicone type (Polyflex 18–23×120mm; Boston Scientific, Natick, Massachusetts, USA).
EMR was performed as described previously [10]. Briefly, animals were anesthetized and a band ligator was used to create a circumferential incision 20cm proximal to the lower esophageal sphincter. Endoscopic forceps were used to dissect free a 10-cm stalk of mucosa which was resected with snare electrocautery. In the control group, no additional therapy was performed. In the remaining animals, the esophageal stent was left uncovered or was wrapped in ECM (Fig.1). A purse-string suture was used to close the distal end and two long sutures were looped through the proximal interstices of the stent. The stent was placed over the endoscope, both were passed over a wire and delivered to fully cover the defect. The long sutures were secured percutaneously to the animal's chin to prevent migration. Bupivicaine (0.5%; Hospira Inc., Lake Forest, Illinois, USA) was injected locally and buprenorphine (5mcg/kg, IM; Reckitt Benckiser Pharmaceuticals Inc., Richmond, Virginia, USA) was administered postoperatively to facilitate pain relief.
Fig.1.
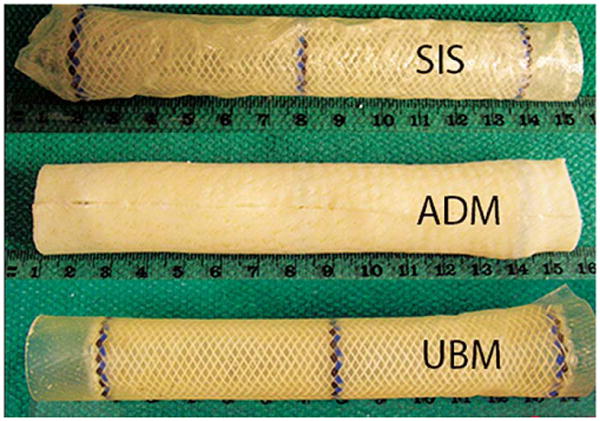
Polyflex stents covered with a biological extracellular matrix (ECM) mesh prior to implantation following esophageal mucosal resection (EMR) in pigs. SIS, small intestine submucosa (Biodesign); ADM, acellular dermal matrix (Strattice); UBM, urinary bladder matrix (Matristem).
Immediately following EMR, fluoroscopic images were recorded. A radiopaque ruler was placed in the field for scale. Animals recovered and resumed a normal diet. Two-dimensional esophagograms were later used to measure the stricture parameters of contraction along the lesion length, reduction in luminal diameter, and dilation proximal to the lesion, by comparing values to those obtained at baseline. Three measurements of diameter were made along the lesion (proximal, middle, and distal). A single diameter measurement was made proximal to the lesion, to calculate the proximal dilation as an indirect indication of stricture resistance. A single measurement was also recorded for calculation of contraction in the longitudinal direction obtainable only once the stent had been removed.
At weekly intervals, animals were sedated (Telazol, 4mg/kg), weighed and assessed for stricture progression. At week 3 stents were removed. Animals displaying symptoms of stricture (regurgitation or poor weight gain) were provided with a liquid diet and were euthanized when the stricture (reduction in luminal diameter) was estimated endoscopically, to exceed 80%. Tissue samples were collected for histologic analysis, fixed in formalin, embedded, sectioned, and stained with hematoxylin and eosin (H&E). The degrees of inflammation, healing and fibrosis were assessed by a GI pathologist blinded to the treatment groups using a semi-quantitative 5-point Likert scale.
It was determined that a sample size of 5 per group was required to detect a 25% smaller stricture size with a significance level of 0.05 and power of 0.80 using a t test. All comparisons between groups were made using a t test or Mann–Whitney rank sum (SigmaStat, version 3.11; Sysstat Software).
Results
All animals (n=25) successfully underwent EMR without complication. Average starting weights and EMR lesions were similar between groups. One animal was excluded as when the tethering sutures were severed the stent migrated into the stomach. No other complications (migrations, obstructions, erosions) were encountered.
The control group rapidly developed strictures whereas in the stent-only group, no stricture formation was detected while the stent was in place (Fig.2 and Fig.3). At week 2, the stentonly group showed significantly less reduction in esophageal diameter (mean 0% vs 77.7%, standard deviation [SD] 12.1%; P=0.008] (Fig.2 and Fig.3). Survival was longer (mean 4.8 vs. 2.4 weeks, P<0.001) compared with controls. All stents were easily removed at week 3, with mild abrasion bleeding.
