Abstract
Excessive alcohol exposure has severe health consequences, and clinical and animal studies have demonstrated that disruptions in the epigenome of somatic cells, such as those in brain, are an important factor in the development of alcohol-related pathologies, such as alcohol-use disorders (AUDs) and fetal alcohol spectrum disorders (FASDs). It is also well known that alcohol-related health problems are passed down across generations in human populations, but the complete mechanisms for this phenomenon are currently unknown. Recent studies in animal models have suggested that epigenetic factors are also responsible for the transmission of alcohol-related pathologies across generations. Alcohol exposure has been shown to induce changes in the epigenome of sperm of exposed male animals, and these epimutations are inherited in the offspring. This paper reviews evidence for multigenerational and transgenerational epigenetic inheritance of alcohol-related pathology through the germline. We also review the literature on the epigenetic effects of alcohol exposure on somatic cells in brain, and its contribution to AUDs and FASDs. We note gaps in knowledge in this field, such as the lack of clinical studies in human populations and the lack of data on epigenetic inheritance via the female germline, and we suggest future research directions.
Keywords: fetal alcohol, preconception alcohol, birth outcome, offspring anxiety behavior, offspring stress response, DNA methylation, germline transmission
1. Introduction
Epigenetics is the milieu of mechanisms that modify gene expression without changing the DNA sequence itself. These mechanisms may involve methylation of DNA, modifications of histones, and small noncoding RNAs. Epigenetics allows for adaptive changes in gene expression that can be quickly implemented but can have long-lasting effects. Much research has focused on the effects of environmental impacts on epigenetic machinery, such as the effects of stress (Franklin et al., 2010; Yao et al., 2014), environmental toxins (Anway, Cupp, Uzumcu, & Skinner, 2005), and drugs of abuse, including alcohol (Finegersh, Rompala, Martin, & Homanics, 2015; Yohn, Bartolomei, & Blendy, 2015) and other drugs (for a review see Walker, Cates, Heller, & Nestler, 2015). Recent evidence also suggests that epigenetic modifications can be carried through the germline and through multiple generations. This type of inheritance is termed “epigenetic inheritance” and stands as a novel mechanism for inheritance. This compelling phenomenon is the subject of much current research, although many questions remain unanswered as to the mechanisms for epigenetic inheritance and for transgenerational epigenetic inheritance. The focus of this paper is to review the latest research on the epigenetic effects of alcohol exposure on the germline and on epigenetic inheritance of alcohol-related pathology. This review also discusses the gaps in knowledge relating to the mechanisms for epigenetic inheritance of alcohol exposure-related pathologies.
2. Alcohol-related pathology
Alcohol-use disorders (AUDs) are a significant psychiatric problem in the United States and worldwide. AUDs are characterized by excessive drinking and abuse of alcohol that leads to adverse social or health consequences for the drinker (Agrawal, Heath, & Lynskey, 2011; APA, 2013). AUDs may involve alcohol dependence or addiction to alcohol, which involves neural adaptations to chronic alcohol use leading to aversive emotional and physical consequences when alcohol use is discontinued, thus maintaining the alcohol abuse to avoid negative consequences (Koob, 2013). AUDs are associated with increased risk for fatal accidents and many serious health problems including depression, stroke, sleep disorders, heart disease, liver disease, and cancer (Rehm et al., 2009; Rehm, Samokhvalov, & Shield, 2013; Shield, Gmel, Patra, & Rehm, 2012). It is estimated that as many as 17 million adults in the US may have AUDs (Kessler et al., 1997; Volpicelli, 2001).
When a pregnant woman abuses alcohol, her exposed children are at a high risk for developing fetal alcohol spectrum disorders (FASDs), which are developmental disorders which can be characterized by diverse symptoms, including delayed growth, craniofacial abnormalities, intellectual impairment, anxiety, depression, and social impairment (Bakoyiannis et al., 2014; Driscoll, Streissguth, & Riley, 1990; Kelly, Day, & Streissguth, 2000). The occurrence and severity of symptoms depend on the duration and developmental timing of alcohol exposure (Alfonso-Loeches & Guerri, 2011). The most severe form of the disorder is called fetal alcohol syndrome (FAS), which is characterized by facial dysmorphologies, impaired growth, and central nervous system dysfunction (Sokol, Delaney-Black, & Nordstrom, 2003). Children that do not meet the full criteria for FAS but show a spectrum of the disorder are included under the umbrella term FASD. Children with FASD suffer long-lasting intellectual, social, and emotional problems, which persist through adulthood. The risk for developing AUDs has been found to “run in families”, due to a combination of social and genetic components, and studies suggest epigenetic factors also play a role in the development and inheritance of alcohol-related health problems. However, the epigenetic mechanisms for heritability of AUDs and familial alcohol-related health defects are far from understood and are discussed in this review.
3. Epigenetics
Epigenetics is the study of changes in gene expression, cell fate, and potentially heritability without any changes in DNA sequence. It can be thought of broadly as a bridge between genotype and phenotype (Goldberg, Allis, & Bernstein, 2007). Epigenetics is known to be regulated by three mechanisms, which interact in complex ways, including 1) DNA methylation, 2) histone modifications, and 3) noncoding RNAs. DNA methylation occurs on the cytosine residue of CpG dinucleotides and is performed by DNA methyltransferases (DNMTs) through the transfer of 5-methylcytosine from the methyl donor S-adenosylmethionine (SAM) to the CpG. In eukaryotes, DNA methylation status is passed down during DNA replication to the daughter strand DNA by the enzyme DNMT1, which performs the “maintenance” methylation activities, ensuring the methylation status of the parent cells is preserved in the daughter cells, while DNMT3a and DNMT3b are “de novo” methylation enzymes, which establish new methylation patterns during embryogenesis (Holliday & Pugh, 1975; Okano, Bell, Haber, & Li, 1999; Riggs, 2002), although recent evidence suggests there is some overlap in the function of maintenance and de novo enzymes (Jones & Liang, 2009). Methylation of DNA is functionally associated with gene silencing and is for the most part limited to so–called “CpG islands”, which are areas rich in CpG dinucleotides and are typically located near the promoter regions of genes and the transcription start site. Methylated DNA at the promoter regions can inhibit transcription by recruiting gene-silencing repressive proteins to the methylated region, such as methyl-CpG-binding protein 2 (MeCP2) and methyl-binding-domain (MBD) proteins (Cedar & Bergman, 2009). In contrast, hypomethylated CpG islands and promoter regions are associated with transcriptionally active histone modifications (discussed further below) and increased accessibility of the DNA transcription factors and RNA polymerase II to the DNA (Zhou, Goren, & Bernstein, 2011). Methylation status is mitotically stable and relatively long lasting, and evidence suggests that methylation status can even be passed on from the germline to the offspring, as this review will discuss. Nonetheless, flexibility in methylation status is also possible, and DNA methylation can also be reversed passively by the loss of 5mC during successive cycles of DNA replication, or by the recently discovered active demethylation of DNA, which can occur by oxidation of 5-methylcytosine by ten-eleven translocation (TET) family enzymes (Kohli & Zhang, 2013). Recent studies also suggest oxidized 5-methylcytosine, 5-hydroxymethylcytosine (5-hmC), may play an important role as an epigenetic mark, although the functional role of 5-hmC is still under investigation (Branco, Ficz, & Reik, 2012).
Alongside changes in DNA methylation, epigenetic machinery also includes a variety of changes in histones, both in post-translational histone modifications and in histone variants (for review see Bannister & Kouzarides, 2011; Kouzarides, 2007; Zhou, Goren, et al., 2011). In eukaryotes, proteins called histones package DNA into units called nucleosomes, which allow for protection and organization of the DNA. The cell has several enzymes that enact numerous covalent modifications on residues of histone tails (for example, methylation, acetylation, phosphorylation, ubiquitylation, and sumoylation), which have been theorized to serve as a “histone code” to allow for either increased transcription or silencing of genes (Strahl & Allis, 2000). Some examples of important modifications include methylation at histone 3 lysine 9 (H3K9me3) and lysine 27 (H3K27me3), which are associated with heterochromatin and gene silencing. In contrast histone 3 lysine 4 methylation (H3K4me2 and H3K4me3), acetylation, and the histone variant H2A.Z are marks associated with euchromatin, active promoters, and increased activation of gene expression (Zhou, Goren, et al., 2011). Histone-modifying enzymes include histone acetyltransferases (HATs), histone deacetylases (HDACs), histone methyltransferases (HMTs), histone demethylases, and kinases that catalyze modifications on the histones, which are then interpreted by chromatin-modifying protein complexes which are able to modify chromatin structure, allowing for decreased or increased accessibility of the transcription machinery to the DNA (Henikoff, 2008; Kouzarides, 2007; Nakao, 2001; Ng & Bird, 1999). While DNA methylation is more permanent, histone modifications are more dynamic and transient, but it is important to stress that histone modifications and changes in DNA methylation are not separate entities, and that they work together to orchestrate transcriptional response and possibly affect epigenetic inheritance (Nakao, 2001; Ng & Bird, 1999).
An additional, a more recently identified mechanism of epigenetic modification includes modulation by noncoding RNAs (ncRNAs), RNAs that are transcribed from DNA but are not translated into protein (for reviews see Berezikov, 2011; Larriba & del Mazo, 2016; and Yan, 2014). Small ncRNAs, which are less than 200 nucleotides (nts) in length, include microRNAs (miRNAs), short interfering RNAs (siRNAs), and Piwi-interacting RNAs (piRNAs), which have been shown to silence gene expression by targeting mRNA transcripts, sometimes via histone and DNA methylation mechanisms, leading to their downregulation (Holoch & Moazed, 2015). Long non-coding (lncRNAs) (>200 nts in length) also participate in epigenetic-mediated gene silencing. Especially, long noncoding intergenic RNA (lincRNAs) are transcribed from regions close to genes that will be silenced, and have been discovered to serve as sequence guides to attract protein complexes to either increase DNA methylation or repressive histone modifications. Examples of the work of lincRNAs include important roles in inactivation of the X chromosome (Ng, Pullirsch, Leeb, & Wutz, 2007) and in genomic imprinting (Gabory, Jammes, & Damdolo, 2010). As ncRNAs participate in important developmental events that affect the primordial germ cells and can also be affected by environmental influences, they could participate, in theory, as an important mechanism in epigenetic inheritance (Larriba & del Mazo, 2016; Yan, 2014).
4. Epigenetic Effects of Alcohol Exposure on Somatic Cells
The effects of alcohol exposure on epigenetic machinery in somatic cells in the organism have been fairly well characterized in recent years. This review will focus on the effects of alcohol exposure on germline cells and evidence for epigenetic inheritance of alcohol-related pathologies. In this section, we will also cover studies on the epigenetic effects of alcohol exposure on somatic cells in humans and in animal models, and the way these epigenetic changes may contribute to the development and maintenance of AUDs and FASDs and other alcohol-related health problems. For comprehensive reviews of the epigenetic mechanisms in the development and maintenance of AUDs and addiction to alcohol, the reader is referred to outstanding reviews by Krishnan, Sakharkar, Teppen, Berkel, & Pandey, 2014; Moonat & Pandey, 2012; and Ponomarev, 2013. For comprehensive reviews of the epigenetic mechanisms in FASD, the reader is referred to excellent reviews by Ramsay, 2010; and Ungerer, Knezovich, & Ramsay, 2013.
