Abstract
The polarity protein Par3/Bazooka (Baz) has been established as a central component of the apical basal polarity system that determines the position of cell-cell junctions in epithelial cells. Consistent with that view, we show that shortly before gastrulation in Drosophila, Baz protein in the mesoderm is down-regulated from junctional sites in response to Snail (Sna) expression. This down-regulation leads to a specific decrease in adherens junctions without affecting other E-Cadherin pools. However, we further show that, interactions between Baz and junctions are not unidirectional. During apical constriction and the internalization of the mesoderm, down-regulation of Baz is transiently blocked as adherens junctions shift apically and are strengthened in response to tension generated by contractile actomyosin. When such junction remodeling is prevented by down-regulating myosin, Baz is lost prematurely in mesodermal epithelium. During such apical shifts, Baz is initially left behind as the junction shifts position, but then re-accumulates at the new location of the junctions. On the dorsal side of the embryo, a similar pattern of myosin activity appears to limit the basal shift in junctions normally driven by Baz that controls epithelium folding. Our results suggest a model where the sensitivity of Baz to Sna expression leads to the Sna-dependent junction disassembly required for a complete epithelium-mesenchymal transition. Meanwhile this loss of Baz-dependent junction maintenance is countered by the myosin-based mechanism which promotes an apical shift and strengthening of junctions accompanied by a transient re-positioning and maintenance of Baz proteins.
1. Introduction
Consistent with their pivotal roles in epithelial identity, morphology and function, systems governing cell polarity and cell adhesion have been shown to interact extensively. Among apical-basal polarity proteins, the scaffold protein Par3/Bazooka (Baz) is distinguished by a localization to the interface between the apical and basal-lateral domains, a position where cell-cell junctions also reside. Increasing evidence points to an essential role for Baz in determining junction morphology and localization (Coopman and Djiane, 2016; Tepass, 2012; West and Harris, 2016). In Drosophila embryos for example, Baz has been implicated in the formation of junctions during the initial establishment of epithelium (Harris and Peifer, 2004), in the basal shift of junctions during the folding of mature epithelium (Wang et al., 2012), and in the planar polarization of junctional components during tissue elongation (Simoes Sde et al., 2010). In the classic epithelium polarization models derived from mammalian cultured cells, Baz is required for the stabilization of small junction clusters, thereby marking the zone where the zonulae adherens will form (Coopman and Djiane, 2016).
Given its essential role in junction formation and maintenance, the targeting of Baz to discrete sites has been extensively studied. These studies suggest that multiple pathways cooperatively localize Baz, with each pathway interacting with different domains of Baz (McKinley et al., 2012; Tepass, 2012; Yu and Harris, 2012). Despite this progress, our understanding in Baz targeting remains incomplete, especially in the context of its interaction with adherens junctions. Although Baz has been shown to physically interact with adherens junction components (Bulgakova et al., 2013; Harris and Peifer, 2005) and appears to always spatially overlap with junction clusters, it is not a core component of adherens junctions and the measured ratio between Baz and junction components in the ectoderm of early fly embryos is well below 1 (Bulgakova et al., 2013; Harris and Peifer, 2005; McGill et al., 2009). The ratio can also differ in different situations and different cell types, suggesting it is not a universal stoichiometric marker for junctions. In the intercalating cells of the Drosophila germband, for example, the ratio is planar polarized and within the same cell can differ at different interfaces (Simoes Sde et al., 2010).
Although polarity protein-mediated processes play a significant role in localizing junctions, the stability, localization and internal organization of junctions are also influenced by the mechanical force they are subjected to (Lecuit and Yap, 2015). In tissue culture cells, for example, the density of E-Cadherin (E-Cad) clusters correlates with the forces that act upon them (Engl et al., 2014) and in fly embryos, actomyosin-generated stress has been shown to influence the endocytosis of E-Cad (Levayer et al., 2011). Our previous study shows that the strength and position of adherens junctions are correlated with individual myosin pulses (Weng and Wieschaus, 2016). Despite our knowledge about both the mechanical and polarity-based mechanisms, it is not known how the two interact in junction formation and remodeling. Under certain circumstances, the two systems could theoretically compensate for each other, whereas in other cases, where they regulate adherens junctions in opposite ways, they may reach a dynamic balance unachievable with only single system operating.
Gastrulating Drosophila embryos provide a useful model system to address these questions. Early Drosophila embryo is composed of a single-layer epithelium and adherens junctions are established at the subapical position as discrete clusters (Fig. 1A–B). Upon gastrulation the mesoderm cells on the ventral side of the embryo are internalized through epithelial folding before undergoing epithelial-mesenchymal transition (EMT). The epithelial folding is driven by the force generated by myosin contraction on the apical surfaces of mesoderm cells which results in apical constriction and cell shape change and eventually internalization of the tissue (Martin et al., 2009). We have shown previously that during apical constriction, adherens junctions in the mesoderm are maintained and remodeled in response to tension force (Weng and Wieschaus, 2016) (Fig. 1C–F). During this process, adherens junctions move from subapical to apical positions and become intensified to sustain the tension (Dawes-Hoang et al., 2005; Kolsch et al., 2007; Weng and Wieschaus, 2016). Although it has been shown that Baz is essential for the formation of these adherens junctions prior to gastrulation (Harris and Peifer, 2004), it is not known what role Baz plays during the apical shift of adherens junctions and how it is coordinated with tension-driven junction remodeling.
Fig. 1. Baz shifts apically in mesoderm during apical constriction.
