Abstract
Purpose of review
To describe recent developments in therapies which target the molecular mechanisms in atopic dermatitis.
Recent findings
Current advances in the understanding of the molecular basis of atopic dermatitis are leading to the stratification of different atopic dermatitis phenotypes. New therapies offer the option to target-specific molecules involved in the pathophysiology of atopic dermatitis. Current new therapies under investigation aim to modulate specific inflammatory pathways associated with distinctive atopic dermatitis phenotypes, which would potentially translate into the development of personalized, targeted-specific treatments of atopic dermatitis.
Summary
Despite the unmet need for well tolerated, effective, and personalized treatment of atopic dermatitis, the current standard treatments of atopic dermatitis do not focus on the individual pathogenesis of the disease. The development of targeted, phenotype-specific therapies has the potential to open a new promising era of individualized treatment of atopic dermatitis.
Keywords: atopic dermatitis, biomarker, phenotype, targeted therapy
INTRODUCTION
Atopic dermatitis is a common inflammatory skin disease affecting up to 25% of children and up to 10% of adults in Western industrialized countries. The disease is clinically characterized by exacerbations and remissions of eczematous skin with inflammation, pruritus and excoriations, scaling, dry skin, and susceptibility for cutaneous bacterial and mycotic infections. Gene polymorphisms and mutations associated with defects of the epidermal barrier function are crucial in patients suffering from atopic dermatitis. Allergens and microbial proteins penetrate the skin subsequently inducing immunoglobulin E (IgE)-mediated sensitizations as part of pathophysiological mechanisms leading to atopic dermatitis; on the other hand about 20% of adult patients suffering from atopic dermatitis are not sensitized to any food or aeroallergens [1].
The standard treatment of atopic dermatitis does not take into account the individual pathogenesis of the disease; according to the guidelines the treatment predominantly focuses on the severity of skin inflammation and consists of topical treatment with corticosteroids and calcineurin inhibitors, ultraviolet light or systemic immunosuppression. Individualized treatment based on the selection of patients with the help of a combination of different phenotypic and immunologic biomarkers represents still a great unmet need which might be approached stepwise by prospective studies systematically studying this aspect together with novel rational-based therapeutic approaches which are or will be on the way in the next years.
Here, we review novel treatment strategies of clinical studies, developments on the way and published during the last year which target key molecules of the immune system altered in atopic dermatitis.
Box 1.
no caption available
ATOPIC DERMATITIS PATHOPHYSIOLOGY
The pathophysiology of atopic dermatitis is complex and therefore not fully understood yet. Damage in the structure and function of the skin barrier enhances the penetration of allergens to the skin and increases the risk of breaking the healthy interaction of the skin with the skin microbiome and environmental factors. In a context of an altered epidermal barrier, antigens encounter epidermal Langerhans cells and inflammatory epidermal dendritic cells, bearing trimeric high-affinity receptor for IgE. The antigens are taken up by these professional antigen presenting cells, initiating sensitization and leading to T-cell driven immune response. The pathophysiology of atopic dermatitis cannot be explained without cutaneous inflammation, which is a hallmark in atopic dermatitis. In the initial, acute, state of atopic dermatitis, T helper 2 and T helper 22 responses are augmented in the skin, with some implication of T helper 17 cells. The mediators produced in this phase contribute to the impairment of the skin barrier and activate different cell types, such as keratinocytes, that enhance the skin inflammation through release of proinflammatory cytokines (Fig. 1). The disease continues its progression with an increased role of Th1 pathways and a still important contribution of T helper 2 cells [2▪,3,4].
FIGURE 1.