Epigenetic Effects of Alcohol Exposure in Adults
In animal models and in human populations, alcohol exposure during adulthood has been shown to affect DNA methylation, histone modifications, and ncRNAs in brain and other tissues, leading to disrupted gene expression and associated pathology (see Table 1 for a summary of studies). In many studies, chronic alcohol exposure has been associated with aberrant DNA methylation, in part by causing dysfunction in folic acid metabolism and deficits in the methyl donor SAM (Blasco et al., 2005; Hamid, Wani, & Kaur, 2009). In addition, alcohol exposure has also been shown to alter DNMT activity, which also leads to changes in DNA methylation (Garro, McBeth, Lima, & Lieber, 1991; Ponomarev, Wang, Zhang, Harris, & Mayfield, 2012; Zhang, Kusumo, Sakharkar, Pandey, & Guizzetti, 2014). In response to alcohol exposure, studies show either hypomethylation or hypermethylation of specific genes, with detrimental health consequences. Recent studies using human post mortem brain tissue and buccal swabs from either persons with AUDs or non-alcoholic controls have shown altered site-specific methylation of regions in long terminal repeat- (LTR) containing retrotransposons (Ponomarev et al., 2012) and in gene pathways involved in immune response, lipid metabolism, inflammation, and gastrointestinal disease (Hagerty, Bidwell, Harlaar, & Hutchinson, 2016) in persons with AUDs, linking alcohol-induced changes in methylation to some of the health issues that often co-occur with alcohol abuse. Examining single nucleotide polymorphisms (SNPs) on the prodynorphin gene, which are associated with alcohol dependence, Taqi and colleagues (2011) found differential methylation on three CPG-SNPs in the dorsolateral prefrontal cortex of post mortem samples from alcoholics, suggesting epigenetic and genetic mechanisms may interact to confer risk to developing alcohol dependence. Finally, aberrant DNA methylation status due to alcohol exposure has been found to be associated with many types of cancers, including liver, colorectal, breast, and upper respiratory cancers (Varela-Rey, Woodhoo, Martinez-Chantar, Mato, & Lu, 2013).
Table 1.
Summary of the Epigenetic Effects of Alcohol in Adult Humans and Animal Models
| Age | Model | ETOH BEC or Dose | Cell Type | Effects | Citation |
|---|---|---|---|---|---|
| Adult | Post-mortem human alcoholic, amygdala and frontal cortex | Not reported | Brain tissue | DNA hypomethylation and H3K4me3 enrichment at GC-rich regions and endogenous retroposons | Ponomarev et al., 2012 |
| Adult | Post-mortem human alcoholic | Not Reported | Brain and buccal tissue | Differential methylation in more than 400 CpGs | Hagerty et al., 2016 |
| Adult | Post-mortem human alcoholic | Not reported | Brain, frontal cortex | Differential methylation of prodynorphin gene SNPs in alcoholics | Taqi et al., 2011 |
| Adult | Post-mortem human alcoholic | Not reported | Brain, frontal cortex | No differences in global methylation in alcoholics, differential methylation in specific promoters in alcoholics | Manzardo et al., 2012 |
| Adult | Post-mortem human alcoholic | Not reported | Brain, cortex | Upregulation of 35 miRNAs in alcoholic brain | Lewohl et al., 2011 |
| Adult | Post-mortem human alcoholic | Not reported | Brain, hippocampus | Overlapping H3K4me3 marks and gene expression changes between cocaine and alcohol users | Zhou et al., 2011 |
| Adult | Human sperm | Not reported | Sperm | Decreased methylation in imprinted genes H19 and IG-DMR in men that consume alcohol | Ouko et al., 2009 |
| Adult | Rat, acute and chronic ethanol exposure | Acute: 91 ± 3.2 mg% Chronic: 177±11 mg% |
Brain, amygdala | Acute alcohol: decrease in HDAC activity and increase in histone H3 and H4 acetylation; Chronic alcohol withdrawal: increase in HDAC activity and decrease in H3 and H4 acetylation | Pandey et al., 2008 |
| Adult | Rat, Alcohol preferring and Non-preferring rats | ~100 ± 5 mg% | Brain, amygdala | Acute alcohol decreased HDAC activity and HDAC2 protein, increased global and gene specific histone acetylation in amygdala | Moonat et al., 2013; Sakharkar et al., 2014 |
| Adult and juvenile (30 days old) | Rat, intermittent for 2 weeks | Dose: 3 g/kg | Brain, prefrontal cortex | In juveniles, alcohol upregulates HAT activity and increases H3 and H4 acetylation and H3(K4) dimethylation in the promotors of cFos, Cdk5, and FosB genes | Pascual et al., 2012 |
| Adult | Mice and rats | ~100 mg% | Brain, nucleus accumbens | Increased DNMT1 protein and gene expression, decreased H4 acetylation | Warnault et al., 2013 |
| Adult | Rat | Dose: 6 g/kg | Tissue from several organs | Increased H3K9 acetylation post- alcohol in liver, lung, spleen, testes, but no change in kidney, brain, heart, stomach, colorectum, pancreas, and vessels | Kim & Shukla, 2006 |
| Adult | Rat | 20 mM | Rat supraoptic nucleus and striatal neurons | Upregulation of miR-9 | Pietrzykowski et al., 2008 |
| Adult (F0 sires and F1 generation) | Rat, chronic alcohol effects on F0 and F1 generation | Dose: 1.1, 3.3 g/kg ETOH for 4 weeks | Sperm, cerebral cortex | Decreased methylation of H19 and Peg3 genes in F0 sperm, and decreased methylation of Peg3 genes in F1 cortex | Liang et al., 2014 |
| Adult (F0 sires and F1 generation) | Mouse chronic alcohol | BEC: >1.0 mg/mL | Sperm of F0 exposed males, somatic cells of F1 offspring | Decreased methylation at two H19 binding sites in F1 offspring; decreased methylation at one CpG of the H19 gene in sperm of F0 males | Knezovich & Ramsay, 2012 |
| Adult (F0 sires and F1 generation) | Mouse chronic alcohol (5 weeks) | BEC: 147.1 ± 7.52 mg/dL | Sperm of F0 exposed males, brain of F1 offspring | Decreased methylation of BDNF promoter in F0 sperm and in VTA of F1 offspring | Finegersh & Homanics, 2014 |
| Adult (F0 sires and F1 generation) | Mouse chronic alcohol (7 weeks) | 0.5, 1, 2, 4 g/kg/day EtOH for 7 weeks | Sperm of F0 exposed males, brain of F1 offspring | Increased methylation of DAT in F0 sperm and in cortex and striatum of F1 offspring | Kim et al., 2014 |
| Adult (F0 sires and F1 generation) | Rats chronic alcohol (9 weeks) | Dose: 6 g/kg 3 times/week BEC: 369.2 ± 30.0 mg/100 mL |
Sperm of F0 exposed males | Decreased cytosine methyltransferase in sperm | Bielawski et al., 2002 |
Changes in histone modifications and miRNAs due to adult alcohol exposure have also been implicated in the development of alcohol addiction and AUDs in animal models and human tissues. In a rodent model, acute ethanol exposure was associated with decreased HDAC activity and increased acetylation of H3 and H4 histones in the amygdala, whereas withdrawal was associated with increased HDAC activity and decreased acetylation of these histones in this brain region (Pandey, Ugale, Zhang, Tang, Prakash, 2008). Withdrawal-induced anxiety behavior, deficits in histone acetylation, and changes in neuropeptides were normalized by administration of an HDAC inhibitor, trichostatin-A, implicating increased amygdala HDAC activity and histone de-acetylation in alcohol withdrawal (Pandey et al., 2008; You, Zhang, Sakharkar, Teppen, & Pandey, 2014). Several other animal studies have also shown that HDAC inhibitors and knockdown of HDAC via siRNA ameliorate alcohol-induced anxiety and alcohol intoxication (Moonat, Sakharkar, Zhang, Tang, & Pandey, 2013; Sakharkar et al., 2014; Warnault, Darcq, Levine, Barak, & Ron, 2013), suggesting that HDAC inhibition has therapeutic potential for the treatment of AUDs. Studies using human post mortem tissue have also found differences in global and promoter-specific H3K4 trimethylation (an activation marker) in brain, indicating chromatin remodeling is associated with AUDs (Ponomarev et al., 2012; Zhou, Yuan, Mash, & Goldman, 2011). Finally, a small number of human and animal studies have noted altered expression of miRNAs as a consequence of chronic alcohol exposure, and suggested a role for these changes in neural plasticity underlying the development of AUDs (Lewohl et al., 2011; Pietrzykowski et al., 2008). (See also Miranda et al., 2010 for a review on the role of miRNAs in alcohol toxicity and in the development of alcohol abuse.)
Epigenetic Effects of Alcohol Exposure During Early Development
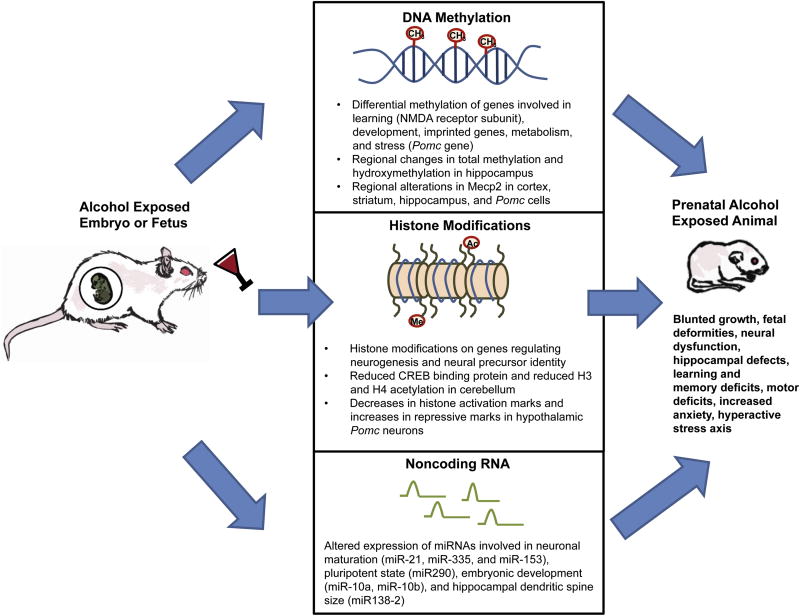
Alcohol exposure during the fetal period has been shown to have a dramatic effect on epigenetic programming, possibly leading to the development of FASDs (see Fig. 1 and Table 2). As also mentioned in Section 3 and Section 6, the genome of the developing embryo undergoes global changes in methylation in order to program cell development in an appropriate manner (Bao & Yan, 2012; Seisenberger et al., 2013). Thus, alcohol exposure during this time period is particularly risky, as it can interfere with normal epigenetic developmental programming. Both animal and human studies show aberrant DNA methylation patterns from exposure to alcohol in utero, because of mechanisms similar to those from adult exposure, such as disruption in folic acid metabolism and deficits in SAM as well as decreased DNMT activity (Garro et al., 1991; Ungerer et al., 2013; Zhang et al., 2014). Animal models have shown global DNA hypomethylation in the fetus (Garro et al., 1991), and also brain regional decreases in methyl-CpG-binding protein Mecp2 in prefrontal cortex and striatum (Kim et al., 2013) and hippocampus (Chen, Ozturk, & Zhou, 2013).