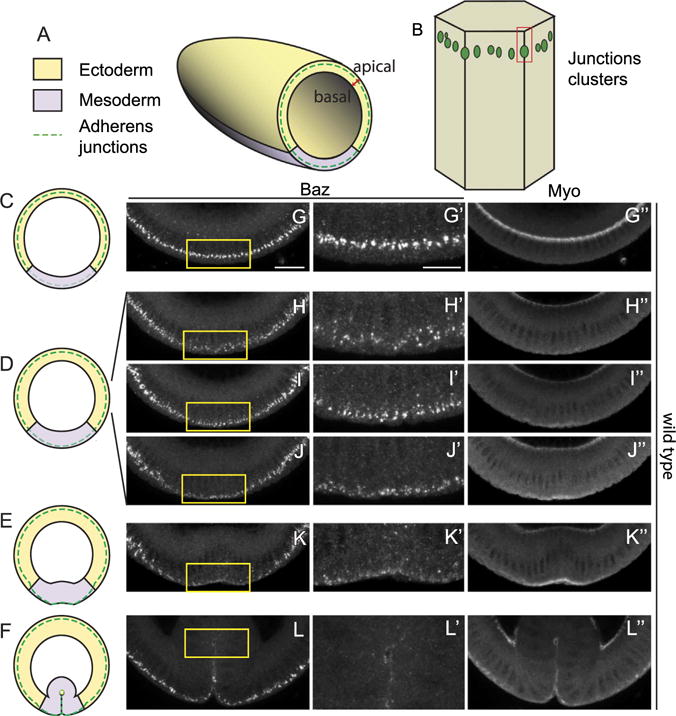
(A) A schematic of a Drosophila embryo during cellularization showing orientation of cross-sections, apical-basal axis of the epithelium, the positions of the future ectoderm and mesoderm and the localization of adherens junctions. (B) Adherens junctions appear as clusters at the subapical position during cellularization. (C–F) Schematics of cross-sections of embryos at different stages: early to mid cellularization (C), late cellularization to early gastrulation (D), bending of mesodermal epithelium (E) and internalization of mesoderm (F). (G–L) Distributions of Baz and myosin during the corresponding stages. (G) Baz localizes at the subapical positions during early to mid cellularization. (H–J) As myosin starts to accumulate, Baz moves from subapical position to apical position. (K) Baz localizes to apical position at low levels when myosin drives the tissue shape change. (L) Baz fades as the mesoderm is internalized.
When apical myosin levels fall at the end of gastrulation, adherens junctions in mesodermal cells disassemble and the cells undergo EMT. This junctional disassembly is developmentally regulated by the expression of Snail (Sna) and is initially countered by tension on the mesodermal junctions generated by myosin contractility (Weng and Wieschaus, 2016). Such tension-dependent junction regulation delays the junction disassembly and ensures that EMT can only happen after the internalization of the tissue despite the early expression of Sna. EMTs are ultimately associated with the loss of polarity systems, but it is unclear how loss of cell polarity is coordinated with the disassembly of junctions.
In this study we follow the distribution of Baz in the early Drosophila embryo during the stages when junctions are under myosin-generated tension and ultimately undergo Sna-mediated disassembly. We show that there is a transient spatial separation of Baz and adherens junctions as the junction shifts apically in response to myosin contraction. The stabilization of junctions by myosin counteracts the Sna-dependent downregulation of Baz in the apically constricting mesodermal epithelium. A similar apical shift of Baz can be triggered by activation of myosin in the cells programed to shift Baz basally and can help fine-tune the position of Baz and junctions.
2. Materials and methods
2.1. Fly stocks and genetics
The maternal driver line mat67;mat15 carries matα4-GAL-VP16 (Hacker and Perrimon, 1998) in homozygous inserts on chromosome II and III. To express Sna in early embryos, virgin females carrying UAS-sna are crossed to mat67;mat15 males and F1 females are crossed to males of appropriate genotypes. The following stocks expressing fluorescent proteins were used: ubi-dE-Cad::GFP (Oda and Tsukita, 2001), ubi-Baz::mCherry (Bosveld et al., 2012). Fog was expressed zygotically by crossing appropriate females to males of UAS-fog (Dawes-Hoang et al., 2005). RNAi lines – sqh RNAi (32439), aCat RNAi (38987 or 33430) and Baz RNAi (35002) – were obtained from TRiP at Harvard Medical School and maternally expressed using mat67;mat15. kr-Gal4 is obtained from B. Shilo’s lab, originally made by M. Leptin lab (Castelli-Gair et al., 1994). snaIIG05 was used to generate homozygous sna mutant embryos identified by the absence of Sna staining. ctaR10/Cyo; T48CC1.2 and hkb-fog are obtained from M. Leptin lab (Barrett et al., 1997; Kolsch et al., 2007). Embryos from stock C(1)DX/fog4a6/Y; hkb-fog(II) are collected and stained for Sex-lethal (Sxl) proteins to distinguish fog mutant and non-fog mutant embryos.
2.2. Live imaging
Embryos were prepared for imaging as previously decribed (Weng and Wieschaus, 2016). Briefly embryos are dechorionated in 50% bleach, and mounted on glass-bottomed Petri dishes without glue. The dish chamber is filled with water and covered by an oxygen-permeable membrane. Images are acquired on a Leica SP5 confocal microscope, with 63X/1.4 NA oil lens. An Argon ion laser and a 561-nm diode laser were used to excite GFP and mCherry respectively. Images for junction and Baz cluster tracking was acquired at zoom 4X and a resolution of 120 nm per pixel, in z-stacks starting from the ventral surface of the embryo to 6 μm deep with 0.5 μm increments, at temporal resolution of 7.5 s per z-stack, with pinhole set at 1 Airy Unit.
2.3. Embryo fixation and fluorescent microscopy
Embryos were dechorionated with 50% bleach and fixed by heat-methanol protocol as described before (Muller and Wieschaus, 1996). Primary antibodies used are mouse anti-Arm (N2-7A1, DSHB, 1:50), mouse anti-Sxl (M18, DSHB, 1:5), rat and guinea pig anti-Sna (1:1000, Wieschaus’ lab), rat anti-Twist (1:1000, Wieschaus’ lab), rabbit anti-Zip (1:100, Wieschaus’ lab), guinea pig anti-Runt (1:1000, Wieschaus’ lab), rabbit and guinea pig anti-Baz (1:500, J. Zallen lab). Secondary antibodies conjugated with Alexa 488, 568, 594 and 647 were used (1:500, Molecular Probe). Stained embryos were sorted and cross-sectioned by hand (using a 26-gauge hypodermic needle) in 50% Aquapolymount/PBST and mounted in Aquapolymount (Polysciences, Warrington, PA). Images are acquired on a Leica SP5 confocal microscope, with 20× or 63×/1.3 NA glycerol immersion lens.
2.4. Scanning EM
Embryos were dechorionated with bleach and fixed for 25 min with 25% glutaraldehyde in 0.1 M cacodylic buffer and heptane. The vitelline membrane was then manually removed in PBS with a needle, and embryos were dehydrated by a gradient of concentration of ethanol (25%, 50%, 75%, 95%, and 100%). Embryos were then incubated for 10 min with a 1:1 mixture of ethanol and Hexamethyldisilazane (HMDS), and then with 100% HMDS twice. After HMDS evaporates completely, and embryos were transferred to the SEM stub and gold palladium coated using a Desk II Sputterer (Denton Vacuum). Samples were imaged using a tabletop scanning EM (TM-1000; Hitachi).