Fig.2.
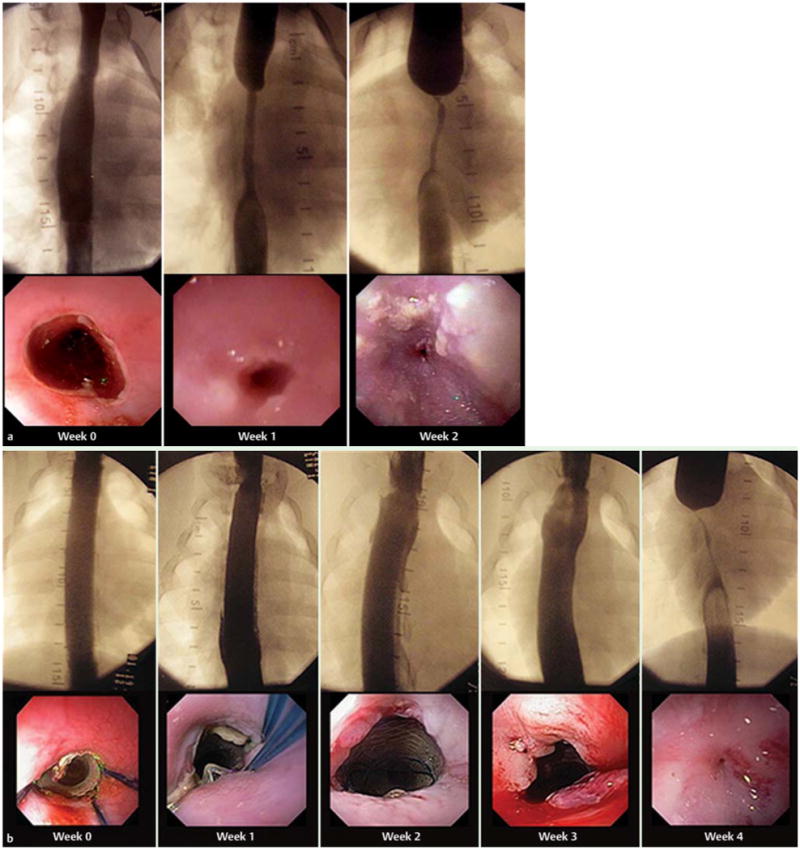
Fluoroscopic and endoscopic view of stricture progression following esophageal mucosal resection in pigs: a in a representative control animal, and b in a representative stent-only animal.
Fig.3.
Use of stents covered with a biological mesh following esophageal mucosal resection in pigs. Percent reduction in esophageal diameter (stricture size) over time for the control and experimental groups. SIS, small intestine submucosa (Biodesign); ADM, acellular dermal matrix (Strattice); UBM, urinary bladder matrix (Matristem).
Comparison between the reduction in diameter (Fig.3), proximal dilation, and lesion length, at week 2 for controls vs. week 4 for stent-only animals (the last week for which all animals remained alive) demonstrated no significant differences.
Similarly to the stent-only group, in groups evaluating ECM, reduction in diameter was significantly less at week 2 compared with controls (0%, P=0.008 for all three groups) (Fig. 3).
Results for each stented group 1 week after stent removal (week 4) are compared with those for controls at 1 week without treatment (week 1) in Table1. Diameter reduction in each of the stented groups was significantly greater than that in the control group after1 week without treatment (stent only, P=0.021; stent +SIS, P=0.008; stent+ADM, P=0.016; stent+UBM, P=0.001; Table1). Similarly, significant differences in longitudinal shortening of the lesion and in proximal dilation were seen in almost all comparisons with the control group (Table1). Compared with the stent-only group, significantly greater stricture formed in the ADMgroup (P=0.016) and the UBM group (P= 0.037). Additionally, a significantly greater contraction in lesion length occurred in the UBM group (P=0.018) compared with the stent group.Ultimately, compared with the stent-only group, the three groups with biologic ECM-covered stents did not show improvement in the measures of stricture formation, namely diameter reduction, proximal dilation, or lesion length (Table1).
Table 1.