Fig. 1.
Role of epigenetic processes in the development of FASD. In vivo and in vitro studies have shown evidence that early developmental exposure to alcohol alters DNA methylation (Bekdash et al., 2013; Chen et al., 2013; Downing et al., 2011; Garro et al., 1991; Govorko et al., 2012; Kim et al., 2013; Liu et al., 2009; Marutha Ravindran & Ticku, 2004; Qiang et al., 2010; Zhang et al., 2014) histone modifications (Bekdash et al., 2013; Guo et al., 2011; Veazey et al., 2013), and miRNAs (Marjonen et al., 2015; Sathyan et al., 2007; Wang et al., 2009). The genes targeted by these modifications are regulators of development, metabolism, neural maturation, learning and memory, motor coordination, and stress. These studies provide evidence that these epigenetic modifications are involved in mediating the development of FASD symptoms.
Table 2.
Summary of the Epigenetic Effects of Alcohol on Early Development
| Age | Model | EtOH Dose or BEC | Cell Type | Effects | Citation |
|---|---|---|---|---|---|
| Fetal | Cultured rat fetal astrocytes | 75 mM | Cultured astrocytes | Decreased DNMT activity, decreased methylation of tPA promoter, decreased DNMT3a protein | Zhang et al., 2014 |
| Fetal | Cultured mouse fetal cortical neurons | 75 mM, 5 days | Cultured neurons | Demethylation of NR2B gene | Marutha Ravindran & Ticku, 2004 |
| Fetal | Cultured mouse fetal cortical neurons | 75 mM, 5 days for chronic intermittent ethanol paradigm | Cultured neurons | Demethylation of NR2B gene | Qiang et al., 2010 |
| Fetal | Mouse, cultured embryo | Dose: 88 mM | Cultured embryo | Altered methylation in many genes involved in metabolism, neural development, imprinting | Liu et al., 2009 |
| Fetal | Mouse, cultured neurospheres | Dose: 13 mM, 26 mM, 70 mM | Cultured neurospheres | Altered activating and repressive histone marks (H3K4me3 and H3K27me3) on genes involved in neural precursor identity and neurogenesis | Veazey et al., 2013 |
| Fetal | Cultured mouse fetal cortical neurospheres | 13 mM or 70 mM EtOH | Cultured neurospheres | High dose suppressed miR-21, -335, -9, and -153; Low dose increased miR-335 | Sathyan et al., 2007 |
| Fetal | Mouse, Gestational day 9–11 | Dose: 3 g/kg | Somatic embryo | DNA hypomethylation, lower methylase activity | Garro et al., 1991 |
| Fetal | Mice, Gestational day 9 | Dose: 5.8 g/kg EtOH | Somatic embryo | Subtle decrease in methylation of Igf2 locus with EtOH | Downing et al., 2011 |
| Fetal | Mouse, Gestational day 6–15 | BEC: ≥0.4 μg/mL EtOH | Brain | Upregulation of many miRNAs, including, miR-10a, miR-10b, miR-9 with EtOH; Downregulation of many miRNAs, including miR-200a, miR-496, miR-296 with EtOH | Wang et al., 2009 |
| Fetal | Mouse, Gestational Day 0.5–8.5 | Dose: ~12 g/kg/day | Brain (hippocampus) | Altered miRNAs expression of miR138-2, miR290, miR16-2; Altered site specific methylation of several CpG islands | Marjonen et al., 2015 |
| Fetal | Mouse and Rat, Gestational day 6–15 | Dose: 0.5, 1, 2, 4 and 6 g/kg/day | Brain | Decreased MeCP2 in prefrontal cortex and striatum | Kim et al., 2013 |
| Fetal | Rat, Gestational day 8–21 | Dose: 5% w/v EtOH, 35% ethanol-derived calories | Brain (hippocampus and cortex) | Altered imprinting patterns and expression of Dio3 | Sittig et al., 2011 |
| Fetal | Mouse, Gestational day 7–16 | BEC: 120–160 mg/dL | Brain (hippocampus) | Altered methylation program during development in hippocampus, decreased Mecp2 and Tet1 | Chen et al., 2013 |
| Fetal | Rats, Gestational day 7–21 | Dose: 6.7% EtOH (v/v), BEC: ~110–120 mg/dL | Brain (hypothalamus) in Pomc cells | Increased MeCP2 protein and DNA binding to Pomc gene | Gangisetty et al., 2014 |
| Neonatal | Rats, postnatal days 2–12 | BEC: 30–80 mM | Brain (cerebellum) | Reduced CREB binding protein, reduced H3 and H4 acetylation with EtOH exposure | Guo et al., 2011 |
| Fetal and preconception | Mouse; Fetal: gestational Day 0.5–8.5; Preconception: 4 days/week for 10 weeks | Dose: 10% EtOH (v/v), ~12 g/kg/day | Tail | Hypermethylation of the Agouti mutation (Avy) locus and transcriptional silencing of the gene | Kaminen-Ahola et al., 2010 |
| Fetal (F1) exposure, F1–3 generations | Rats, Gestational day 7–21 | Dose: 6.7% EtOH (v/v), BEC: ~110–120 mg/dL | Brain (hypothalamic neurons), sperm | Increased methylation of the Pomc gene promoter in hypothalamus and sperm cells in F1-F3 offspring | Govorko et al., 2012; Bekdash et al., 2013 |
| Fetal (F1) exposure, F2 offspring | Mouse, Gestational day 10–18 | Dose: 0.5 g/kg/day EtOH | Sperm, brain | Decrease in methylation of H19 gene in sperm of exposed F1 offspring, Decrease in methylation of H19 gene in brain of F2 offspring | Stouder et al., 2011 |
| Human children | Human children with FASD and healthy control children | Not reported | Buccal epithelial cells | 658 differentially methylated sites in FASD children compared to controls, overlapping with 95 different genes; increased methylation on genes relevant to neurodevelopment | Portales-Casamar et al., 2016 |
Numerous studies also show differential methylation on specific genes with functional consequences in gene expression and neural development (for a comprehensive review see Ungerer et al., 2013). Using in vitro models of chronic alcohol exposure, decreased DNA methylation in cultured fetal mouse neurons was found near the gene for the NMDA receptor subunit NR2B, a receptor involved in the development of AUDs (Marutha Ravindran & Ticku, 2004; Qiang et al.. 2010). Changes in methylation coincided with increased expression of NR2B, demonstrating functional consequences (Marutha Ravindran & Ticku, 2004; Qiang et al., 2010). As the NMDA receptor plays a major role in neural development (Constantine-Paton, Cline, & Debski, 1990) and in learning and memory (Li & Tsien, 2009), it is possible that methylation changes in NR2B may play a role in the development of FASD pathology and cognitive symptoms, although further research using in vivo methods is needed to confirm this hypothesis. An in vivo study showed prenatal alcohol exposure altered the normal developmental methylation and hydroxymethylation gradient in the hippocampus, which was associated with reduced hippocampus size and developmentally delayed formation of mature neurons in the dentate gyrus, suggesting that altered developmental methylation patterns in hippocampus due to alcohol exposure may play a role in the neural and intellectual deficits seen in FASD (Chen et al., 2013). Another study utilizing whole embryo culture showed alcohol-induced changes in methylation on chromosomes 7, 10, and X, which correlated with neural tube defects. Specifically, increased methylation of genes known to play a role in metabolism, such as Cyp4f13, and decreased methylation of genes known to play a role in development (Nlgn3, Elavl2, Sox21, and Sim1) and the imprinted gene Igf2r, were observed in alcohol-exposed embryos (Liu, Balaraman, Wang, Nephew, & Zhou, 2009). Other studies have also shown changes in methylation of imprinted genes including Igf2 (Downing et al., 2011). Thus, alteration in methylation of these genes may play an important role in the development of neural dysfunction in FASD, as they play a key role in normal neural development. As people with FASD are more likely to suffer from anxiety disorders, studies in our lab focus on the role of epigenetic modifications in stress-axis disruption due to fetal alcohol exposure. Studies in our lab have shown that rats exposed to fetal alcohol show hypermethylation of the proopiomelanocortin (Pomc) gene promoter region in the hypothalamus, and also reduced expression of this gene. These changes correlate with reduced production of the peptide encoded by the Pomc gene, β-endorphin (BEP), a negative regulator of the stress axis, and increased stress response in alcohol-exposed animals (Bekdash, Zhang, & Sarkar, 2013; Govorko, Bekdash, Zhang, & Sarkar, 2012). Fetal alcohol-induced Pomc promotor hypermethylation, reduced Pomc expression, and hyper-stress response were normalized by gestational choline supplementation, a dietary source of methyl, suggesting that altered Pomc methylation may have been due in part to deficits in available methyl donors (Bekdash et al., 2013). In addition, fetal alcohol-induced decreases in Pomc expression also coincided with increased transcriptional repressor MeCP2 in Pomc cells in the hypothalamus. Also, lentiviral knockdown of MeCP2 normalized the decreases in Pomc and normalized the aberrant hormonal response to stress in fetal alcohol-exposed animals (Gangisetty, Bekdash, Maglakelidze, & Sarkar, 2014). Finally, DNA methylation is also differentially altered in human populations exposed to in utero alcohol, as a recent study of methylation status of buccal swabs from children with FASD showed hypermethylation in genes implicated in neurodevelopment and neurological diseases such as anxiety, epilepsy, and autism-spectrum disorders (Portales-Casamar et al., 2016).