2.5. Imaging processing and analysis
ImageJ (http://rsb.info.nih.gov/ij/) was used to adjust brightness and contrast across the whole image for better visualization. Quantitative analysis was done using MATLAB (MathWorks).
To quantify the ratio between Baz and Arm, 15 μm stacks of images of cross-sectioned embryos were used. As the intensity of cytoplasmic background contributes to more than 97% of the total intensity of the non-zero pixels, the mean plus three standard deviations of all the nonzero pixels was used as the threshold to remove cytoplasmic background. The intensity value of each pixel was normalized by the mean of non-zero pixels to minimize the difference between embryos due to immunostaining efficiency or laser power changes. Non-zero pixels after thresholding of Arm images were converted into a binary mask to quantify junctional Baz intensity. Ratios between Baz and Arm intensity for each embryo were the mean ratio at each non-zero pixel after thresholding. Ratios of different tissues were quantified in cropped images containing the tissue of interest while the thresholding and normalization was done in the whole images. At least three embryos were quantified for each stage.
To quantify junction and Baz total intensity in embryos developing from cellularization to early gastrulation, a 15 μm thick z-stack was imaged with a temporal resolution of 1 min. When the images are process for quantifying the junction intensity, a region of interest is drawn at each time point to always include the same cells (about 5×10 cells). The total intensity curve was obtained by summing all the pixel intensities at each time point and averaging the values over three embryos. Images are processed to subtract the background before quantitative analysis. In experiments to measure Baz, cytoplasmic intensities are subtracted so that only clustered signals are quantified. Only stacks above the basal junction layer are quantified to exclude signals from those junctions. Non-junctional E-Cad signal comes mainly from the non-junctional membrane pool whose intensity is measured. The mean of this pool plus two standard deviations was used as the background value.
3. Results
3.1. Myosin-triggered apical shifts in junctional components transiently decouple Baz and junction clusters during mesodermal invagination
We have previously shown that upon apical constriction, junction clusters move apically in response to myosin activation on the apical surface of the mesodermal cells during gastrulation (Weng and Wieschaus, 2016) (Fig. 1A–F). This junction repositioning is correlated with myosin in terms of developmental timing: it occurs at the same time when myosin activation is initiated. To understand whether and how Baz position changes in respect to myosin activity during this same period, we first used cross-sections of fixed embryos to examine the localization of Baz in mesoderm. Consistent with previous reports, Baz localizes at subapical position during cellularization when myosin is not activated on the apical surface of the mesoderm (Fig. 1G). As myosin starts to accumulate on the apical surfaces of the mesodermal cells upon gastrulation, Baz begins to move apically in those cells, appearing to localize between subapical and apical positions (Fig. 1H–J). Baz becomes apically localized when epithelium shape changes become apparent and eventually fades as the tissue is completely internalized (Fig. 1K–L). This suggests that Baz shows a positional change temporally correlated to myosin activation, very similar to the junction positional change as we reported previously (Weng and Wieschaus, 2016). To compare Baz and junctions in their repositioning in detail, we tracked both Baz and E-Cad simultaneously in living embryos (Fig. 2A). Prior to the repositioning, Baz and E-Cad clusters overlap. However as an E-Cad cluster starts to move apically, the associated Baz puncta are left behind and quickly diminish (enlarged image in Fig. 2A′). As the junction cluster reaches its final apical position, Baz is restored to the junction, albeit at low levels (Fig. 2A, yellow arrows). This transient separation and reduction in Baz level prompted us to test if a similar separation of Baz and junctional components can be observed following junctional shifts in tissues not programmed to undergo Sna-mediated EMT. To produce junctional shifts in the ectoderm we induced apical myosin by ectopically expressing folded gastrulation (fog), a secreted factor expressed in the mesoderm (Costa et al., 1994). In the ectoderm of wild type embryos, Baz and Armadillo (Arm, Drosophila β-Catenin) overlap at the subapical position at late cellularization and early gastrulation stages (Fig. 2B–C). However in the ectoderm of embryos with the ectopic myosin activation, when junctions are in the process of shifting apically but have not reached the final position, junctions show a more apical localization than Baz clusters (Fig. 2D). In embryos at later stages when junctions have completed their apical shift, Baz again overlaps with junctions (Fig. 2E). These data suggest that in response to the apical myosin activation, adherens junctions are mobilized first while Baz lags behind but eventually relocates to the new junction sites.
Fig. 2. Baz follows adherens junctions during their apical shift.
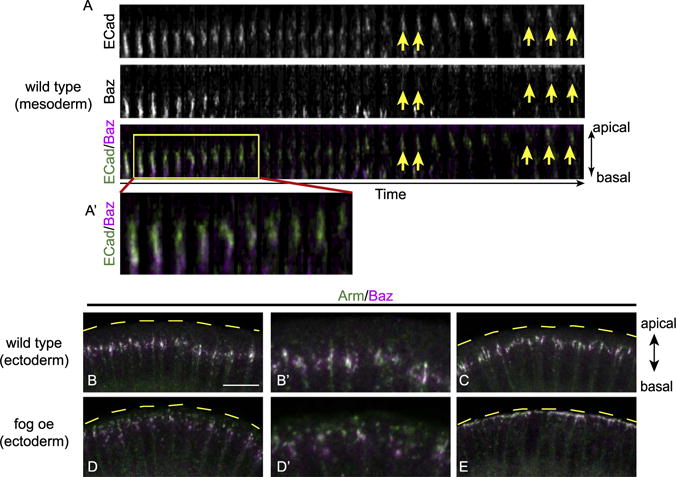
(A) Tracking of an individual junction (GFP) and Baz (mCherry) cluster in wild type mesoderm during apical constriction. Tiles of 3-D reconstructed images show that the junction cluster moves apically while Baz is first left behind, diminishes and then recovers at the junctional region at lower levels (yellow arrows). (A′) The enlarged image of the boxed area. Tiles are 7.5 s apart in time. (B–C) Junctions in wild type ectoderm stay at subapical position from cellularization to gastrulation. (D) When myosin is activated in ectoderm cells by expressing Fog, there is a transient separation of junctions and Baz. (E) When junctions reach the most apical edge of the cell, Baz again overlaps with junctions. B′ and D′ are high-magnification images from B and D. Images are maximum projections from 6 μm thick image stacks. Scale bar: 10 μm.