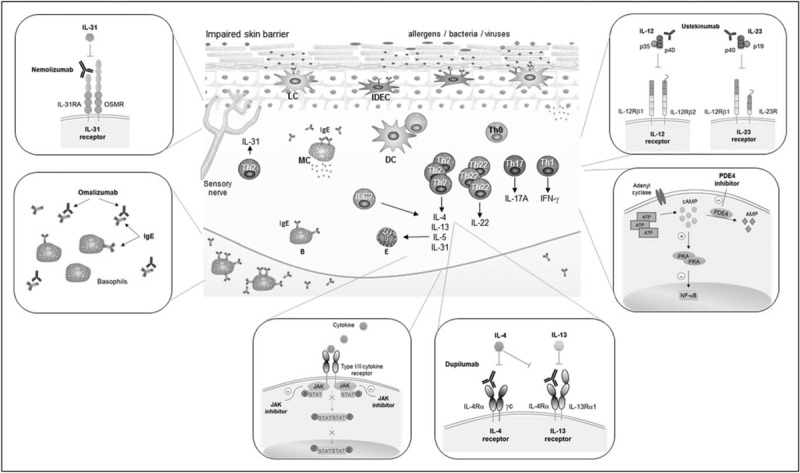
AD immunopathology and mechanisms of action of targeted therapies. AD immunopathology (central picture): damage in the skin barrier promotes the penetration of allergens into the skin and facilitate the entrance of microbial products. Antigens are taken up by LC and IDEC, initiating the sensitization and T-cell driven immune response. In the acute phase, Th2 and Th22 responses are augmented with contribution of Th17. The proinflammatory mediators produced in this phase further contribute to the impairment of the skin barrier and to the activation of different cell types that enhance the skin inflammation. Progression into chronicity involves an increased role of the Th1 pathway, but with important contributions of other T-cell subpopulations. Targeted therapies (external pictures, counter-clockwise from top left). Mechanism of action of nemolizumab (anti-IL-31 receptor antibody); omalizumab (anti-IgE antibody); JAK inhibitors; dupilumab (anti-IL-4/IL-13 receptors antibody); PDE4 inhibitors; and ustekinumab (anti-IL-12/-23p40 antibody). AD, atopic dermatitis; B, basophil; DC, dendritic cell; E, eosinophil. IDEC, inflammatory dendritic epidermal cells; ILC, innate lymphoid cell; LC, Langerhans cells; MC, mast cell; PDE, phosphodiesterase; Th, T-helper.
ATOPIC DERMATITIS PHENOTYPES AND BIOMARKERS
Atopic dermatitis can be stratified according to different criteria, such as age, severity, complications, and other factors. During the last decade great advances have been made in the clinical characterization of atopic dermatitis phenotypes. However, identification of different biomarkers that characterize each phenotype is essential for the development of individualized atopic dermatitis therapies.
ATOPIC DERMATITIS IN CHILDHOOD AND ADULTHOOD
It is well established that the typical clinical features and courses as well as trigger factors of atopic dermatitis might differ with age. Biomarkers which might characterize childhood and adulthood atopic dermatitis or other age states of atopic dermatitis have yet not been identified. In adults, the acute phase of atopic dermatitis has been recently linked to a strong T helper 2/T helper 22 activation and the contribution of T helper 17 cells, whereas the chronic phase was characterized by a marked T helper 1 polarization, although the T helper 2 pathway had still an important role in this phase. Data from a recent study provided evidence for a differential T-cell polarization in pediatric atopic dermatitis skin and adults with atopic dermatitis. More T helper 17-related cytokines and antimicrobial peptides were detectable in the skin of atopic dermatitis children than in the skin of adults with atopic dermatitis. T helper 9/interleukin (IL)-9, IL-33, and innate markers were enhanced in pediatric atopic dermatitis skin. The activation of the T helper 2 and T helper 22 axis was observable in both [5▪▪]. The findings obtained in the skin of atopic dermatitis children differed in part from the results obtained from peripheral blood, where a high T helper cell 2 activation within the skin-homing T cells could be found [6,5▪▪]. In serum, higher concentration of IL-31 and IL-33 was found in atopic dermatitis children as compared with adults with atopic dermatitis, whereas no differences could be found in thymic stromal lymphopoietin serum concentrations between both age groups [7].
ATOPIC DERMATITIS EXTRINSIC AND INTRINSIC
The differentiation of atopic dermatitis in extrinsic or intrinsic is based on total and specific serum IgE levels and a history of family or personal atopy, factors that are characteristic for extrinsic atopic dermatitis. Previous studies suggested that extrinsic atopic dermatitis had a predominant T helper 2 polarization compared with intrinsic atopic dermatitis phenotype. However, the concept has been recently challenged by the observation that mRNA levels of the T helper 2 cytokines IL-4, IL-5, IL-13, and IL-31 were augmented in the skin lesions of both atopic dermatitis phenotypes. mRNA levels of interferon (IFN)-γ, however, were increased in lesional intrinsic atopic dermatitis skin, together with the regulatory T-cell marker forkhead box protein 3. Factors that seem to be important for the decreased production of IgE in the intrinsic atopic dermatitis phenotype. In addition, T-cell subsets such as T helper 17 and T helper 22 were found to be augmented in intrinsic atopic dermatitis, with a higher immune activation in intrinsic atopic dermatitis compared with extrinsic atopic dermatitis [1]. In line with these observations, a recent study assessed the differences of gene expression in the skin of patients with mild extrinsic or intrinsic atopic dermatitis, psoriasis and healthy study participants. The expression of genes related to inflammation were increased in intrinsic atopic dermatitis compared with extrinsic atopic dermatitis [8].