In addition to alterations in methylation, data from animal models show fetal alcohol exposure also causes chromatin remodeling, including changes in histone acetylation and methylation, and changes in miRNA expression, which contribute to altered gene expression and the neurodevelopmental deficits in FASD (for review see Ungerer et al., 2013). In a study utilizing ethanol-exposed cultured mouse fetal neurospheres, it was found that alcohol exposure alters activating and repressive histone modifications (H3K4me3 and H3K27me3, respectively) enrichment at genes involved in neural precursor identity and neurogenesis, such as nestin, Igf1, Dlx2, HoxA1, and Pax6, with some of these genes also showing differences in transcription (Veazey, Carnahan, Muller, Miranda, Golding, 2013). Thus, alcohol-induced post-translational histone modifications may alter expression of key regulatory genes involved in brain development to produce the neural dysfunction in FASD. An in vivo study rats exposed to alcohol neonatally (third-trimester human equivalent) showed decreased CREB binding protein, a histone acetyltransferase, and reduced H3 and H4 acetylation in the developing cerebellum (Guo et al., 2011). The researchers noted that these epigenetic changes in cerebellum might underlie cerebellar dysfunction and motor impairment in FASD. In the previously mentioned studies from our lab, fetal alcohol-induced increases in hypothalamic Pomc promoter methylation and decreased Pomc expression coincided with changes in histone modifications in Pomc cells, including decreases in histone activation marks (H3K4me3, acetylated H3K9, phosphorylated H3S10), and increases in repressive marks (H3K9me2 and histone methyltransferases G9a and Setdb1) (Bekdash et al., 2013). Treatment of fetal alcohol-exposed animals with HDAC inhibitor trichostatin-A also normalized Pomc expression and aberrant hormonal response to immune stress (Govorko et al., 2012). Thus, histone modifications appear to be crucial to some of the neurobiological defects produced with fetal alcohol exposure.
In addition, a few pioneering studies indicate fetal alcohol produces alterations in miRNAs that regulate neurodevelopment. Using cultured mouse fetal cortical neurospheres, high dose alcohol was found to suppress the expression of miR-21, miR-335, and miR-153, which were shown to synergistically regulate genes involved in neuronal maturation and proliferation, which are known to be aberrant in FASD (Sathyan, Golden, & Miranda, 2007). In an in vivo model, prenatal exposure to alcohol induced severe fetal teratogenesis and learning impairment in offspring, which was correlated with increased expression of several miRNAs, including miR-10a, miR-10b, miR-9, and decreased expression of miR-200a, miR-496, and miR-296 in brain (Wang et al., 2009). It is possible these miRNAs may play a role in the observed teratogenesis and learning deficits in the alcohol-exposed animals, but further studies are needed to confirm this mechanism. Finally, early gestational exposure to ethanol resulted in differential expression of miR138-2, miR290, and miR16-2 in hippocampus (Marjonen et al., 2015). It was noted by the researchers that miR290 has been connected with embryonic gene regulation (Houbaviy, Murray, & Sharp, 2003) and the pluripotent stem cell state (Tata, Tata, Kühl, & Sirbu, 2011), while miR138-2 is associated with dendritic spine size in the hippocampus (Siegel et al., 2009). These studies suggest miRNAs participate in the development of alcohol-induced neural defects, but more studies are needed on this topic to determine a role for ncRNAs in the development of FASDs.
5. Epigenetic Inheritance
Epigenetic inheritance is the phenomenon that occurs when so-called epimutations, or changes in gene expression that do not involve actual changes to the DNA sequence, are carried via the germline to the offspring, potentially through multiple generations. Epigenetic inheritance has been shown to occur from exposure of the parental or F0 organism to multiple environmental stimuli, including changes in diet, psychological stress, exposure to toxins, exposure to drugs of abuse, and exposure to alcohol, on which this review will focus. It is important to distinguish between “germline-dependent” from “germline-independent” epigenetic inheritance. Germline-dependent epigenetic inheritance occurs when the first generation organism (F0) is exposed to an environmental stimulus and his/her germ cells acquire epimutations that are then passed on to the next generation offspring (F1), affecting its germline and somatic cell epigenome in this generation in the absence of any external stimuli factors. In turn, these epimutations can then be transferred to the next generation (F2) through the germline, to alter F2’s somatic cells’ gene expression to express the same phenotype in the absence of external factors, and so on. In order for “transgenerational” inheritance to occur, the phenotype must be passed on from the F0 generation to the F3 generation along the female germline or from the F0 generation to the F2 generation along the male germline (Jirtle & Skinner, 2007; Skinner, 2008, 2011). This is because the germ cells of the F0 generation would be affected directly by exposure to the environmental factor (i.e., alcohol exposure), and thus any effects on the F1 generation would be due to the direct effects of alcohol on the germline. In the case of exposed females, if the F0 female is pregnant, both her germ cells and the germ cells of the developing F1 embryo(s) would be exposed, so it is necessary for the epimutation to be transmitted to the F3 generation in order to meet the criteria for “transgenerational” epigenetic inheritance. Epigenetic inheritance that does not meet the criteria for “transgenerational” inheritance might be termed “multigenerational” inheritance instead (Skinner, 2008).
“Germline-independent” epigenetic inheritance is another phenomenon, and occurs because of behavioral parental factors when the effects of environmental insults (i.e., alcohol exposure) affect the uterine environment or the maternal behavior of the exposed F0 female, and thus induce epimutations in the F1 generation due to changes in the prenatal environment and/or maternal care, independent of any effect on the germline (Grossniklaus, Kelly, Ferguson-Smith, Pembrey, & Lindquist, 2013; Sarkar, 2016; Weaver, 2007). These epimutations may then be transmitted again through altered parental behavior by the adult F1 animals to the F2 offspring, and so on. A specific example of this effect was found in the epigenetic effects on the offspring of good versus negligent maternal care in rats. Neonatal rats that received high levels of licking and grooming from their mothers, an indicator of good maternal care, showed decreased methylation at a binding site in the promotor of the glucocorticoid receptor gene and increased expression of the gene in the hippocampal region of the brain, which was associated with resilience in response to stress (Weaver, 2007; Weaver et al., 2004). In addition, cross fostering of pups from high-licking and grooming dams to low-licking and grooming dams reversed this effect, demonstrating that this epigenetic effect was dependent on maternal care, and was thus germline-independent.
Mechanisms for Epigenetic Inheritance
Understanding the mechanisms of multigenerational or transgenerational epigenetic inheritance of alcohol-related disease is complex, for several reasons. First, as germline-dependent and germline-independent mechanisms exist, one must discriminate between these two mechanisms. In humans, epigenetic inheritance of alcohol-related disease in humans most likely involves both germline-dependent and germline-independent mechanisms of transmission, as having an AUD is known to impact the quality of maternal behavior, and social factors such as parental abuse and neglect and early life environment and adversity play an important role in developing AUDs in offspring later in life (Hill, Blow, Young, & Singer, 1994). Germline-dependent mechanisms for epigenetic inheritance of alcohol-related disease also exist based on human and animal studies. Notably, in animal models, the parental care effects can be eliminated by fostering offspring to un-exposed surrogate mothers, and thus, it can be determined whether the epimutations observed are due to germline-dependent or germline-independent mechanisms. This review will focus on germline-dependent mechanisms for epigenetic inheritance of alcohol-related health problems.
In addition, pinpointing specific epigenetic mechanisms for germline-dependent inheritance of alcohol-related disease has been difficult to date, and these mechanisms are still poorly understood. Theoretically, it would seem that transmission of epimutations through the germline are rare, as embryonic cells, including primordial germ cells, go through two waves of demethylation during early development, thus losing most epigenetic information from the germ cells encoded in methylations (Bao & Yan, 2012; Seisenberger et al., 2013). Nonetheless, some sections of DNA, such as imprinted genes in the germline, do keep their methylation status inherited from one of the parents and escape the demethylation waves during early development. The methylation status in these regions is thus preserved and then transmitted to the offspring from the parent. A well-known example of imprinted genes is the Igf2/H19 gene cluster (Nordin, Bergman, Halje, Engstrom, & Ward, 2014). Studies suggest that the number of imprinted genes might be fairly high, especially in brain, suggesting that genomic imprinting could be a common mechanism for epigenetic inheritance (Gregg et al., 2010). In addition, some non-imprinted genomic elements, such as IAPs, also escape the demethylation during development (Lane et al., 2003). Sperm cells also lose most of their histone epigenetic histone marks, as most histones are swapped for protamines during spermatogenesis. However, some histones in sperm are retained, which allows for an additional possible mechanism for germline-dependent epigenetic inheritance (Bench, Friz, Corzett, Morse, & Balhorn, 1996; Gatewood, Cook, Balhorn, Bradbury, & Schmid, 1987). It was also found that C. elegans embryos retained the repressive histone mark H3K27me from oocytes, indicating that epigenetic marks from the female germline might also be transmitted to the next generation (Gaydos, Wang, & Strome, 2014). In addition, recent studies suggest epigenetic inheritance may also involve transmission of epimutations through ncRNAs (Ashe et al., 2012; Larriba & del Mazo, 2016; Yan, 2014). Notably, alcohol exposure does affect epigenetic machinery in germline cells, and some studies have shown evidence for multigenerational and transgenerational epigenetic inheritance of alcohol-related health problems, although much more future research in this area is warranted. These studies are reviewed below.
6. Evidence for Epigenetic Inheritance of Alcohol-related Pathology
Human Studies: Multigenerational Effects
It is known that alcohol abuse and AUDs tend to be passed down across generations in human populations, although the mechanisms are hard to interpret in these observational studies. Having a parent or other relative with an AUD increases the risk for and the severity of alcohol abuse (Cotton, 1979; Kendler et al., 2015; Morean, Corbin, Sinha, & O’Malley, 2009; Sjoerds et al., 2013; Turner et al., 1993; Worobec et al., 1990), although environmental and social factors alongside genetic factors play a role in this relationship (Enoch & Goldman, 1999; Hill et al., 1994; Nieratschker, Batra, & Fallgatter, 2013). The heritability of alcohol dependence is estimated to be between 30%–70% as estimated from studies of twins, in which one twin was separated from the other and adopted, thus controlling the effects of similar environment (Agrawal & Lynskey, 2008). Several genetic factors including genes for alcohol metabolism, such as alcohol dehydrogenase, and genes for neuromodulators, including dopamine systems, serotonin systems, and opioid systems, have been found to be correlated to alcohol dependence (Demers, Bogdan, & Agrawal, 2014; Kreek, Nielsen, & LaForge, 2004).
It has been noted that genetic variants do not appear to explain all the heredity in complex disorders (such as AUDs) (Manolio et al., 2009), and perhaps epigenetic inheritance might be able to “fill in the blanks” to help better explain the inheritance of complex diseases (Bohacek, Gapp, Saab, & Mansuy, 2013; Danchin et al., 2011). In addition to genetic variants, some studies have also opened the possibility of epigenetic factors for inherited transmission of alcohol-related health problems. It has long been noted that children born to fathers with AUDs show symptoms similar to children with FASD, including cognitive deficits, although they are not directly exposed to alcohol as is the case of children born to mothers who drank during pregnancy (Gabrielli & Mednick, 1983; Lemoine, Harousseau, Borteyru, & Menuet, 2003). For women who drink prior to pregnancy, the risk is also increased for her children for lower birth weight (Nykjaer et al., 2014) and mental retardation (Roeleveld, Vingerhoets, Zielhuis, & Gabreëls, 1992). Data on possible epigenetic mechanisms for these phenomena in humans are lacking. In one recent study, heavy alcohol consumption has been shown to be correlated with a decrease in methylation of imprinted genes H19 and IG-DMR in the sperm of men (Ouko et al., 2009). This novel study suggests a possible mechanism for the epigenetic transmission of alcohol-related health problems to the offspring via imprinted genes on sperm cells.