Stricture formation after esophageal mucosal resection (EMR) in pigs: effect of use of stents covered with a biological extracellular matrix (ECM) mesh. Control animals after one week (week 1), and treatment groups after one week without stent (week 4). SIS, small intestine submucosa (Biodesign); ADM, acellular dermal matrix (Strattice); UBM, urinary bladder matrix (Matristem).
| Control (week 1) | Stent-only (week 4) | Stent with biological mesh (week 4) | |||
|---|---|---|---|---|---|
| +SIS | +ADM | +UBM | |||
| Number of animals, n | 5 | 5 | 5 | 4 | 5 |
| Esophageal diameter, % reduction, mean (SD) | 62.2 (12.9) | 82.0 (8.3)1 | 86.5 (7.5)1 | 94.4 (1.9)1,2 | 94.0 (6.9)1,2 |
| Lesion length, % of baseline length, mean (SD) | 77.6 (12.4) | 49.6 (11.7)1 | 43.0 (25.1) | 38.5 (8.8)1 | 32.0 (6.2)1,2 |
| Proximal dilation, percent, mean (SD) | 128.2 (6.2) | 203.5 (19.8)1 | 198.6 (24.6)1 | 203.7 (25.6)1 | 195.9 (30.4)1 |
P<0.05; at week 4 vs. Control at week 1
P<0.05; at week 4 vs. Stent-only at week 4.
Histologic examination of the resected mucosal tissue showed normal-appearing epithelium, lamina propria, muscularis mucosae, and superficial submucosa. Necropsy specimens proximal to the EMR site showed normal-appearing full-thickness esophagus with no pathologic alterations. Specimens from the site of the stricture were more heterogeneous, demonstrating evidence of ulceration, granulation, repair, and fibrosis. Evidence of re-epithelialization was present in some cases. (Fig.4). None of the samples showed evidence of residual ECM. Nostatistical differences were noted between groups in terms of histologic findings.
Fig.4.
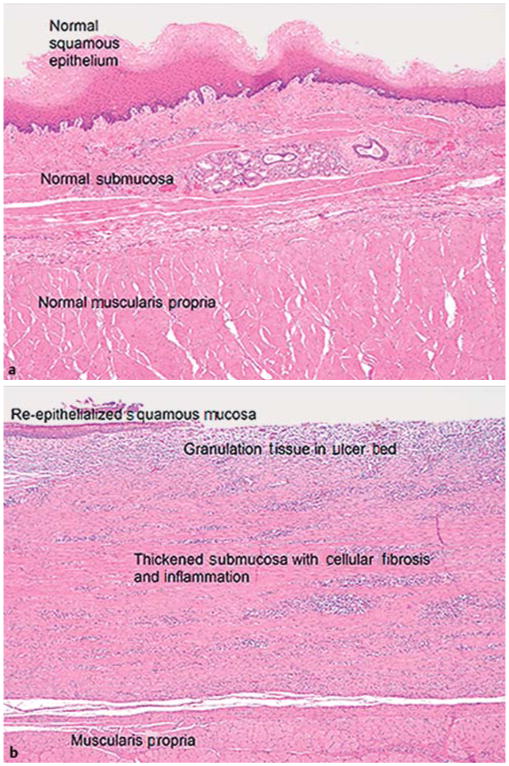
Esophageal mucosal resection in pigs. Histologic sections for a representative animal (hematoxylin and eosin [H&E];× 40 original magnification): a native esophagus; b area of stricture.
Discussion
ECM has been investigated extensively with regard to modifying the tissue healing response in numerous anatomic sites, and has been used with success in various surgical specialties. After creation of an EMR lesion, stents with three commercially available ECM products were applied to assess the effect on healing. Unstented control animals quickly developed clinically significant stricture after circumferential EMR [10]. The placement of a silicone stent alone or with ECM maintained the lumen and delayed the time to deterioration. Unfortunately, stricture formation occurred shortly after stent removal. Ultimately, the maximal degree of luminal narrowing, contraction of the lesion, or proximal dilation was not altered by stent or ECM placement. Moreover, stricture in control animals one week after EMR was actually less than in treated animals one week after stent removal (week 4). This suggests that, although esophageal narrowing was resisted by the stent, the ECM did not provide a substrate to attenuate the fibrotic process or prevent ultimate contraction. Histologic analysis of the lesion in all groups provided evidence of complex fibrotic processes while demonstrating no differences in inflammation, collagen deposition, fibroblast cellularity, and markers of healing such as neovascularization, further indicating that stenting whether alone or with ECM did not alter the underlying remodeling that occurred post EMR.
Our attempt to advance this field, by providing a simple means of ECM application to EMR lesions, was not as successful as had been anticipated on the basis of the success of other animal studies. However, Badylak et al. [7] demonstrated that xenogeneic ECM can reduce stricture formation after partial circumferential EMR in a canine model, but that the use of ECM scaffolds as a circumferential graft resulted in early stricture. Likewise, the success of Nieponice et al. [8] using ECM scaffolds was achieved with a shorter defect (5-cm) than in our model (9–11-cm). Our robust circumferential 10-cm resection may have exceeded the capability of ECM to be effective.