ATOPIC DERMATITIS SEVERITY
In terms of atopic dermatitis severity, atopic dermatitis can be categorized into mild, moderate, or severe. Several studies have tried to establish correlations between different biomarkers and atopic dermatitis severity. However because of the heterogeneous nature of atopic dermatitis, the establishment of such correlations is complex. A recent study has suggested that the integration of biomarkers from lesional and nonlesional atopic dermatitis skin together to blood biomarkers gave the best correlation with atopic dermatitis severity measured by scoring atopic dermatitis (SCORAD) index score [9▪]. Another study found that a combination of four serum biomarkers: thymus and activation-regulated chemokine (TARC)/CC chemokine ligand 17 (CCL17), pulmonary and activation-regulated chemokine (PARC)/CC chemokine ligand 18 (CCL18), IL-22, and sIL-2R, correlated better with atopic dermatitis severity (six area six sign atopic dermatitis score) than individual biomarkers [10]. However, TARC/CCL17, a chemokine involved in skin homing of CC chemokine receptor 4-expressing T cells, has been postulated as a reliable serum marker for atopic dermatitis severity in a recent meta-analysis [11]. Further studies in large cohorts of patients would be needed to explore potential biomarkers for atopic dermatitis severity.
THERAPEUTIC STRATEGIES
Targeted therapies are expected to open a new promising era for personalized treatment of atopic dermatitis. The new therapies aim to target-specific inflammatory pathways involved in the pathophysiology of atopic dermatitis, such as the T helper 2 and inflammatory axis, through modulation of cytokines, receptors, and other molecules involved. New therapies also aim to target pathways such as T helper 1, T helper 17, and T helper 22, which play an important role in the cutaneous inflammatory response. Components of the skin barrier represent another promising therapeutic target.
ANTI-IL-4 RECEPTOR ANTIBODY: DUPILUMAB
Dupilumab is a human mAb binding to the shared alpha subunit of the IL-4 and IL-13 receptors, and modulating the activation of T cells because of IL-4 and IL-13 pathways (Fig. 1). The receptor is additionally expressed on dendritic cells, keratinocytes, or eosinophils. Dupilumab induced a dose-dependent improvement of the molecular signature in atopic dermatitis skin in in-vitro studies. Suppression of mRNA expression of genes involved in the activation of T cells, dendritic cells or eosinophils was observed [12]. Early clinical trials of dupilumab have shown clinical improvement in adults with moderate-to-severe atopic dermatitis [13–15]. The effect of dupilumab on atopic dermatitis has been recently investigated in two large phase-III-trials [16▪▪]. In both trials adults suffering from atopic dermatitis inadequately controlled by topical treatment were included. Patients were treated over a period of 16 weeks with placebo or with 300 mg dupilumab administered weekly or in 2-week intervals. The primary endpoint of both trials was the investigator's global assessment (IGA) scale, a validated scoring system for the severity of atopic dermatitis. The results of both studies were similar and a reduction of 2 points or more in IGA was observed at week 16 in 38%/36% of patients treated with dupilumab every 2 weeks, in 37/36% of patients treated with dupilumab in weekly intervals and only 10/8% of patients of the placebo group. In both trials there was a 75% reduction of the eczema area and severity index (EASI) in patients treated with dupilumab following one of the two dosing regimes [16▪▪].
Overall both phase-III-studies demonstrated a significant improvement of the severity of atopic dermatitis, a reduction of pruritus and improved quality of life compared to placebo. In March 2017, the US Food and Drug Administration (FDA) approved dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults.