Human Studies: Transgenerational Effects
Studies investigating the possibility of transgenerational inheritance of alcohol-related health problems are also lacking. In one study, it was found that having a grandparent with major depressive disorder, which often co-occurred with substance abuse including alcohol abuse, greatly increased the risk of the individual having major depressive disorder himself or herself (Olino et al., 2008). Likewise with FASD, grandmothers of children with fetal alcohol syndrome or partial fetal alcohol syndrome were found to have a much greater rate of history of alcohol abuse, suggesting a generational phenomenon in FASDs (Kvigne, Leonardson, Borzelleca, & Welty, 2008). Of course, epigenetic inheritance is impossible to conclude in this case, as social factors were also likely at play, and the mothers drank during pregnancy, but the authors speculated that the risk for FASD seemed to be magnified with the incidence of grandparental alcohol abuse. These clinical studies hint at the possibility of multigenerational and transgenerational inheritance of psychiatric disorders and alcohol-related health problems, but they do not establish a mechanism for the inheritance of these disorders. Clearly, more clinical studies are needed to examine the family tree of persons with AUDs or FASDs to investigate a generational pattern for alcohol-related problems. In addition, incidences of other health problems, including stress-related and psychiatric problems in persons with a familial history of AUDs or FASDs might be studied. Finally, future studies could also examine the possibility and mechanisms of epigenetic inheritance of AUDs in humans.
Animal Studies: Multigenerational Effects
Although human studies of generational transfer of alcohol-related health problems is lacking, there are many studies showing multigenerational and a small number of studies showing transgenerational effects of alcohol exposure in animal models (for excellent reviews, see also Finegersh et al., 2015; Sarkar, 2016; and Yohn et al., 2015). Many studies have shown that paternal preconception alcohol exposure has detrimental effects on the F1 offspring behavior and neurobiology in mouse and rat models. Preconception paternal alcohol exposure results in reduced organ weight and an increase in the number of “runts” in litters in rat offspring (Abel, 1993b). F1 offspring also show neurobiological alterations, including increased cortical thickening (Jamerson, Wulser, & Kimler, 2004), decreased cortical and striatal dopamine transporter expression (Kim et al., 2014), decreased expression of cortical and striatal MeCP2 (Kim et al., 2014), and decreased hypothalamic β-endorphin (Cicero et al., 1990). Paternal exposure to alcohol results in several behavioral abnormalities in the F1 offspring, including altered learning in the passive avoidance and radial arm maze tasks (Abel & Lee, 1988; Abel & Tan, 1988; Wozniak, Cicero, Kettinger, & Meyer, 1991). In addition, preconception paternal alcohol also results in behaviors resembling psychopathology in humans, including decreased grooming, decreased fear, increased aggression, decreased attention, increased impulsivity, increased hyperactivity, and increased anxiety (Abel, 1994; Kim et al., 2014; Liang et al., 2014; Meek, Myren, Sturm, & Burau, 2007). In addition, studies have also found hormonal alterations from paternal preconception alcohol in rats. Male mouse F1 offspring show decreased corticosterone response to stress, suggesting altered hypothalamic-pituitary-adrenal axes in these animals (Rompala, Finegersh, & Homanics, 2016), and also show reduced adult levels of testosterone (Abel & Lee, 1988). Finally, studies have shown paternal preconception increased sensitivity to amphetamine (Abel, 1993a) and decreased alcohol drinking, but increased sensitivity to alcohol (Finegersh & Homanics, 2014). Notably, the effects of preconception paternal alcohol exposure on F1 offspring were shown to be sexually dimorphic in many cases. These studies show, for the most part, that alcohol drinking in F0 males results in physiological and neurological abnormalities, disrupted learning, abnormal behavior, and increased sensitivity to drugs and alcohol in F1 offspring, findings which resemble deficits shown in human FASD populations and noted in children of fathers with AUDs.
While these studies examined the effects of paternal preconception alcohol exposure on offspring, studies in our lab have recently shown that maternal preconception exposure also negatively affects offspring health, leading to an increase in adult offspring vulnerability to stress (Jabbar et al., 2016). Specifically, maternal alcohol exposure 3 weeks prior to conception results in several defects in adult F1 offspring, including increased hormonal stress response to an immune stressor, increased hypothalamic corticotropin-releasing hormone, decreased hypothalamic β-endorphin, and altered methylation status on CpG islands in stress regulatory genes in various brain areas. Interestingly, F0 maternal behavior was not altered by preconception alcohol, and fostering F1 offspring from alcohol-treated dams to control dams did not change their enhanced stress response, suggesting the detrimental effects on the F1 offspring were not due to changes in F0 maternal behavior. In addition, the vulnerabilities in F1 offspring of preconception alcohol-treated dams were normalized by neonatal treatment with a DNMT blocker 5-azadeoxycytidine, suggesting the stress abnormalities observed in F1 offspring may have been inherited via epigenetic mechanisms, such as altered methylation at promoters of stress regulatory regions. Further studies of oocytes from preconception alcohol-treated dams are underway to determine a germline mechanism for the inheritance of stress vulnerabilities in F1 offspring.
In addition, future studies are needed to determine whether the effects of preconception maternal alcohol are transgenerational. In addition to preconception alcohol effects on offspring, recent rat studies have also shown that grandmaternal ethanol consumption during pregnancy has profound effects on behavior and endocrinology of second-generation progeny. Exposure to ethanol in utero has been shown to cause profound metabolic, endocrine, and behavioral disruptions in the exposed F1 offspring, and recent studies from the Redei laboratory have also shown these effects are carried over into the unexposed F2 generation. Particularly, exposure to prenatal ethanol was shown to cause insulin resistance in both the F1 and F2 generations, but the phenotype was dependent on the sex of the exposed F1 parent and of the F2 offspring (Harper, Tunc-Ozcan, Graf, & Redei, 2014). In addition, grandmaternal alcohol consumption was shown to cause a fear memory deficit and altered expression of hippocampal thyroid hormone-regulated type 3 deiodinase (Dio3) and neurogranin (Nrgn) expression in the F1-exposed offspring and in the matrilinear F2 offspring, suggesting these deficits may have been carried over via the female germline (Tunc-Ozcan, Harper, Graf, & Redei, 2016). In both studies, thyroxine (T4) administration to the F0 mother during ethanol exposure alleviated the detrimental metabolic and behavioral effects on the F2 generation, suggesting that alcohol-disrupted thyroid function in the F0 female was in part responsible for the multigenerational effects of ethanol exposure.
Animal Studies: Transgenerational Effects
One of the first studies to examine the transgenerational effects of alcohol was done in the early 20th century using ethanol vapor-exposed male and female guinea pigs that were then bred with normal control animals over several generations (Stockard & Papanicolaou, 1918). The researchers found the effects of alcohol exposure affected offspring as far as the F4 generation, causing decreased weight, behavioral changes, increased mortality, and fertility problems in some portion of the descendants. The cause of this phenomenon was hypothesized to be damage to the germline.
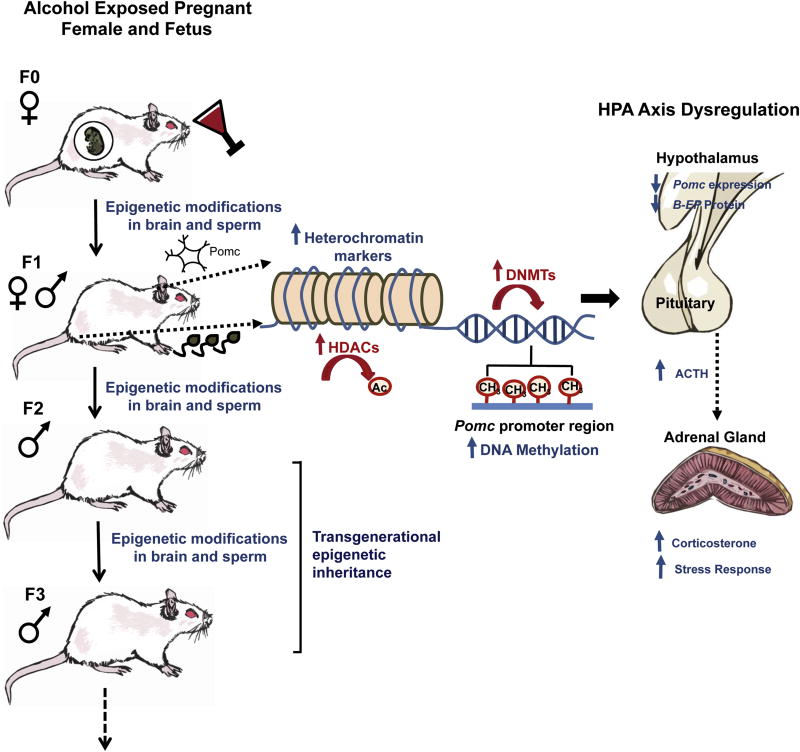
Nearly a century later, studies in our lab investigated whether deficits in hypothalamic Pomc expression due to fetal alcohol exposure could be passed down to subsequent generations. It was found that F1-F3 male progeny of the male germline, but not female germline, did show consistent decreases in hypothalamic Pomc expression with concomitant increases in Pomc methylation (Govorko et al., 2012). Importantly, the hypermethylation of the Pomc gene in the hypothalamus was accompanied by a similar hypermethylation of Pomc in sperm cells in the F1-F3 males, demonstrating that the deficit in Pomc expression was carried from generation to generation via the male germline (see Fig. 2). Changes in methylation and expression of Pomc were accompanied by decreased production of the Pomc-encoded protein BEP, a negative regulator of the stress axis, and increased hypothalamic-pituitary-adrenal response to stress. These studies show that the negative effects of alcohol exposure on the stress axis can be transgenerationally inherited over several generations and, in the case of Pomc deficiency, are transmitted via methylation changes on the male germline.
Fig. 2.
Mechanisms for transgenerational inheritance of fetal alcohol-induced epigenetic marks on the Pomc gene and relation to stress axis disruption. Fetal alcohol exposure induces epigenetic modifications on the Pomc gene and hyper-stress response in male and female F1 offspring. These epigenetic changes and functional defects are carried on to the F2 and F3 generations via the male, but not female, germline (Govorko et al., 2012). Specifically, fetal alcohol exposure leads to increased repressive epigenetic marks on histones in Pomc-producing hypothalamic neurons including increased de-acetylation by HDACs. Fetal alcohol exposure also leads to increased levels of DNMTs and increased methylation of the Pomc promotor, leading to reduced hypothalamic expression of Pomc, reduced hypothalamic production of the protein β-endorphin (β-EP), and increased stress response. Hypermethylation of the Pomc gene in the hypothalamus is accompanied by a similar hypermethylation of Pomc in sperm cells in the F1-F3 males.