The reduction in stricture size observed in their study, however, may also be attributable to their method of preparation of the UBM or to the attachment method (surgical adhesive). In the current study, the lack of significant reduction in stricture in the ECM groups may have been due to the technique used to deliver the ECM to the esophagus rather than to the material itself. The mucosal secretions and dynamic environment natural to the esophagus are detrimental for ECM engraftment. Endoscopic images showed that the stent and esophagus wall move at least partly independently, and that the ECM appears to be more affixed to the stent than to the esophagus. Although the purpose of the stent was to apply radial force to the ECM to ensure adequate contact with the esophageal wall, this may not have occurred sufficiently. The use of a surgical adhesive instead of a stent to affix the ECM may circumvent this limitation and offers potential for future study. Likewise, additional tissue biopsies taken during weekly surveillance could have provided evidence of ECM integration but were not collected to avoid interfering with the healing process. In vitro studies of wound contraction and pharmacologic inhibition of fibrosis may benefit this field.
Perhaps because of the technical approach, rather than the biologic material itself, delivery of a commercially available biological ECM substrate on a stent, in this model, did not alter the wound remodeling that occurred and ultimately resulted in substantial stricture formation.
Acknowledgments
Partial funding of this project was provided by Olympus America, Center Valley, Pennsylvania, USA. The Polyflex stents, Biodesign SIS matrix, Strattice acellular dermal matrix, and Matristem urinary bladder matrix used in this study were graciously provided by Boston Scientific (Natick, Massachusetts, USA), Cook Medical (Bloomington, Indiana, USA), Lifecell (Bridgewater, New Jersey, USA), and Acell Corporation (Columbia, Maryland, USA), respectively.
Competing interests: S.J. Schomisch serves as a consultant for Novelmed Therapeutics. J. L. Ponsky serves as a consultant for US Endoscopy. J. M. Marks receives an honorarium as a consultant for Olympus, WL Gore, and G.I. Supply, and for serving on the advisory board for Apollo Endosurgery.
Footnotes
None of the other authors have relationships to disclose.
References
- 1.Pech O, Ell C. Endoscopic therapy of Barrett's esophagus. Curr Opin Gas-troenterol. 2009;25:405–411. doi: 10.1097/MOG.0b013e32832d9b71. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer DE, Sharma VK. Endoscopic ablation of Barrett's esophagus using the Halo system. Dig Dis. 2008;26:280–284. doi: 10.1159/000177009. Epub 2009 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieth M, Ell C, Gossner L, et al. Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy. 2004;36:776–781. doi: 10.1055/s-2004-825802. [DOI] [PubMed] [Google Scholar]
- 4.Rhee PH, Friedman CD, Ridge JA, et al. The use of processed allograft dermal matrix for intraoral resurfacing: an alternative to split-thickness skin grafts. Arch Otolaryngol Head Neck Surg. 1998;124:1201–1204. doi: 10.1001/archotol.124.11.1201. [DOI] [PubMed] [Google Scholar]
- 5.Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg. 2012;130:44S–53S. doi: 10.1097/PRS.0b013e31825f2215. [DOI] [PubMed] [Google Scholar]
- 6.Silverman RP. Acellular dermal matrix in abdominal wall reconstruction. Aesthet Surg J. 2011;31:24S–9S. doi: 10.1177/1090820X11418090. [DOI] [PubMed] [Google Scholar]
- 7.Badylak S, Meurling S, Chen M, et al. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097–1103. doi: 10.1053/jpsu.2000.7834. [DOI] [PubMed] [Google Scholar]
- 8.Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–296. doi: 10.1016/j.gie.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Cobb MA, Badylak SF, Janas W, et al. Histology after dural grafting with small intestinal submucosa. Surg Neurol. 1996;46:389–393. doi: 10.1016/s0090-3019(96)00202-9. [DOI] [PubMed] [Google Scholar]
- 10.Pauli EM, Schomisch SJ, Furlan JP, et al. Biodegradable esophageal stent placement does not prevent high-grade stricture formation after circumferential mucosal resection in a porcine model. Surg Endosc. 2012;26:3500–3508. doi: 10.1007/s00464-012-2373-6. [DOI] [PMC free article] [PubMed] [Google Scholar]