ANTI-IL-31 RECEPTOR ANTIBODY: NEMOLIZUMAB OR CIM331
Itch and subsequent scratching is a trigger of atopic dermatitis. IL-31 has been described as a mediator inducing intense pruritus [17]. T helper 2 cells are the predominant producers of IL-31 whose receptor A is expressed by keratinocytes and a subset of dorsal root ganglion neurons. IL-31 has been found to induce pruritus by activation of sensory nerves in the skin [18]. Pruritus promotes exacerbation of atopic dermatitis status, sleeping disorders, with a negative impact on the patients’ quality of life. Nemolizumab is a humanized mAb that binds IL-31 receptor A in certain cells such as neurons, to block the binding of IL-31 and inhibit IL-31 signaling (Fig. 1).
In a randomized, double-blind, placebo-controlled phase I/Ib study, nemolizumab was used for the treatment of 36 patients with moderate-to-severe atopic dermatitis despite treatment with topical corticosteroids. A single subcutaneous administration was well tolerated and pruritus visual analogue scale decreased significantly in all dosing groups compared with placebo [19]. In a phase II, randomized, multicenter, double-blind, placebo-controlled trial, nemolizumab was evaluated in adults with moderate-to-severe atopic dermatitis for a period of 12 weeks. The primary endpoint was the percentage of improvement from baseline and week 12 for the score on visual analogue scale. In total, 216 patients completed the study, which showed a significant, dose-dependent improvement of pruritus in all the groups that received nemolizumab every 4 weeks compared with the placebo group [20▪▪].
ANTI-IGE ANTIBODY: OMALIZUMAB
Omalizumab is a humanized monoclonal anti-IgE antibody binding to the Cε3 domain of IgE blocking the binding of free serum IgE antibodies to the membrane of effector cells (Fig. 1). Efficacy is proven in severe allergic asthma and in urticaria, diseases for which the antibody is already registered.
In a case series, nine patients suffering from recalcitrant atopic dermatitis, defined as persisting atopic dermatitis despite treatment with more than four different drugs, were treated with a fixed dose of omalizumab subcutaneously. Based on physicians’ assessment, 62.5% of the patients experienced a benefit of the treatment, overall 50% had a good or excellent response to the mAb. A limitation of this study was that the severity of atopic dermatitis was not assessed using a validated scoring system (SCORAD, IGA) [21]. In a literature search the authors identified 26 studies which report about the efficacy of treatment of atopic dermatitis with omalizumab in a total of 174 patients. A beneficial effect was documented in 74.1% of all patients, with a degree of improvement from little to complete response. Omalizumab was safe and well tolerated [21]. However, the efficacy was not validated in phase III trials. Therefore, the use of omalizumab cannot be recommended in general in the clinical practice.
Another mAb targeting IgE, ligelizumab, which binds to the Cε3 domain of the IgE molecule with a higher affinity compared with Omalizumab [22], is currently under investigation for atopic dermatitis treatment.
ANTI-IL-5 ANTIBODY: MEPOLIZUMAB
Mepolizumab is a fully humanized monoclonal anti-IL-5 antibody registered for the treatment of severe eosinophilic asthma. Efficacy has also been demonstrated for chronic rhinosinusitis, eosinophilic oesophagitis and hypereosinophilia. In patients suffering from severe atopic dermatitis, two injections of the antibody were ineffective instead of a significantly reduced number of peripheral blood eosinophils in the active treatment group [23]. In a double-blind placebo-controlled study on 42 patients, mepolizumab did not show any effect on the result of atopy patch testing with extracts of house dust mite, grass pollen, or cat allergen. A significant reduction of blood eosinophils was observed in the active group. In skin biopsies taken from patch tests the influx of eosinophils into the tissue was not significantly reduced compared with the placebo group [24]. Limitation of both trials was the very short therapy with only two shots of mepolizumab within an interval of 1 week.