3.2. Sna expression is required for post-transcriptional downregulation of Baz in the mesoderm primordium but not for separation of Baz and junctions
During the early to mid stages of cellularization, the intensity ratios between Baz and Arm localized at the junctional region are similar in mesoderm and ectoderm (Fig. 3A and E). However, when the embryos reach late cellularization stages to early gastrulation, the Baz:Arm ratio becomes lower in the mesoderm cells compared to that of the ectoderm cells and continues to decrease as apical constriction proceeds (Fig. 3B–D and E). To investigate how this shift in ratio occurs, we tracked the level of Baz in live embryos, excluding cytoplasmic signal so that only clustered Baz signal at the junctional region is quantified. During late cellularization, the decrease in localized Baz levels occurs simultaneously with the previously reported decline in junctional E-Cad (Weng and Wieschaus, 2016) (Fig. 3F–G). As the trend in E-Cad level reverses upon the onset of apical constriction, the rate of Baz loss continues initially but the loss slows down and Baz levels eventually plateau without showing the dramatic increase observed for E-Cad levels (Fig. 3F–G). This suggests that Baz shows a delayed and milder response to apical constriction compared to E-Cad. This decline in Baz level is specific to mesoderm primordium: in the whole-mount preparation, it is evident that the domain of downregulated Baz is geometrically matched to the Sna expression domain (Fig. 3H). When Sna is eliminated, Baz localization patterns are identical in ectoderm and mesoderm as marked by Twist staining, indicating Sna is responsible for the Baz downregulation in the mesoderm (Fig. 3I). The Sna-dependence of Baz loss is consistent with Sna’s role in downregulating polarity proteins to promote EMT of mesodermal epithelium following the internalization of those cells.
Fig. 3. Sna downregulates Baz in mesoderm cells prior to gastrulation.
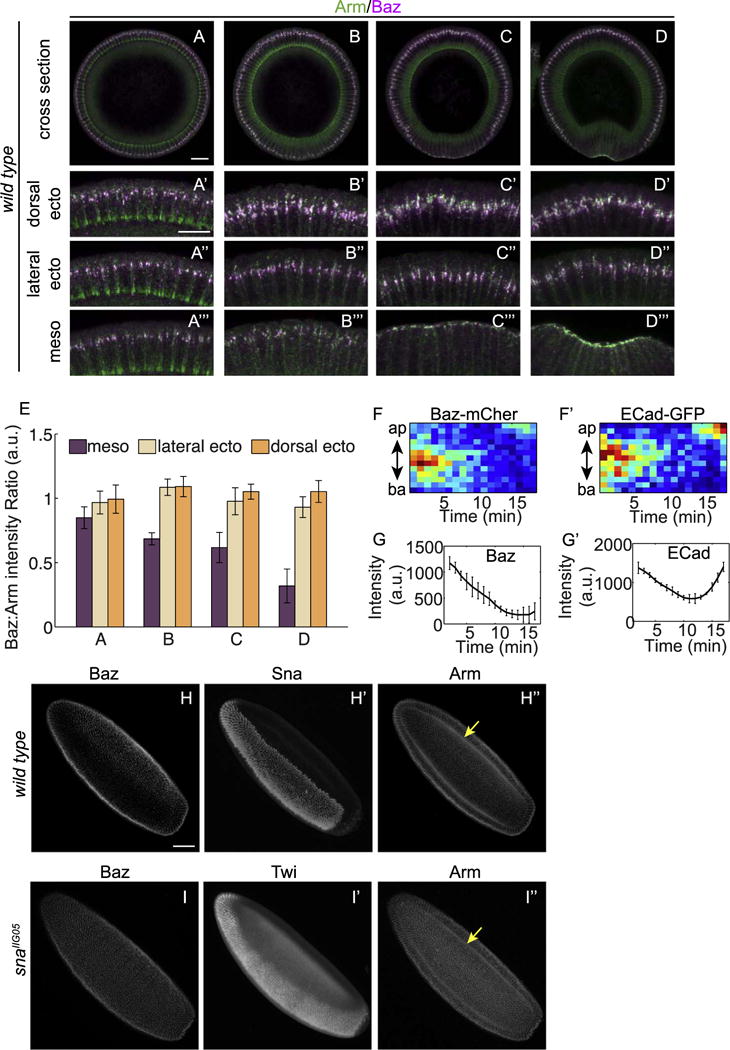
(A–D) Wild type embryos at different stages of development from mid-cellularization (A), late cellularization (B), to gastrulation (C–D). Zoom-in images show cells from the dorsal (A′–D′), lateral (A″–D″) and ventral (A‴–D‴) regions. Images have been rotated so that the cells are of the same orientation with the apical side up. All zoom-in images are of the same setting to allow comparing the ratio between Baz and Arm in the three regions of the same embryo. Compared to dorsal and lateral sides, the merged images of ventral cells become increasingly greener indicating the Baz:Arm ratio becomes lower as the embryos develop. Scale bars: 20 μm (A–D) and 10 μm (A′–D′, A″–D″, A‴–D‴). (E) Ratios between Baz and Arm fluorescent intensity in mesoderm and ectoderm of fixed embryos at similar stages as embryos in A–D. For the ratios at each stage, at least three embryos are quantified. (F) Kymograph of Baz-mCherry (F) and E-Cad-GFP (F′) in ventral cells from late cellularization to gastrulation. (G) Total intensity of Baz-mCherry and E-Cad-GFP in ventral cells from later cellularization to gastrulation. Average values from 3 embryos are shown. (H) In wild type embryos, Baz is downregulated in mesoderm cells identified by their Sna staining (H′). (I) In sna mutant embryos Baz pattern is indistinguishable between ectoderm and mesoderm, in this cases, identified by Twist (Twi) staining (I′). Scale bar: 50 μm.
The classic mechanism for Sna to downregulate cell polarity is to transcriptionally repress junctional components and polarity genes such as Crumbs (Lamouille et al., 2014). However transcriptional repression is not sufficient to explain the downregulation of Baz observed in junctional sites during gastrulation. This is because Baz at this stage is largely derived from maternal deposition and the Baz::mCherry used in the live imaging is expressed under the ubiquitin promoter but is still removed from the junctional region in the mesoderm (Fig. 2A). Therefore it is likely that one or several Sna target genes are directly responsible for the removal of maternally supplied Baz protein from the junctional zone in mesodermal cells.