7. Possible Mechanisms for Germline-dependent Epigenetic Inheritance of Alcohol-related Pathology
Knowledge about possible mechanisms of epigenetic inheritance of alcohol-related pathology is limited to date, but studies investigating alcohol’s effects on the germline have offered promising directions for the research. Notably, alcohol exposure is known to have a direct detrimental effect on the male germline in humans (Emanuele & Emanuele, 1998; Muthusami & Chinnaswamy, 2005). In order to investigate possible mechanisms for epigenetic germline-dependent inheritance, recent human and animal studies have focused investigation on alcohol-induced changes in methylation along the male germline. As mentioned previously, in human populations, heavy alcohol consumption has been shown to be correlated with a decrease in methylation of imprinted genes H19 and IG-DMR in the sperm of men (Ouko et al., 2009). In animal models, it was also found that fetal alcohol exposure decreased methylation on the H19 gene in the alcohol-exposed F1 generation, and that this decreased methylation pattern on H19 was also observed in the brains of the F2 generation, demonstrating epigenetic inheritance of methylation changes in this imprinted gene (Stouder, Somm, & Paoloni-Giacobino, 2011). In another animal study, it was found that alcohol exposure in F0 sires decreased methylation on the H19- and Peg3-imprinted genes in sperm, and that similar decreases in methylation on Peg3 were also found in the cerebral cortices of the F1 offspring (Liang et al., 2014). These changes in Peg3 methylation in offspring brain were associated with aberrant circling behavior. These studies all offer evidence that alcohol-induced epigenetic transmission of pathology can be transmitted to the offspring, specifically via imprinted genes along the male germline. However, another study found paternal alcohol exposure was associated with decreased methylation at two H19 binding sites in F1 offspring, but only decreased methylation at one CpG of the H19 gene in the sperm of alcohol-exposed F0 sires (Knezovich & Ramsay, 2012). The authors suggested that the imprinted gene H19 does seem to play a role in epigenetic transmission of paternal alcohol exposure effects to the offspring, but the mechanism may not be specifically via changes in methylation at this gene in sperm, but rather, different mechanisms such as sperm-borne RNA-mediated effects or histone modifications. Thus, further studies on the mechanisms of epigenetic inheritance involving the H19 imprinted region are warranted.
Other studies have investigated the effects of alcohol on methylation of non-imprinted genes in the germline and its relation to epigenetic inheritance of alcohol-induced health problems. As discussed previously, our lab found increased methylation of the Pomc gene in sperm and hypothalamus of the F1-F3 male descendants of fetal alcohol exposed rats (see Fig. 2) (Govorko et al., 2012). This study shows that aberrant methylation due to alcohol can be passed down to offspring. The reason why methylation patterns on this gene along the male germline can be preserved and escape reprogramming during development is unknown. A possible explanation for this would be, since the phenotype is only passed down along the male germline and Pomc expression is regulated by the non-pairing region of the Y chromosome (Botbol et al., 2011), alcohol exposure leads to epigenetic modifications on the Y chromosome that are spared from reprogramming during development and are then passed down to subsequent generations (Sarkar, 2016). Future studies are needed to address this possibility.
Other studies have also found methylation changes in specific non-imprinted genes in the sperm of the alcohol-exposed F0 sires that were then transmitted to somatic brain cells of the F1 offspring generation. Finegersh and colleagues found that alcohol exposure in F0 males decreased methylation of the brain-derived neurotrophic factor (BDNF) promoter in sperm, a methylation change which was also present in the ventral tegmental area of the brain of F1 offspring (Finegersh & Homanics, 2014). This change in BDNF methylation was also accompanied by increased VTA BDNF expression in F1 offspring, as well as decreased preference for and consumption of alcohol but increased sensitivity to the behavioral effects of alcohol. Thus, this study shows a functional change in offspring of sires exposed to alcohol, which may be due to an inherited change in methylation on the BDNF gene that was transmitted across the male germline. In another study, increased methylation of the dopamine transporter (DAT) gene was found in both the sperm of F0 alcohol-exposed sires and in the cortical and striatal regions of the brains of F1 offspring, and F1 offspring showed a decrease in DAT mRNA and protein in these brain regions (Kim et al., 2014). Alongside these methylation changes in DAT, decreases in expression of two regulators of methylation machinery, DNMT1 and MeCP2, were also found in the brains of F1 offspring, suggesting that changes in these regulators may play a role in the epigenetic inheritance of the methylation changes in DAT. In addition, the F1 offspring whose sires were exposed to alcohol were found to have increased hyperactivity, inattentiveness, and impulsive behaviors, resembling human ADHD symptoms. This study also shows another potential mechanism for the cross-generational inheritance of the behavioral disruptions observed in the offspring of fathers who drink heavily, specifically changes in methylation on the DAT gene on the male germline. Together, these studies provide evidence that alcohol-induced methylation changes on non-imprinted genes can be transmitted along the male germline across generations, and that these changes might mediate the multi-generational dysfunctional effects of alcohol on the offspring. Future studies are also needed to address the mechanisms required for epigenetic inheritance of specific methylation changes on these non-imprinted genes.
It is interesting to note that in all these cases, aberrant methylation of genes is observed in the germline and in somatic tissue of offspring. As discussed in Section 4, alcohol is known to affect methylation machinery, leading to severe health consequences, such as cancer (Varela-Rey et al., 2013). Particularly, alcohol has been shown to decrease activity of DNMT1 in somatic tissue (Ponomarev et al., 2012) and reduce cytosine methyltransferase mRNA in sperm (Bielawski, Zaher, Svinarich, & Abel, 2002). Alcohol is also known to reduce levels of the methyl donor SAM, leading to hypomethylation of somatic tissue (Lu & Mato, 2005). Alcohol’s effects on these enzymes in the germline could potentially be a mechanism for the observed changes in methylation in sperm and for the epigenetic inheritance of these methylation changes. These mechanisms need to be investigated for future studies.
While these studies have shown evidence for alcohol-induced epigenetic inheritance by methylation changes along the male germline, it remains a likely possibility that other mechanisms exist, such as sperm-mediated epigenetic inheritance of histone modifications and ncRNAs. Alcohol is known to modify histones (Moonat & Pandey, 2012) and to change levels of several miRNAs (Miranda et al., 2010) in somatic cells, and it is possible these processes occur in germline cells as well and may play a role in epigenetic inheritance. Indeed, epigenetic inheritance via histone modifications and ncRNAs have been identified in other models (Ashe et al., 2012; Gaydos et al., 2014; Larriba & del Mazo, 2016; Yan, 2014). In C. elegans, the H3K27me mark can be transmitted via germ cells to subsequent generations (Gaydos et al., 2014). Also in C. elegans, Ashe and colleagues (2012) found that a piRNA-triggered mechanism could maintain epigenetic memory across many generations. Similar mechanisms might be at play in epigenetic inheritance of alcohol-related pathologies also.
While many studies have focused on mechanisms of male germline-dependent inheritance of alcohol-related pathology, there are few studies to date that have investigated female germline-dependent epigenetic inheritance. Can alcohol exposure in females result in epimutations in oocytes that can be transmitted to future generations? Recent studies discussed in Section 6 showing that grandmaternal alcohol consumption caused behavioral and gene expression changes in the matrilinear F2 offspring suggest that some alcohol-induced functional changes may be carried over via the female germline (Tunc-Ozcan et al., 2016). Oocytes have been shown to be sensitive to the effects of alcohol (Cuthbertson 1983; Emanuele, Wezeman, & Emanuele, 2002). Also, as mentioned in the previous paragraph, histone marks in oocytes were found to be passed down to offspring in C. elegans (Gaydos et al., 2014), but whether this epigenetic inheritance occurs in mammals needs to be investigated. This question presents an open avenue for investigation, and is pertinent for understanding the epigenetic transmission of alcohol-related pathology.
8. Summary and Future Studies
In summary, animal and human studies demonstrate exposure to alcohol during early development and later in life leads to changes in the epigenome, including changes in DNA methylation, histone modifications, and ncRNAs, and these studies suggest these epigenetic changes play a role in the development of FASDs and AUDs. Future studies on the role of epigenetic processes in the development of alcohol-related pathology should build on these studies to determine precise mechanisms for the development of these pathologies and possible targets for treatment. In addition, animal and human studies show evidence that pathologies due to alcohol exposure can be inherited in offspring via epigenetic mechanisms. Multigenerational and transgenerational epigenetic inheritance of alcohol-related pathologies has been demonstrated in animal models, but the mechanisms for this inheritance are still under investigation. Animal studies have shown that a mechanism for epigenetic inheritance of alcohol-related pathologies occurs via methylation changes in imprinted and non-imprinted genes via the male germline, but more studies are needed to fully understand this mechanism. In addition, further studies are needed to assess the possibility of alternative germline mechanisms for multigenerational epigenetic inheritance, including the possibility of transference via histone modifications and ncRNAs, and inheritance via the female germline. Current animal studies are limited by the fact that most only go as far as the F1 generation to examine the effects of alcohol exposures on subsequent generations, and future studies might examine F2 and F3 generations for evidence of transgenerational epigenetic inheritance. Finally, studies on epigenetic inheritance of alcohol-related disorders in human populations are lacking, and future clinical studies could focus on descendants of persons with AUDs to better understand multigenerational and transgenerational inheritance of alcohol-related pathology. These future studies are crucial to a complete understanding of the phenomenon and mechanisms for epigenetic inheritance of alcohol-related health problems.
Highlights.
Alcohol-related health problems are often passed down across generations in human populations.
Recent studies in animal models have suggested that epigenetic factors are partially responsible for the transmission of alcohol-related pathologies across generations.
This review summarizes evidence for multigenerational and transgenerational epigenetic inheritance of alcohol-related pathology through the germline.