JANUS KINASE INHIBITORS: TOFACITINIB
Several cytokines utilize the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway for intracellular signaling. Intracellular JAK proteins associate with cytokine receptors I/II and become activated after the recognition by the specific receptor of extracellular ligands. Upon activation of JAK proteins, phosphorylation, dimerization, and translocation to the nucleus of specific STAT proteins takes place. The JAK protein family is formed by JAK1, JAK2, JAK3, and tyrosine kinase 2. Each JAK protein associates with multiple receptors of cytokines relevant in several inflammatory diseases [25]. JAK-STAT pathway has been described to be essential for T helper 2 cell differentiation; in particular JAK1, JAK3 and STAT6 are involved in signaling of IL-4. Tofacitinib citrate, a JAK1/3 inhibitor approved for the treatment of rheumatoid arthritis, is currently under investigation for its therapeutic potential in atopic dermatitis (Fig. 1). Tofacitinib taken orally showed a marked clinical improvement of six patients with moderate-to-severe atopic dermatitis treated with the drug. Although the study lacks placebo group and blinding, it implies that tofacitinib might represent a potential therapeutical agent for atopic dermatitis [26]. More recently, the efficacy of topical tofacitinib in 69 adults with mild-to-moderate atopic dermatitis was evaluated in a phase IIa, randomized, double-blind, vehicle-controlled trial. Significantly higher reduction of the EASI scores in the group treated with tofacitinib 2% ointment than the control group as well as a significant reduction of pruritus was observed [27].
PHOSPHODIESTERASE 4 INHIBITORS: CRISABOROLE AND OPA-15406
Phosphodiesterase 4 (PDE4) regulates the production of inflammatory cytokines. Under normal conditions, intracellular second messenger cyclic AMP (cAMP) is present in considerable quantities and PDE4 mediates its consumption. However, in patients with atopic dermatitis, the activity of PDE4 in circulating inflammatory cells is increased with decrease of cAMP and overexpression of proinflammatory cytokines. Inhibition of PDE4 activity led to a better regulation of cAMP levels with reduction of proinflammatory cytokine mediator release (Fig. 1). In line with these observations, PDE4 inhibiting agents have been analyzed for their therapeutical potential in atopic dermatitis. That is the case for crisaborole, a topical compound that has been recently approved by the FDA for atopic dermatitis treatment. Other PDE4 inhibitors such as the topical medication OPA-15406 (also known as MM36), or apremilast, a systemic PDE4 inhibitor are currently under investigation [28▪].
Crisaborole is a small, lipophilic, boron-based molecule with an effective skin penetration. Early clinical trials showed that crisaborole topical ointment is well tolerated with low risk for systemic side-effects, as the molecule is quickly metabolized into its inactive metabolites [29–32]. In a phase III multicenter, randomized, double-blind, vehicle-controlled clinical study, crisaborole was assessed for its efficacy and safety in patients with 2 years of age or older with mild-to-moderate atopic dermatitis. Primary endpoint was an Investigator's Static Global Assessment (ISGA) score of clear or almost clear skin with 2 grade or more improvement from baseline at day 29 of treatment. Significantly more crisaborole-treated patients achieved success on the ISGA score than vehicle-treated patients. ISGA score success was achieved earlier in crisaborole-treated patients than in those treated with the vehicle. Improvement of pruritus was greater and earlier in crisaborole-treated patients than in the vehicle treated group [33▪]. Another topical PDE4 inhibitor, OPA-15406, has been investigated in a phase II, randomized, double-blind, vehicle-controlled, dose-finding trial. Patients from 10 to 70 years of age with mild or moderate atopic dermatitis were included. The primary endpoint IGA score of 0 or 1 with a 2 grade or greater reduction in atopic dermatitis severity was achieved in the OPA-15406 treated group with the highest concentration assessed. The levels of OPA-15406 in blood were negligible [34▪].
ANTI-IL-12/-23 P40 ANTIBODY: USTEKINUMAB
Although many agents under investigation for atopic dermatitis therapy use the T helper 2 axis as a target, atopic dermatitis cannot be explained exclusively in the context of T helper 2 pathway activation. T helper 1, T helper 17, and T helper 22 pathways have also been shown to play an important role in the cutaneous inflammatory response in atopic dermatitis. In that respect, the IL-12/IL23p40 antagonist ustekinumab suppresses T helper 1, T helper 17, and T helper 22 activation, by blocking the cytokines IL-12 and IL-23, targeting a common subunit (p40) shared by both cytokines (Fig. 1). Ustekinumab is registered for the treatment of psoriasis and is under investigation as a potential therapeutic drug in atopic dermatitis. In a phase II, placebo-controlled, double-blinded, single-center clinical trial, 33 patients suffering from moderate-to-severe atopic dermatitis were treated with either ustekinumab or placebo. The primary endpoint was SCORAD 50 at 16 weeks, which is the proportion of study participants who achieved 50% improvement or higher at 16 weeks from baseline SCORAD. Higher SCORAD 50 responses in the ustekinumab group compared with placebo were found at week 12 and 16, but did not reach statistical significance. Interestingly, by the use of gene expression analysis, a robust modulation of T helper 1, T helper 17, and T helper 22 pathway ustekinumab was found [35].