3.3. Sna is sufficient to induce Baz loss in non-mesodermal tissues
To test whether Sna is sufficient to lower Baz in non-mesodermal tissues where other mesoderm factors such as Twist are not expressed, we ectopically expressed Sna in the whole embryo. In embryos with global Sna expression, Baz levels are severely reduced in the ectoderm, but are maintained at levels comparable to wild type in the mesoderm, where Sna is naturally expressed and myosin is activated (Fig. 4A–B, A′–B′). The contrasting behaviors of Baz in ectoderm and mesoderm parallel the previously reported behaviors of subapical adherens junctions following ectopic Sna-expression (Fig. 4A″-B″, A‴-B‴) (Weng and Wieschaus, 2016). The down-regulation of junctions following Sna expression is restricted to adherens junctions and does not target other Cadherin/Catenin complexes such as the basal junctions located above cellularization front, and Cadherin/Catenin molecules diffusing freely on plasma membrane (Fig. 4A‴–B‴). This is in contrast to the phenotype of embryos depleted for core junction components. In α-Catenin RNAi embryos, all forms of Cadherin/Catenin complex are undetectable using anti-Arm antibody (Fig. 4C″–C‴). Despite this drastic loss of all junctional material, Baz appears to be recruited to the membrane at the subapical position of the ectoderm cell even though it shows a broader distribution along apical-basal axis than it does in wild type (Fig. 4C–C′). Thus junctions are not essential for membrane targeting of Baz, although they may play a role in refining its distribution. In mesodermal cells of these embryos it is difficult to assess the Baz localization, because myosin contraction combined with loss of cell junctions in mesoderm leads to the rupture and aggregation of apical membranes and thus it no longer reflects physiological conditions. Furthermore, the localized Baz in α-Catenin RNAi embryos is still sensitive to ectopically expressed Sna, suggesting that Sna downregulates Baz in a junction-independent pathway (Fig. 4D–D‴).
Fig. 4. Sna expression is sufficient to disassemble junctions via downregulation of Baz.
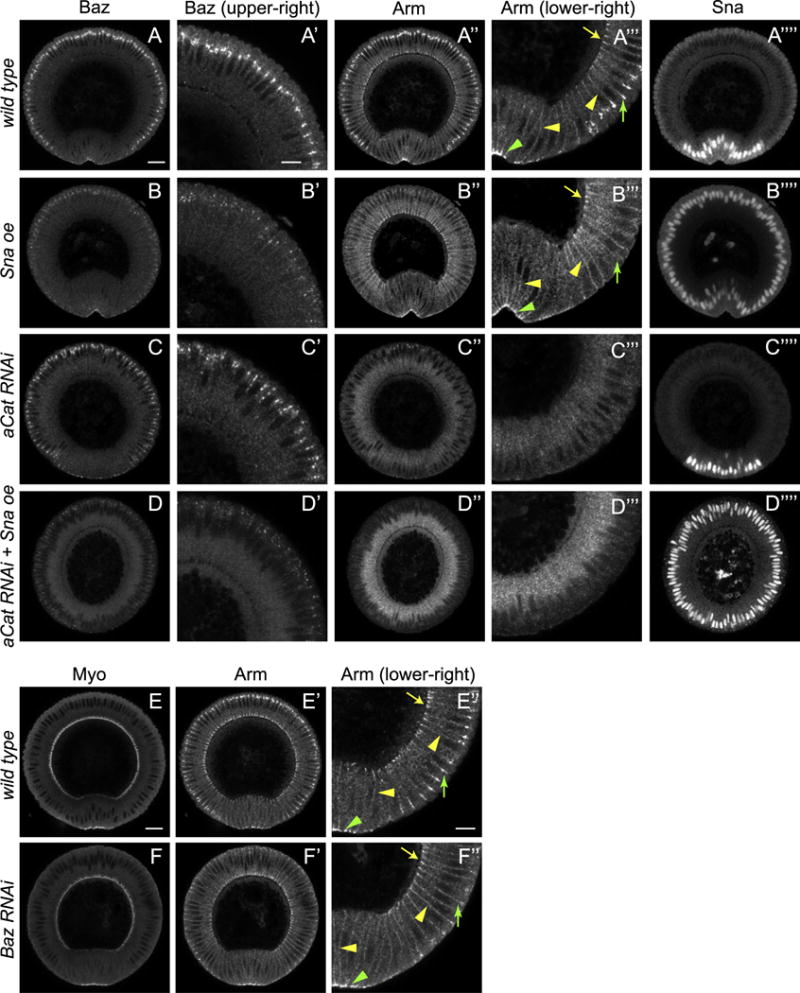
(A–D) Cross-sections of fixed embryos of different genotypes showing localization patterns of Baz and Arm. (A″-D″) High-magnification images showing different Cadherin/Catenin pools. Green arrowhead: adherens junctions in ventral mesoderm cells. Green arrow: adherens junctions in ectoderm cells. Yellow arrowhead: non-junctional membrane associated Cadherin/Catenin. Yellow arrow: basal junctions. Basal junctions are only present in the ectoderm at this stage as cellularization is finished in mesoderm cells. (A‴–D‴) High-magnification images showing Baz distributions. (E–F) Compared to wild type (E), depletion of Baz (F) leads to the loss of adherens junctions in ectoderm cells without affecting myosin activation (E–F) and other Cadherin/Catenin pools (E″–F″). It thus phenocopies ectopic Sna expression. Arrow annotations are the same with A–D. Scale bars: 20 μm (A–D, A′–D′, A⁗–D⁗, E–F, E′–F′) and 10 μm (A″–D″, A‴–D‴, E″–F″).
The difference in junction phenotypes between ectopic Sna expression and depletion of junctional components suggests that Sna’s specificity for adherens junctions might be achieved by downregulating a factor that is specifically important for adherens junction formation at the subapical position. Baz is a strong candidate for such a factor: it can be downregulated by Sna as shown above and it colocalizes with Cadherin/Catenin only at the site of downregulation - at adherens junction sites, but not at basal junctions or generally in the plasma membrane (Fig. 4A and A′). In agreement with this hypothesis, loss of Baz phenocopies ectopic Sna expression: the adherens junctions in ectoderm are lost but the basal junctions and membrane Cadherin/Catenin complex in all tissues remain intact and the adherens junctions in the apically constricting mesoderm can also be detected (Fig. 4E–F″). These data point to a model where Sna expression blocks Baz targeting to the subapical sites and thereby indirectly drives a transient loss of junctions until apical constriction starts.