Acknowledgments
This work is partly supported by NIH grants R37AA08757, R01AA011591 to DKS, and F32AA023434 grant to LC. We would like to thank Aaron DeLaRosa for his assistance in illustrating the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Paternal alcohol exposure and hyperactivity in rat offspring: effects of amphetamine. Neurotoxicology and Teratology. 1993a;15:445–449. doi: 10.1016/0892-0362(93)90063-t. [DOI] [PubMed] [Google Scholar]
- Abel EL. Rat offspring sired by males treated with alcohol. Alcohol. 1993b;10:237–242. doi: 10.1016/0741-8329(93)90042-m. [DOI] [PubMed] [Google Scholar]
- Abel EL. Effects of physostigmine on male offspring sired by alcohol-treated fathers. Alcoholism: Clinical and Experimental Research. 1994;18:648–652. doi: 10.1111/j.1530-0277.1994.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Lee JA. Paternal alcohol exposure affects offspring behavior but not body or organ weights in mice. Alcoholism: Clinical and Experimental Research. 1988;12:349–355. doi: 10.1111/j.1530-0277.1988.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Tan SE. Effects of paternal alcohol consumption on pregnancy outcome in rats. Neurotoxicology and Teratology. 1988;10:187–192. doi: 10.1016/0892-0362(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Heath AC, Lynskey MT. DSM-IV to DSM-5: the impact of proposed revisions on diagnosis of alcohol use disorders. Addiction. 2011;106:1935–1943. doi: 10.1111/j.1360-0443.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Critical Reviews in Clinical Laboratory Sciences. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Ashe A, Sapetschnig A, Weick EM, Mitchell J, Bagijn MP, Cording AC, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi: 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakoyiannis I, Gkioka E, Pergialiotis V, Mastroleon I, Prodromidou A, Vlachos GD, et al. Fetal alcohol spectrum disorders and cognitive functions of young children. Reviews in the Neurosciences. 2014;25:631–639. doi: 10.1515/revneuro-2014-0029. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Research. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Yan W. Male germline control of transposable elements. Biology of Reproduction. 2012;86:162, 1–14. doi: 10.1095/biolreprod.111.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcoholism: Clinical and Experimental Research. 2013;37:1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench GS, Friz AM, Corzett MH, Morse DH, Balhorn R. DNA and total protamine masses in individual sperm from fertile mammalian subjects. Cytometry. 1996;23:263–271. doi: 10.1002/(SICI)1097-0320(19960401)23:4<263::AID-CYTO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Berezikov E. Evolution of microRNA diversity and regulation in animals. Nature Reviews. Genetics. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- Bielawski DM, Zaher FM, Svinarich DM, Abel EL. Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcoholism: Clinical and Experimental Research. 2002;26:347–351. [PubMed] [Google Scholar]
- Blasco C, Caballería J, Deulofeu R, Lligoña A, Parés A, Lluis JM, et al. Prevalence and mechanisms of hyperhomocysteinemia in chronic alcoholics. Alcoholism: Clinical and Experimental Research. 2005;29:1044–1048. doi: 10.1097/01.alc.0000169265.36440.ee. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Gapp K, Saab BJ, Mansuy IM. Transgenerational epigenetic effects on brain functions. Biological Psychiatry. 2013;73:313–320. doi: 10.1016/j.biopsych.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Botbol M, Roubertoux PL, Carlier M, Trabado S, Brailly-Tabard S, Perez-Diaz F, et al. Modulation of brain β-endorphin concentration by the specific part of the Y chromosome in mice. PLoS One. 2011;6:e16704. doi: 10.1371/journal.pone.0016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature Reviews. Genetics. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nature Reviews. Genetics. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ozturk NC, Zhou FC. DNA methylation program in developing hippocampus and its alteration by alcohol. PLoS One. 2013;8:e60503. doi: 10.1371/journal.pone.0060503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Adams ML, O’Connor L, Nock B, Meyer ER, Wozniak D. Influence of chronic alcohol administration on representative indices of puberty and sexual maturation in male rats and the development of their progeny. The Journal of Pharmacology and Experimental Therapeutics. 1990;255:707–715. [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annual Review of Neuroscience. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. Journal of Studies on Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- Cuthbertson KS. Parthenogenetic activation of mouse oocytes in vitro with ethanol and benzyl alcohol. The Journal of Experimental Zoology. 1983;226:311–314. doi: 10.1002/jez.1402260217. [DOI] [PubMed] [Google Scholar]
- Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nature Reviews. Genetics. 2011;12:475–486. doi: 10.1038/nrg3028. [DOI] [PubMed] [Google Scholar]
- Demers CH, Bogdan R, Agrawal A. The Genetics, Neurogenetics and Pharmacogenetics of Addiction. Current Behavioral Neuroscience Reports. 2014;1:33–44. doi: 10.1007/s40473-013-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing C, Johnson TE, Larson C, Leakey TI, Siegfried RN, Rafferty TM, et al. Subtle decreases in DNA methylation and gene expression at the mouse Igf2 locus following prenatal alcohol exposure: effects of a methyl-supplemented diet. Alcohol. 2011;45:65–71. doi: 10.1016/j.alcohol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicology and Teratology. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Emanuele MA, Emanuele NV. Alcohol’s effects on male reproduction. Alcohol Health and Research World. 1998;22:195–201. [PMC free article] [PubMed] [Google Scholar]
- Emanuele MA, Wezeman F, Emanuele NV. Alcohol’s effects on female reproductive function. Alcohol Research & Health. 2002;26:274–281. [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. The Psychiatric Clinics of North America. 1999;22:289–299. viii. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Finegersh A, Homanics GE. Paternal alcohol exposure reduces alcohol drinking and increases behavioral sensitivity to alcohol selectively in male offspring. PLoS One. 2014;9:e99078. doi: 10.1371/journal.pone.0099078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Rompala GR, Martin DI, Homanics GE. Drinking beyond a lifetime: New and emerging insights into paternal alcohol exposure on subsequent generations. Alcohol. 2015;49:461–470. doi: 10.1016/j.alcohol.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. Epigenetic transmission of the impact of early stress across generations. Biological Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. Bioessays. 2010;32:473–480. doi: 10.1002/bies.200900170. [DOI] [PubMed] [Google Scholar]
- Gabrielli WF, Jr, Mednick SA. Intellectual performance in children of alcoholics. The Journal of Nervous and Mental Disease. 1983;171:444–447. doi: 10.1097/00005053-198307000-00009. [DOI] [PubMed] [Google Scholar]
- Gangisetty O, Bekdash R, Maglakelidze G, Sarkar DK. Fetal alcohol exposure alters proopiomelanocortin gene expression and hypothalamic-pituitary-adrenal axis function via increasing MeCP2 expression in the hypothalamus. PLoS One. 2014;9:e113228. doi: 10.1371/journal.pone.0113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro AJ, McBeth DL, Lima V, Lieber CS. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1991;15:395–398. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Gatewood JM, Cook GR, Balhorn R, Bradbury EM, Schmid CW. Sequence-specific packaging of DNA in human sperm chromatin. Science. 1987;236:962–964. doi: 10.1126/science.3576213. [DOI] [PubMed] [Google Scholar]
- Gaydos LJ, Wang W, Strome S. Gene repression. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science. 2014;345:1515–1518. doi: 10.1126/science.1255023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biological Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, et al. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U, Kelly WG, Ferguson-Smith AC, Pembrey M, Lindquist S. Transgenerational epigenetic inheritance: how important is it? Nature Reviews. Genetics. 2013;14:228–235. doi: 10.1038/nrg3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Crossey EL, Zhang L, Zucca S, George OL, Valenzuela CF, et al. Alcohol exposure decreases CREB binding protein expression and histone acetylation in the developing cerebellum. PLoS One. 2011;6:e19351. doi: 10.1371/journal.pone.0019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerty SL, Bidwell LC, Harlaar N, Hutchison KE. An Exploratory Association Study of Alcohol Use Disorder and DNA Methylation. Alcoholism: Clinical and Experimental Research. 2016;40:1633–1640. doi: 10.1111/acer.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development. The FEBS Journal. 2009;276:2175–2191. doi: 10.1111/j.1742-4658.2009.06959.x. [DOI] [PubMed] [Google Scholar]
- Harper KM, Tunc-Ozcan E, Graf EN, Redei EE. Intergenerational effects of prenatal ethanol on glucose tolerance and insulin response. Physiological Genomics. 2014;46:159–168. doi: 10.1152/physiolgenomics.00181.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nature Reviews. Genetics. 2008;9:15–26. doi: 10.1038/nrg2206. [DOI] [PubMed] [Google Scholar]
- Hill EM, Blow FC, Young JP, Singer KM. Family history of alcoholism and childhood adversity: joint effects on alcohol consumption and dependence. Alcoholism: Clinical and Experimental Research. 1994;18:1083–1090. doi: 10.1111/j.1530-0277.1994.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nature Reviews. Genetics. 2015;16:71–84. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Developmental Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- Jabbar S, Chastain LG, Gangisetty O, Cabrera MA, Sochacki K, Sarkar DK. Preconception Alcohol Increases Offspring Vulnerability to Stress. Neuropsychopharmacology. 2016;41:2782–2793. doi: 10.1038/npp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamerson PA, Wulser MJ, Kimler BF. Neurobehavioral effects in rat pups whose sires were exposed to alcohol. Brain Research. Developmental Brain Research. 2004;149:103–111. doi: 10.1016/j.devbrainres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews. Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nature Reviews. Genetics. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, et al. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genetics. 2010;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicology and Teratology. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Edwards A, Myers J, Cho SB, Adkins A, Dick D. The predictive power of family history measures of alcohol and drug problems and internalizing disorders in a college population. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2015;168B:337–346. doi: 10.1002/ajmg.b.32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Kim P, Choi CS, Park JH, Joo SH, Kim SY, Ko HM, et al. Chronic exposure to ethanol of male mice before mating produces attention deficit hyperactivity disorder-like phenotype along with epigenetic dysregulation of dopamine transporter expression in mouse offspring. Journal of Neuroscience Research. 2014;92:658–670. doi: 10.1002/jnr.23275. [DOI] [PubMed] [Google Scholar]
- Kim P, Park JH, Choi CS, Choi I, Joo SH, Kim MK, et al. Effects of ethanol exposure during early pregnancy in hyperactive, inattentive and impulsive behaviors and MeCP2 expression in rodent offspring. Neurochemical Research. 2013;38:620–631. doi: 10.1007/s11064-012-0960-5. [DOI] [PubMed] [Google Scholar]
- Knezovich JG, Ramsay M. The effect of preconception paternal alcohol exposure on epigenetic remodeling of the h19 and rasgrf1 imprinting control regions in mouse offspring. Frontiers in Genetics. 2012;3:10. doi: 10.3389/fgene.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Current Topics in Behavioral Neurosciences. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Medicine. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. The epigenetic landscape of alcoholism. International Review of Neurobiology. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvigne VL, Leonardson GR, Borzelleca J, Welty TK. Characteristics of grandmothers who have grandchildren with fetal alcohol syndrome or incomplete fetal alcohol syndrome. Maternal and Child Health Journal. 2008;12:760–765. doi: 10.1007/s10995-007-0308-y. [DOI] [PubMed] [Google Scholar]
- Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- Larriba E, del Mazo J. Role of Non-Coding RNAs in the Transgenerational Epigenetic Transmission of the Effects of Reprotoxicants. International Journal of Molecular Science. 2016;17:452. doi: 10.3390/ijms17040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Children of alcoholic parents--observed anomalies: discussion of 127 cases. Therapeutic Drug Monitoring. 2003;25:132–136. doi: 10.1097/00007691-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of microRNAs in brain of human alcoholics. Alcoholism: Clinical and Experimental Research. 2011;35:1928–1937. doi: 10.1111/j.1530-0277.2011.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Tsien JZ. Memory and the NMDA receptors. The New England Journal of Medicine. 2009;361:302–303. doi: 10.1056/NEJMcibr0902052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Diao L, Liu J, Jiang N, Zhang J, Wang H, et al. Paternal ethanol exposure and behavioral abnormities in offspring: associated alterations in imprinted gene methylation. Neuropharmacology. 2014;81:126–133. doi: 10.1016/j.neuropharm.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4:500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol. 2005;35:227–234. doi: 10.1016/j.alcohol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Henkhaus RS, Butler MG. Global DNA promoter methylation in frontal cortex of alcoholics and controls. Gene. 2012;498:5–12. doi: 10.1016/j.gene.2012.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjonen H, Sierra A, Nyman A, Rogojin V, Gröhn O, Linden AM, et al. Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLoS One. 2015;10:e0124931. doi: 10.1371/journal.pone.0124931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Ticku MK. Changes in methylation pattern of NMDA receptor NR2B gene in cortical neurons after chronic ethanol treatment in mice. Brain Research. Molecular Brain Research. 2004;121:19–27. doi: 10.1016/j.molbrainres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Meek LR, Myren K, Sturm J, Burau D. Acute paternal alcohol use affects offspring development and adult behavior. Physiology & Behavior. 2007;91:154–160. doi: 10.1016/j.physbeh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Pietrzykowski AZ, Tang Y, Sathyan P, Mayfield D, Keshavarzian A, et al. MicroRNAs: master regulators of ethanol abuse and toxicity? Alcoholism: Clinical and Experimental Research. 2010;34:575–587. doi: 10.1111/j.1530-0277.2009.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Pandey SC. Stress, epigenetics, and alcoholism. Alcohol Research. 2012;34:495–505. [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biological Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Sinha R, O’Malley SS. Parental history of anxiety and alcohol-use disorders and alcohol expectancies as predictors of alcohol-related problems. Journal of Studies on Alcohol and Drugs. 2009;70:227–236. doi: 10.15288/jsad.2009.70.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertility and Sterility. 2005;84:919–924. doi: 10.1016/j.fertnstert.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Nakao M. Epigenetics: interaction of DNA methylation and chromatin. Gene. 2001;278:25–31. doi: 10.1016/s0378-1119(01)00721-1. [DOI] [PubMed] [Google Scholar]
- Ng HH, Bird A. DNA methylation and chromatin modification. Current Opinion in Genetics & Development. 1999;9:158–163. doi: 10.1016/s0959-437x(99)80024-0. [DOI] [PubMed] [Google Scholar]
- Ng K, Pullirsch D, Leeb M, Wutz A. Xist and the order of silencing. EMBO Reports. 2007;8:34–39. doi: 10.1038/sj.embor.7400871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieratschker V, Batra A, Fallgatter AJ. Genetics and epigenetics of alcohol dependence. Journal of Molecular Psychiatry. 2013;1:11. doi: 10.1186/2049-9256-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Bergman D, Halje M, Engstrom W, Ward A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Proliferation. 2014;47:189–199. doi: 10.1111/cpr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer C, Alwan NA, Greenwood DC, Simpson NA, Hay AW, White KL, et al. Maternal alcohol intake prior to and during pregnancy and risk of adverse birth outcomes: evidence from a British cohort. Journal of Epidemiology and Community Health. 2014;68:542–549. doi: 10.1136/jech-2013-202934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Olino TM, Pettit JW, Klein DN, Allen NB, Seeley JR, Lewinsohn PM. Influence of parental and grandparental major depressive disorder on behavior problems in early childhood: a three-generation study. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:53–60. doi: 10.1097/chi.0b013e31815a6ae6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research. 2009;33:1615–1627. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. The Journal of Neuroscience. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I. Epigenetic control of gene expression in the alcoholic brain. Alcohol Research. 2013;35:69–76. [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. The Journal of Neuroscience. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portales-Casamar E, Lussier AA, Jones MJ, MacIsaac JL, Edgar RD, Mah SM, et al. DNA methylation signature of human fetal alcohol spectrum disorder. Epigenetics Chromatin. 2016;9:25. doi: 10.1186/s13072-016-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Denny A, Chen J, Ticku MK, Yan B, Henderson G. The site specific demethylation in the 5′-regulatory area of NMDA receptor 2B subunit gene associated with CIE-induced up-regulation of transcription. PLoS One. 2010;5:e8798. doi: 10.1371/journal.pone.0008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay M. Genetic and epigenetic insights into fetal alcohol spectrum disorders. Genome Medicine. 2010;2:27. doi: 10.1186/gm148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. Journal of Hepatology. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Riggs AD. X chromosome inactivation, differentiation, and DNA methylation revisited, with a tribute to Susumu Ohno. Cytogenetic and Genome Research. 2002;99:17–24. doi: 10.1159/000071569. [DOI] [PubMed] [Google Scholar]
- Roeleveld N, Vingerhoets E, Zielhuis GA, Gabreëls F. Mental retardation associated with parental smoking and alcohol consumption before, during, and after pregnancy. Preventive Medicine. 1992;21:110–119. doi: 10.1016/0091-7435(92)90010-f. [DOI] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Homanics GE. Paternal preconception ethanol exposure blunts hypothalamic-pituitary-adrenal axis responsivity and stress-induced excessive fluid intake in male mice. Alcohol. 2016;53:19–25. doi: 10.1016/j.alcohol.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, et al. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. The International Journal of Neuropsychopharmacology. 2014;17:1207–1220. doi: 10.1017/S1461145714000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK. Male germline transmits fetal alcohol epigenetic marks for multiple generations: a review. Addiction Biology. 2016;21:23–34. doi: 10.1111/adb.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. The Journal of Neuroscience. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2013;368:20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield KD, Gmel G, Patra J, Rehm J. Global burden of injuries attributable to alcohol consumption in 2004: a novel way of calculating the burden of injuries attributable to alcohol consumption. Population Health Metrics. 2012;10:9. doi: 10.1186/1478-7954-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nature Cell Biology. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittig LJ, Shukla PK, Herzing LB, Redei EE. Strain-specific vulnerability to alcohol exposure in utero via hippocampal parent-of-origin expression of deiodinase-III. FASEB Journal. 2011;25:2313–2324. doi: 10.1096/fj.10-179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Van Tol MJ, Van den Brink W, Van der Wee NJ, Van Buchem MA, Aleman A, et al. Family history of alcohol dependence and gray matter abnormalities in non-alcoholic adults. The World Journal of Biological Psychiatry. 2013;14:565–573. doi: 10.3109/15622975.2011.640942. [DOI] [PubMed] [Google Scholar]
- Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reproductive Toxicology. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Stockard CR, Papanicolaou GN. Further studies on the modification of the germ-cells in mammals: the effect of alcohol on treated guinea pigs and their descendants. Journal of Experimental Zoology. 1918;26:119–226. [Google Scholar]
- Stouder C, Somm E, Paoloni-Giacobino A. Prenatal exposure to ethanol: a specific effect on the H19 gene in sperm. Reproductive Toxicology. 2011;31:507–512. doi: 10.1016/j.reprotox.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Sheedy D, Harper C, Alkass K, et al. Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addiction Biology. 2011;16:499–509. doi: 10.1111/j.1369-1600.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Tata NR, Kühl M, Sirbu IO. Identification of a novel epigenetic regulatory region within the pluripotency associated microRNA cluster, EEmiRC. Nucleic Acids Research. 2011;39:3574–3581. doi: 10.1093/nar/gkq1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Harper KM, Graf EN, Redei EE. Thyroxine administration prevents matrilineal intergenerational consequences of in utero ethanol exposure in rats. Hormones and Behavior. 2016;82:1–10. doi: 10.1016/j.yhbeh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunc-Ozcan E, Sittig LJ, Harper KM, Graf EN, Redei EE. Hypothesis: genetic and epigenetic risk factors interact to modulate vulnerability and resilience to FASD. Frontiers in Genetics. 2014;5:261. doi: 10.3389/fgene.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WM, Cutter HS, Worobec TG, O’Farrell TJ, Bayog RD, Tsuang MT. Family history models of alcoholism: age of onset, consequences and dependence. Journal of Studies on Alcohol. 1993;54:164–171. doi: 10.15288/jsa.1993.54.164. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Knezovich J, Ramsay M. In utero alcohol exposure, epigenetic changes, and their consequences. Alcohol Research. 2013;35:37–46. [PMC free article] [PubMed] [Google Scholar]
- Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC. Alcohol, DNA methylation, and cancer. Alcohol Research. 2013;35:25–35. [PMC free article] [PubMed] [Google Scholar]
- Veazey KJ, Carnahan MN, Muller D, Miranda RC, Golding MC. Alcohol-induced epigenetic alterations to developmentally crucial genes regulating neural stemness and differentiation. Alcoholism: Clinical and Experimental Research. 2013;37:1111–1122. doi: 10.1111/acer.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR. Alcohol abuse and alcoholism: an overview. The Journal of Clinical Psychiatry. 2001;62(Suppl 20):4–10. [PubMed] [Google Scholar]
- Walker DM, Cates HM, Heller EA, Nestler EJ. Regulation of chromatin states by drugs of abuse. Current Opinion in Neurobiology. 2015;30:112–121. doi: 10.1016/j.conb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LL, Zhang Z, Li Q, Yang R, Pei X, Xu Y, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Human Reproduction. 2009;24:562–579. doi: 10.1093/humrep/den439. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling--a novel strategy to control excessive alcohol drinking. Translational Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let’s call the whole thing off. Epigenetics. 2007;2:22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Worobec TG, Turner WM, O’Farrell TJ, Cutter HS, Bayog RD, Tsuang MT. Alcohol use by alcoholics with and without a history of parental alcoholism. Alcoholism: Clinical and Experimental Research. 1990;14:887–892. doi: 10.1111/j.1530-0277.1990.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L, 3rd, Meyer ER. Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology (Berl) 1991;105:289–302. doi: 10.1007/BF02244324. [DOI] [PubMed] [Google Scholar]
- Yan W. Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance. Molecular and Cellular Endocrinology. 2014;398:24–30. doi: 10.1016/j.mce.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Robinson AM, Zucchi FC, Robbins JC, Babenko O, Kovalchuk O, et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Medicine. 2014;12:121. doi: 10.1186/s12916-014-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Progress in Biophysics and Molecular Biology. 2015;118:21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. The International Journal of Neuropsychopharmacology. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kusumo H, Sakharkar AJ, Pandey SC, Guizzetti M. Regulation of DNA methylation by ethanol induces tissue plasminogen activator expression in astrocytes. Journal of Neurochemistry. 2014;128:344–349. doi: 10.1111/jnc.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nature Reviews. Genetics. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Yuan Q, Mash DC, Goldman D. Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6626–6631. doi: 10.1073/pnas.1018514108. [DOI] [PMC free article] [PubMed] [Google Scholar]