SKIN BARRIER DAMAGE REPAIR
Basis therapy is aimed to repair the damaged skin barrier in atopic dermatitis to prevent the penetration of allergens into the skin and subsequent IgE-mediated sensitization. Basis therapy has been demonstrated to prevent the development of atopic dermatitis in newborns [36]. In total, 124 newborns at high risk for the development of atopic dermatitis were either randomized to a control group or to the intervention group where the use of emollients at least once a day was recommended. After 6 months, the incidence of atopic dermatitis was 43% in the control group but only 22% in the intervention group (P = 0,017). Therefore a consequent basis therapy can prevent the development of atopic dermatitis in high-risk children.
If basis therapy is able to repair the skin barrier and to prevent the penetration of allergens into the skin and therefore prevent subsequent IgE-mediated sensitization, a defect of the skin barrier may be a biomarker for the efficacy of basis therapy. There is a need for clinical trials demonstrating that the effect of basis therapy is higher in patients with a skin barrier defect compared with those without such a defect.
CONCLUSION
Personalized medicine in atopic dermatitis, in comparison to other diseases, is still at a very early stage. The current management of atopic dermatitis does not take into account the spectrum of clinical and immunologic subtypes, which leads to an unmet need for well tolerated, effective and personalized treatment of the disease. The development of a pathophysiology-based classification of atopic dermatitis is the requirement for a rational use of patient tailored therapy. In that context, biomarkers would help to define atopic dermatitis phenotypes in order to identify potential targets for new therapeutic strategies. The increased knowledge generated in recent years regarding the molecular basis of atopic dermatitis is expected to contribute to the definition of specific biomarkers or group of biomarkers that categorize the heterogeneous clinical phenotypes in atopic dermatitis.
New therapeutic approaches aim to affect target structures involved in the pathophysiology of the disease in a phenotype-specific way. Atopic dermatitis phenotypes with a strong implication of the T helper 2 inflammatory axis, would potentially benefit from therapies based in the modulation of T helper 2-related cytokines or their receptors. That is the case for the recently FDA approved anti-IL-4 receptor antibody dupilumab for the treatment of moderate-to-severe atopic dermatitis. New therapies also aim to target pathways such as T helper 1, T helper 17, and T helper 22, which play an important role in the cutaneous inflammatory response in specific atopic dermatitis phenotypes. Basis therapy able to repair the skin barrier and to prevent the penetration of allergens is also currently under investigation.
Acknowledgements
The study was supported by Cluster of Excellence ImmunoSensation of the German Research Foundation (DFG), a BONFOR Grant of the University of Bonn and CK-CARE.
Financial support and sponsorship
N.N. received lectures fees from Novartis.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Suárez-Fariñas M, Dhingra N, Gittler J, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol 2013; 132:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪.Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–1122. [DOI] [PubMed] [Google Scholar]; Thorough summary of the latest knowledge of the clinical and molecular aspects of atopic dermatitis.
- 3.Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 2013; 68:974–982. [DOI] [PubMed] [Google Scholar]
- 4.Cabanillas B, Novak N. Atopic dermatitis and filaggrin. Curr Opin Immunol 2016; 42:1–8. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Esaki H, Brunner PM, Renert-Yuval Y, et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol 2016; 138:1639–1651. [DOI] [PubMed] [Google Scholar]; Interesting study that characterizes the differences of the skin phenotype of pediatric atopic dermatitis, controls and adults with atopic dermatitis.
- 6.Czarnowicki T, Esaki H, Gonzalez J, et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J Allergy Clin Immunol 2015; 136:941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol 2016; 30:1930–1938. [DOI] [PubMed] [Google Scholar]
- 8.Martel BC, Litman T, Hald A, et al. Distinct molecular signatures of mild extrinsic and intrinsic atopic dermatitis. Exp Dermatol 2016; 25:453–459. [DOI] [PubMed] [Google Scholar]
- 9▪.Ungar B, Garcet S, Gonzalez J, et al. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J Invest Dermatol 2017; 137:603–613. [DOI] [PubMed] [Google Scholar]; The study identified an unbiased set of biomarkers reflecting the disease activity of atopic dermatitis.