3.4. Apical activation of myosin is necessary and sufficient for the apical shift of Baz and the maintenance of Baz in the presence of Sna
Despite the drastic decrease in the membrane recruitment of Baz when Sna is expressed, the downregulation reaches a plateau upon apical constriction of the mesoderm and junctional Baz is never completely lost during apical constriction and internalization of the mesoderm cells (Fig. 3F–G). We tested whether the contracting myosin is required for the maintenance of Baz. Indeed when myosin is severely knocked down, Baz becomes undetectable in the mesoderm at later cellularization or early gastrulation stage, although it is not significantly affected in the ectoderm where Sna is not expressed (Fig. 5A–B). A similarly strong impact on Baz levels is observed in concertina; T48 embryos, where apical myosin activation is blocked but the myosin level is unchanged, suggesting that it is the activation of apical myosin contraction that rescues Baz localization (Fig. 5C). Not only is myosin activation necessary for the transient maintenance of Baz in the mesoderm cells, it is sufficient to drive the apical shift of Baz in the ectoderm independent of Sna expression and it protects Baz from Sna-induced loss in those tissues (Fig. 5D–E). Despite these correlations between myosin activity and Baz localization, the response of Baz to myosin appears to be more delayed and much milder than that of adherens junctions as discussed previously (Fig. 3F–G). This suggests that the interaction between Baz and myosin may be less direct than that between junctions and myosin whereas Sna may act more directly on Baz levels than junction levels. This would explain why, in the presence of Sna expression, myosin activity can reorganize and enhance junctions but is not enough to promote a significant increase in Baz levels. One possible mechanism would be that junctions supported by alternative mechanism such as apical myosin tension may require less Baz for stabilization and may even recruit Baz at lower levels. This idea is consistent with the possibility that loss of Baz induced by Sna expression or Baz RNAi, though sufficient to remove junctions from non-contracting ectoderm cells, fails to eliminate junctions from apically constricting mesodermal cells (Fig. 4E′–F″).
Fig. 5.
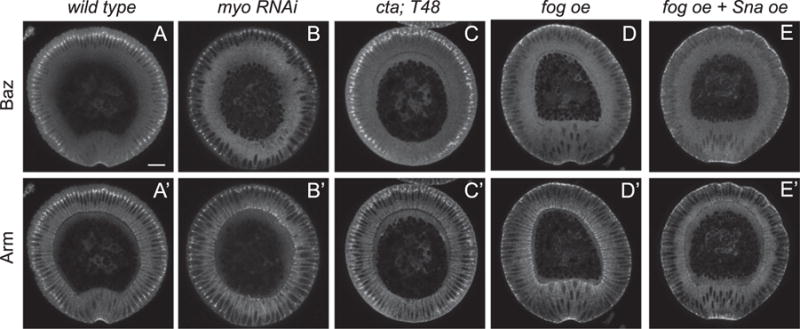
Myosin is necessary to maintain Baz localization in mesodermal cells and ectopic activation of myosin relocates Baz to the apical surface. (A) Arm and Baz staining in the wild type embryo. (B) Loss of Sqh (the myosin regulatory light chain) leads to loss of Baz and junctions in the ventral mesoderm cells while ectoderm cells are largely unaffected. (C) Failure in activating myosin by removing cta and T48 leads to similar loss of Baz and junctions specifically in the mesoderm. (D) Activation of myosin by Fog overexpression leads to relocation of Baz and junctions to the apical edge of the cell. (E) Baz is maintained in the presence of Sna expression when Fog is expressed simultaneously. Scale bar: 20 μm.
3.5. Myosin-dependent Baz apical shift can override the basal shift program driven by polarity protein imbalance
Fog expression activates apical myosin and is prominent in ventral mesoderm and posterior endoderm to drive morphogenetic changes (Costa et al., 1994). However we also find Fog expressed at a low level in the trunk region of the embryo, raising the possibility that it may play a role regulating junctions and Baz in those tissues as well. Intriguingly, this ectoderm expression appears to exclusively in an ectodermal domain between the cells that initiate the two dorsal transverse furrows as identified by Runt staining (Fig. 6A) (Wang et al., 2012). Dorsal furrow formation is driven by a basal shift of junctions associated with the downregulation of basal lateral protein Par1 and a relocation of Baz (Wang et al., 2012). For normal morphology, the basal shift must first occur in the initiator cells and spread progressively to neighboring cells thereby promoting the formation of a furrow. The expression of Fog in neighboring cells but not in initiator cells led us to test the possibility that the resultant induction of myosin contractility might block the basal shift of junctions in neighboring cells, thereby restricting the initial shift to the initiator cells. In these experiments a Kr promoter/enhancer was used to drive the expression of Fog in a broader domain that includes the initiator cells but still minimized the effect in other tissues. The flattened region indicates myosin is activated from the cephalic furrow to the posterior midgut (Fig. 6C). Consistent with a negative impact on polarity-induced basal shifts, when Fog is expressed in the entire dorsal region, the Baz basal shift normally observed in the initiator cells is blocked and the formation of dorsal folds was completely abolished (Fig. 6B–C). To test whether the weak expression in cells between the initiator cells may contribute to the continued apical localization of junctions in those cells, we examined dorsal fold formation in fog mutant embryos with posterior Fog expression restored by the hkb-fog transgene. This transgene rescues fog’s phenotype in the posterior midgut and thereby simplifies analysis of its effect on the dorsal folds (Barrett et al., 1997). Compared to control embryos, fog mutants often show deeper dorsal folds, especially the anterior fold (Fig. 6D–E). This supports the idea that in the absence of fog-activated apical myosin, the basal shift in junctions may spread to more cells and as a result more cells are incorporated into the furrows. The weak activation of myosin normally induced by the low level of Fog may prevent more cells entering the fold either through cell-autonomously counteracting the basal shift of adherens junctions induced by Baz basal repositioning, or through strengthening junction-actin link to resist the non-cell autonomous mechanical propagation of junction shift, a mechanism that has been proposed to keep the anterior fold shallower than the dorsal fold (Wang et al., 2013). These data suggest that myosin activation may play a role in fine-tuning the position of junctions especially when in combination with a mechanism that shifts junctions to an opposite direction, and therefore may represent an additional insurance to optimize the furrow formation in that region.
Fig. 6. Fog expression between dorsal folds optimizes the morphology of the epithelial folds.
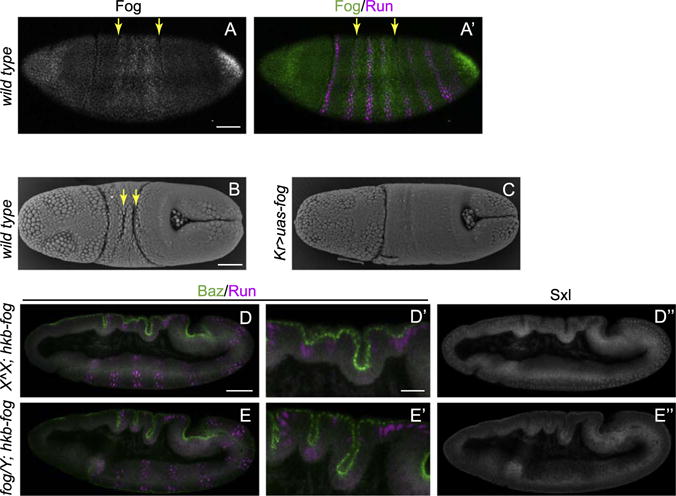
(A) Fog is expressed at low levels between the future dorsal folds, whose positions are indicated by the second and fifth Runt stripes (yellow arrows). (B–C) Scanning EM images show two transverse furrows (yellow arrows) form in the wild type embryo (B) but are abolished in the embryos with Fog expressed throughout the dorsal ectoderm (C). (D–F) Dorsal folds are deeper in the fog mutant embryos carrying hkb-fog compared to the embryos expressing hkb-fog alone. Male embryos carrying a fog mutant X chromosome and a Y chromosome are identified by the absence of nuclear Sex-lethal (Sxl) staining. Scale bars: 50 μm (A–C, D–E, D″–E″) and 20 μm (D′–E′).
4. Discussion
Adherens junctions in Drosophila mesodermal cells experience multiple regulatory remodeling through different stages of development. Like all junctions in the embryo (Harris and Peifer, 2004), mesodermal junctions are initially built at sites of Baz accumulation (Fig. 7A). We show that as Baz levels fall at the subapical sites in response to Sna expression, adherens junctions specifically disassemble without affecting other E-Cad pools (Fig. 7B). Upon activation of myosin contraction to drive mesoderm invagination, adherens junction level is rescued and enhanced, and Baz loss is postponed (Fig. 7C). Finally when the mesoderm is completely internalized and myosin activity is lost, Baz continued to be removed under the expression of Sna. Meanwhile adherens junctions, no longer maintained through myosin-based mechanism, eventually disassemble (Fig. 7D). The final loss of both Baz and junctions allows the EMT to proceed.
Fig. 7. Relationships between Baz, adherens junctions and myosin during cellularization and gastrulation.
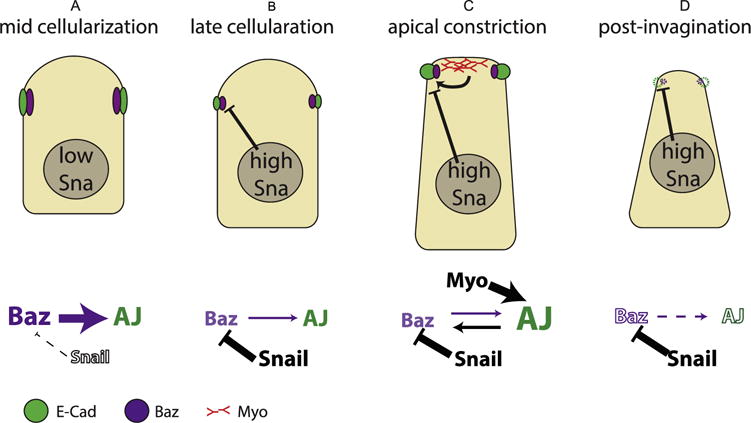
(A) During early to mid cellularization, Baz localization at the subapical position determines the position of adherens junctions. (B) At late cellularization, the expression of Sna leads to the downregulation of Baz which in turn results in lower level of adherens junctions. (C) Upon myosin activation, myosin-dependent junctions maintenance and strengthening leads to an increase in junction levels and delayed Baz loss in the presence of Sna expression. Due to myosin network engaged with adherens junctions, cells are apically constricted and apical-basally elongated (He et al., 2014). (D) As myosin fades after the internalization of mesodermal tissues, both adherens junctions and Baz are eventually lost.
During the relocation of adherens junctions in apically constricting cells, Baz does not precede but instead follows adherens junction. This differs from most previously reported models where the localization of Baz determines that of adherens junctions. One case that is similar to the results reported here occurs during the initial phase when two cultured cells make contact and establish polarization. In such cases, it is the formation of the small E-Cad clusters that triggers the subsequent recruitment of Baz to the contact site (Coopman and Djiane, 2016). These two cases have one feature in common: namely, that the membranes between neighbor cells are not yet apposed at the site where future junctions will form. Making the needed new membrane contacts might utilize interaction between E-Cad molecules on adjacent cells, thereby stabilizing cell-cell contacts. In contrast, Baz is not a transmembrane protein and has not been shown to mediate cell-cell interaction. Therefore in cases where closing a physical gap between future contact sites is necessary, junctions may play an earlier role than Baz.
We also find that adherens junctions contribute to the precision of Baz localization. Our result confirmed previous findings that without junctional components, Baz is still localized to the subapical region of the cell (Harris and Peifer, 2004). However, the distribution of Baz clusters in such cases is broader than that in the wild type. This suggests that a junction-independent mechanism targets Baz to a broad subapical region, whereas the subsequent junction formation creates a sub-region that is more favorable for Baz to localize and thus further refines Baz localization.
The post-transcriptional mechanism downstream of Sna that regulates Baz localization is not known, but is likely to be performed by Sna’s transcriptional target genes. A post-transcriptional suppression of Baz and adherens junctions may be more rapid than direct transcriptional control of junctional subunits and particularly beneficial in the fast developing Drosophila embryos where gene products that are required early like Baz and junctional components are maternally deposited.