- 10.Thijs JL, Nierkens S, Herath A, et al. A panel of biomarkers for disease severity in atopic dermatitis. Clin Exp Allergy 2015; 45:698–701. [DOI] [PubMed] [Google Scholar]
- 11.Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol 2015; 15:453–460. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton JD, Suárez-Fariñas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014; 134:1293–1300. [DOI] [PubMed] [Google Scholar]
- 13.Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014; 371:130–139. [DOI] [PubMed] [Google Scholar]
- 14.Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016; 387:40–52. [DOI] [PubMed] [Google Scholar]
- 15.Simpson EL, Gadkari A, Worm M, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J Am Acad Dermatol 2016; 75:506–615. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375:2335–2348. [DOI] [PubMed] [Google Scholar]; This is the most recent controlled study that evaluated the efficacy of the anti-IL-4 receptor antibody dupilumab for the treatment of atopic dermatitis.
- 17.Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allergy Immunol 2016; 51:263–292. [DOI] [PubMed] [Google Scholar]
- 18.Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014; 133:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemoto O, Furue M, Nakagawa H, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol 2016; 174:296–304. [DOI] [PubMed] [Google Scholar]
- 20▪▪.Ruzicka T, Hanifin JM, Furue M, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017; 376:826–835. [DOI] [PubMed] [Google Scholar]; This is the most recent controlled study that evaluated the efficacy of the anti-IL-31 receptor antibody nemolizumab for the treatment of atopic dermatitis.
- 21.Holm JG, Agner T, Sand C, Thomsen SF. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol 2017; 56:18–26. [DOI] [PubMed] [Google Scholar]
- 22.Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy 2014; 44:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldhoff JM, Darsow U, Werfel T, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 2005; 60:693–696. [DOI] [PubMed] [Google Scholar]
- 24.Oldhoff JM, Darsow U, Werfel T, et al. No effect of antiinterleukin-5 therapy (mepolizumab) on the atopy patch test in atopic dermatitis patients. Int Arch Allergy Immunol 2006; 141:290–294. [DOI] [PubMed] [Google Scholar]
- 25.Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol 2017; 76:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol 2015; 73:395–399. [DOI] [PubMed] [Google Scholar]
- 27.Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 2016; 175:902–911. [DOI] [PubMed] [Google Scholar]
- 28▪.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017; 139:S65–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study summarizes current research about the pathophysiology of atopic dermatitis and knowledge about targeted therapies.
- 29.Zane LT, Kircik L, Call R, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure study. Pediatr Dermatol 2016; 33:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murrell DF, Gebauer K, Spelman L, Zane LT. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol 2015; 14:1108–1112. [PubMed] [Google Scholar]
- 31.Stein Gold LF, Spelman L, Spellman MC, et al. A phase 2, randomized, controlled, dose-ranging study evaluating crisaborole topical ointment, 0.5% and 2% in adolescents with mild to moderate atopic dermatitis. J Drugs Dermatol 2015; 14:1394–1399. [PubMed] [Google Scholar]
- 32.Tom WL, Van Syoc M, Chanda S, Zane LT. Pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2% in adolescents with atopic dermatitis: an open-label phase 2a study. Pediatr Dermatol 2016; 33:150–159. [DOI] [PubMed] [Google Scholar]
- 33▪.Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016; 75:494.e4–503.e4. [DOI] [PubMed] [Google Scholar]; Two phase III studies that evaluated the efficacy of the inhibitor crisaborole in patients aged 2 years or older with mild-to-moderate atopic dermatitis.
- 34▪.Hanifin JM, Ellis CN, Frieden IJ, et al. OPA-15406, a novel, topical, nonsteroidal, selective phosphodiesterase-4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): a phase-II randomized, double-blind, placebo-controlled study. J Am Acad Dermatol 2016; 75:297–305. [DOI] [PubMed] [Google Scholar]; The study evaluates the clinical activity of the topical PDE4 inhibitor OPA-15406 in patients with mild-to-moderate atopic dermatitis.
- 35.Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol 2017; 26:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134:818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]