Downregulating junctions at the protein level, however, may not be unique to Drosophila gastrulation and may represent an essential step in the EMT of other organisms. It is shown that during mouse gastrulation, the NIK/p38 pathway is required to downregulate E-Cad proteins in parallel to Sna’s repression of E-Cad transcription (Zohn et al., 2006). Loss of function of the NIK/p38 pathway leads to defects in the EMT of mesoderm. Furthermore, in Drosophila gastrulation, the transcriptional suppression of E-Cad is shown to be not essential for EMT as the EMT of mesoderm proceeds even with elevated level of E-Cad transcription and proteins, suggesting the critical role of post-transcriptional regulation of adherens junctions (Schafer et al., 2014). Although transcriptional suppression of E-Cad is a common theme in EMT, complete elimination of E-Cad production can lead to defect in EMT. For example, it is shown that in both Zebrafish and fly, continual recycling of E-Cad and re-establishment of cell-cell contact is essential for the cohesive migration of mesenchymal cells, implying the importance of E-Cad regulation at the protein level in mesenchymal cells (Campbell and Casanova, 2015; Ulrich et al., 2005; West and Harris, 2016).
The junction-independent localization of Baz during cellularization of Drosophila embryos has been shown to be mediated by additive effort of multiple pathways, including polarization of cytoskeleton, polarity protein Par1, PDZ domain-mediated transport and Rap1/afidin pathway (Benton and Johnston, 2003; McKinley and Harris, 2012; Tepass, 2012; Yu and Harris, 2012). Although removing any of those pathways clearly affects Baz localization, the effects are quantitatively weaker than the ones we observe on subapical Baz levels when Sna is expressed. It will be interesting to test whether a subset of the pathways known to play a role in localizing Baz can be suppressed by Sna, or if Sna’s effect relies on other unknown genes. It will also be interesting to test how the loss of Baz at gastrulation impacts other polarity proteins and whether other polarity proteins are suppressed by Sna through the same or different post-transcriptional mechanisms.
5. Conclusion
We demonstrate that, during gastrulation of Drosophila embryos, Sna expression downregulates polarity protein Baz which in turn results in junction disassembly at protein levels. On the other hand, apical myosin contraction counteracts junction disassembly by reorganizing junctions which in turn repositions and prevents complete loss of polarity protein Baz. While Sna functions through a conventional model where polarity protein Baz determines the position of junction formation, myosin acts more directly on junctions which results in Baz following junctions’ cues. These two counterbalancing forces allow maintenance of junctions in mesoderm cells for their successful collective internalization without delaying other functions of Sna.
Acknowledgments
We thank the M. Leptin, Y. Balleiche, B. Shilo and Bloomington Drosophila Stock Center for fly stocks. We thank J. Zallen for sharing antibodies. We thank members of the Wieschaus and Schupbach labs, especially for discussion and helpful comments. We thank O. Devergne, M. Swan and J. Lee for comments on the manuscript. We are indebted to the staff of the Imaging Facilities of the Princeton Molecular Biology department. This work was supported in part by Grant Number 5R37HD15587 from NICHD to E.F.W. and New Jersey Cancer Postdoctoral Fellowship and NIH K99 grant to M.W., as well as funds from the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflicts of interest.
References
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Benton R, Johnston DS. A conserved oligomerization domain in Drosophila Bazooka/PAR-3 Is important for apical localization and epithelial polarity. Curr Biol. 2003;13:1330–1334. doi: 10.1016/s0960-9822(03)00508-6. [DOI] [PubMed] [Google Scholar]
- Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet PL, Marcq P, Graner F, Bellaiche Y. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–727. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- Bulgakova NA, Grigoriev I, Yap AS, Akhmanova A, Brown NH. Dynamic microtubules produce an asymmetric E-cadherin–Bazooka complex to maintain segment boundaries. J Cell Biol. 2013;201:887–901. doi: 10.1083/jcb.201211159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Casanova J. A role for E-cadherin in ensuring cohesive migration of a heterogeneous population of non-epithelial cells. Nat Commun. 2015;6:7998. doi: 10.1038/ncomms8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- Coopman P, Djiane A. Adherens junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol Life Sci. 2016;73:3535–3553. doi: 10.1007/s00018-016-2260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. Folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Biol. 2014;16:587–594. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Doubrovinski K, Polyakov O, Wieschaus E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature. 2014;508:392–396. doi: 10.1038/nature13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- Levayer R, Pelissier-Monier A, Lecuit T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol. 2011;13:529–540. doi: 10.1038/ncb2224. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, McKinley RF, Harris TJ. Independent cadherin-catenin and Bazooka clusters interact to assemble adherens junctions. J Cell Biol. 2009;185:787–796. doi: 10.1083/jcb.200812146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley RFA, Harris TJC. Displacement of basolateral Bazooka/PAR-3 by regulated transport and dispersion during epithelial polarization in Drosophila. Mol Biol Cell. 2012;23:4465–4471. doi: 10.1091/mbc.E12-09-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley RFA, Yu CG, Harris TJC. Assembly of Bazooka polarity landmarks through a multifaceted membrane-association mechanism. J Cell Sci. 2012;125:1177–1190. doi: 10.1242/jcs.091884. [DOI] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E. Armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- Schafer G, Narasimha M, Vogelsang E, Leptin M. Cadherin switching during the formation and differentiation of the Drosophila mesoderm – implications for epithelial-to-mesenchymal transitions. J Cell Sci. 2014;127:1511–1522. doi: 10.1242/jcs.139485. [DOI] [PubMed] [Google Scholar]
- Simoes Sde M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–685. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schötz E-M, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech P-H, Heisenberg C-P. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-Cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Wang YC, Khan Z, Kaschube M, Wieschaus EF. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature. 2012;484:390–393. doi: 10.1038/nature10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Khan Z, Wieschaus EF. Distinct Rap1 activity states control the extent of epithelial invagination via alpha-catenin. Dev Cell. 2013;25:299–309. doi: 10.1016/j.devcel.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Wieschaus E. Myosin-dependent remodeling of adherens junctions protects junctions from Snail-dependent disassembly. J Cell Biol. 2016;212:219–229. doi: 10.1083/jcb.201508056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JJ, Harris TJC. Cadherin Trafficking for Tissue Morphogenesis: Control and Consequences. Traffic. 2016 doi: 10.1111/tra.12407. bn/a-n/a. [DOI] [PubMed] [Google Scholar]
- Yu CG, Harris TJC. Interactions between the PDZ domains of Bazooka (Par-3) and phosphatidic acid: in vitro characterization and role in epithelial development. Mol Biol Cell. 2012;23:3743–3753. doi: 10.1091/mbc.E12-03-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]


